Abstract
IMPORTANCE
Screening for osteoporosis with bone mineral density (BMD) is recommended for older adults. It is unclear whether repeating a BMD screening test improves fracture risk assessment.
OBJECTIVES
To determine whether changes in BMD after 4 years provide additional information on fracture risk beyond baseline BMD and to quantify the change in fracture risk classification after a second BMD measure.
DESIGN, SETTING, AND PARTICIPANTS
Population-based cohort study involving 310 men and 492 women from the Framingham Osteoporosis Study with 2 measures of femoral neck BMD taken from 1987 through 1999.
MAIN OUTCOMES AND MEASURES
Risk of hip or major osteoporotic fracture through 2009 or 12 years following the second BMD measure.
RESULTS
Mean age was 74.8 years. The mean (SD) BMD change was −0.6% per year (1.8%). Throughout a median follow-up of 9.6 years, 76 participants experienced an incident hip fracture and 113 participants experienced a major osteoporotic fracture. Annual percent BMD change per SD decrease was associated with risk of hip fracture (hazard ratio [HR], 1.43 [95% CI, 1.16 to 1.78]) and major osteoporotic fracture (HR, 1.21 [95% CI, 1.01 to 1.45]) after adjusting for baseline BMD. At 10 years’ follow-up, 1 SD decrease in annual percent BMD change compared with the mean BMD change was associated with 3.9 excess hip fractures per 100 persons. In receiver operating characteristic (ROC) curve analyses, the addition of BMD change to a model with baseline BMD did not meaningfully improve performance. The area under the curve (AUC) was 0.71 (95% CI, 0.65 to 0.78) for the baseline BMD model compared with 0.68 (95% CI, 0.62 to 0.75) for the BMD percent change model. Moreover, the addition of BMD change to a model with baseline BMD did not meaningfully improve performance (AUC, 0.72 [95% CI, 0.66 to 0.79]). Using the net reclassification index, a second BMD measure increased the proportion of participants reclassified as high risk of hip fracture by 3.9% (95% CI, −2.2% to 9.9%), whereas it decreased the proportion classified as low risk by −2.2% (95% CI, −4.5% to 0.1%).
CONCLUSIONS AND RELEVANCE
In untreated men and women of mean age 75 years, a second BMD measure after 4 years did not meaningfully improve the prediction of hip or major osteoporotic fracture. Repeating a BMD measure within 4 years to improve fracture risk stratification may not be necessary in adults this age untreated for osteoporosis.
Bone mineral density (BMD) testing is important in the management of osteoporosis. Along with clinical risk factors for fracture, BMD is incuded in the World Health Organization Fracture Risk Assessment Tool (FRAX),1 a screening test that estimates the 10-year absolute risk of hip and major osteoporotic fracture. Guidelines for initiating pharmacologic treatment for osteoporosis are based on BMD in conjunction with risk classification scores.2
Despite the utility of BMD, the value of repeating a BMD screening test is unclear.3–5 Given limitations in the precision of BMD testing, the US Preventive Services Task Force recommends waiting a minimum of 2 years to obtain a second BMD measure but notes that “longer intervals may be necessary to improve fracture prediction.”6 Data are inconsistent on whether BMD change improves fracture prediction beyond baseline BMD.7–11
Currently, Medicare reimburses for BMD screening every 2 years regardless of baseline BMD and without a restriction on the number of repeat tests. Among Medicare beneficiaries 75 years or older, an estimated 417 080 (5%) without recent BMD testing receive a BMD screening test in a given year.12 Twenty-two percent of screened beneficiaries receive a repeat BMD test within 3 years,12 on average 2.2 years apart.13
Given the priority of reducing health care costs while improving quality of care, it is important to determine whether repeat BMD screening is useful. Our primary objective was to determine whether BMD change throughout 4 years provides additional information on hip and major osteoporotic fracture risk after accounting for baseline BMD in women and men from the Framingham Osteoporosis Study. We also examined whether BMD change predicts fracture differently according to clinical risk factors, such as age and fracture risk score. Finally, we quantified the change in fracture risk classification after a second BMD measure.
Methods
Participants
This study was approved by the Hebrew SeniorLife institutional review board. All participants gave written informed consent. The Framingham original cohort began in 1948 with enrollment of a two-thirds sample of residents living in Framingham, Massachusetts.14 Since 1948, these participants have been examined every 2 years. From 1987 through 1999, all surviving participants were invited for 3 BMD tests, approximately 4 years apart. Participants of our study included members with 2 BMD measures. The mean time between measures was 3.7 years (range, 2.4–6.0 years). We followed up participants from the second BMD measure until death or through 2009 or 12 years of follow-up.
Because individuals with a hip fracture are recommended for pharmacologic treatment of osteoporosis regardless of BMD,2 we excluded members with a hip fracture prior to the second test.
Bone Mineral Density
Bone mineral density of the femoral neck was measured using a dual-photon absorptiometer from 1987 through 1991 and a dual-energy x-ray absorptiometer from 1992 through 1999 (both manufactured by Lunar Corp). The BMD was measured on different scanners for 91.0% of participants, with adjustments made using the cross-calibration of the 2 instruments.15 The coefficients of variation for the individual scanners ranged from 1.7% to 2.6% at the femoral neck.
Hip and Major Osteoporotic Fracture
Hip fractures were defined as fractures of the proximal femur and confirmed with medical records.16 Fractures at sites other than the hip were not confirmed by medical record review. We considered 2 outcomes: hip and major osteoporotic fracture (hip, spine, forearm, or shoulder).
Clinical Characteristics
Information on clinical characteristics was obtained from the Framingham Study examination closest to the first and second BMD tests. Weight, measured to the nearest pound (in light clothing), and height (without shoes), measured to the nearest quarter-inch, were used to calculate body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). Change in weight was calculated using measurements at the time of the first and second BMD test. History of fracture included any self-reported fracture (except fingers, toes, skull, or face) occurring as an adult.
Information on smoking, glucocorticoid use (any current use), and alcohol use was ascertained by a standardized questionnaire and medication review. Excess alcohol was defined as 27 grams or more of alcohol per day1 and calculated from self-reported weekly wine, beer, and cocktail intake. Rheumatoid arthritis was determined by a physician following a history and physical examination. No information on family history of hip fracture was collected; thus, we assumed all participants had no parental history of hip fracture.
Fracture Risk Score
Information on clinical characteristics and BMD was provided to the World Health Organization to calculate risk scores using FRAX, version 3.6. Risk score of hip and major osteoporotic fracture were modeled in 3 ways: using (1) historic clinical characteristics and baseline BMD, (2) updated clinical characteristics and baseline BMD, and (3) updated clinical characteristics and the second BMD measure. Model 2 is most representative of what a clinician faces when deciding whether to order a repeat BMD test.
Statistical Analysis
We used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% CIs to estimate the association between percentage and absolute BMD change (per sex-specific SD) and risk of incident hip and major osteoporotic fracture, separately. Models were adjusted for updated clinical characteristics and baseline BMD. The proportional hazards assumption was violated at about 13 years’ follow-up; thus, we truncated follow-up at 12 years for participants with longer follow-up (n = 261). We estimated the risk difference of fracture if the BMD change equaled the mean change in the population vs 1 SD below the mean with 10 years’ follow-up.17
In a secondary analysis, we used negative binomial regression to estimate the association between percentage and absolute BMD change and rate of hip and major osteoporotic fracture over 12 years.
We used unconditional logistic regression models with receiver operating characteristic (ROC) curves to compare the models assessing risk of hip and major osteoporotic fracture using baseline BMD and BMD change. We performed analyses stratified by age, sex, weight loss, BMI, baseline T score, and fracture risk score (calculated with updated clinical characteristics and baseline BMD). We formally tested for interactions by including a product term in the regression models. Significance testing was 2-sided, and statistical significance was defined as P ≤ .05.
We plotted the risk score of hip fracture with updated clinical characteristics and baseline BMD vs risk score with updated clinical characteristics and the second BMD measure. We used the net reclassification index (NRI)18 to quantify the change in risk classification after a second BMD measure. We used clinically meaningful cut points to classify risk.19 High risk was defined as an individual with a risk score of hip fracture of 3% or greater or a risk score of major osteoporotic fracture of 20% or greater. Low risk was defined as an individual with a risk score of hip fracture less than 3% or risk score of major osteoporotic fracture less than 20%. We used SAS (SAS Institute Inc, version 9.3) for all analyses.
Results
A total of 1766 members of the Framingham Osteoporosis Study were alive in January 1992, when participants began a second round of BMD testing. We excluded 931 participants (52.7%) without 2 BMD tests and 33 participants (1.9%) with hip fracture before the second BMD test. Excluded participants were older (mean [SD], 82.1 years [6.4] vs 78.8 years [4.5]), more likely to be women (68.7% vs 61.4%), yet had a similar BMI (mean [SD], 26.0 [4.8] vs 26.8 [4.6]) compared with those included in our analytic sample.
Participants in our study included 310 men and 492 women. The mean age at the baseline BMD test was 74.8 years. At baseline, 21.9% of participants had a T score of −1.0 or higher and 52.7% of participants had a T score between −1.01 and −2.49. The mean BMD change was −0.6% per year and ranged from 5.6% per year (gain) to −9.0% per year (loss).
The median follow-up after the second BMD test was 9.6 years (interquartile range [IQR], 5.3–12 years). During follow-up, 113 participants (14.1%) experienced 1 or more major osteoporotic fractures (88 hip, 24 spine, 5 shoulder, and 33 forearm fractures). Participants who experienced a hip or major osteoporotic fracture were more likely to be women, to report a prior fracture, and to have a lower BMI and baseline BMD than participants without a major osteoporotic fracture (Table 1). On average, participants who experienced a major osteoporotic fracture lost 0.006 g/cm2 or 0.9% per year compared with 0.005 g/cm2 or 0.6% per year in participants without a fracture.
Table 1.
Participant Characteristics of the Framingham Osteoporosis Study With 2 Measures of BMD Characterized Hip or Major Osteoporotic Fractures During 12 Years of Follow-up
| Characteristicsa | No. (%) of Participants | |||
|---|---|---|---|---|
| All Participants (N = 802) | No Major Osteoporotic Fracture (n = 689) | Major Osteoporotic Fractureb (n = 113) | Hip Fracture (n = 76) | |
| Age at baseline BMD, mean (SD), y | 74.8 (4.5) | 74.9 (4.5) | 74.5 (4.3) | 74.8 (4.4) |
| Age at second BMD measure, mean (SD), y | 78.8 (4.5) | 78.8 (4.5) | 78.5 (4.2) | 78.8 (4.3) |
| Women | 492 (61.4) | 397 (57.6) | 95 (84.1) | 62 (81.6) |
| Prior fracture | 256 (31.9) | 204 (29.6) | 52 (46.0) | 31 (40.8) |
| BMI, mean (SD) | 26.8 (4.6) | 27.0 (4.5) | 25.4 (4.9) | 25.2 (5.4) |
| Weight change between baseline and second BMD measure, mean (SD), lb | −2.3 (9.8) | −2.3 (10.0) | −1.8 (8.1) | −2.5 (8.2) |
| Current smoker | 63 (7.9) | 52 (7.6) | 11 (9.7) | 10 (13.2) |
| Excess alcohol use | 60 (7.5) | 53 (7.7) | 7 (6.2) | 4 (5.3) |
| Glucocorticoid use | 26 (3.2) | 22 (3.2) | 4 (3.5) | 2 (2.6) |
| Rheumatoid arthritis | 8 (1.0) | 7 (1.0) | 1 (0.9) | 0 |
| Baseline | ||||
| BMD, mean (SD), g/cm2c | 0.79 (0.14) | 0.80 (0.14) | 0.71 (0.12) | 0.70 (0.13) |
| T scored | ||||
| ≥−1.0 | 176 (21.9) | 169 (24.5) | 7 (6.2) | 4 (5.3) |
| −1.01 to −2.49 | 423 (52.7) | 375 (54.4) | 48 (42.5) | 29 (38.2) |
| ≤−2.5 | 203 (25.3) | 145 (21.0) | 58 (51.3) | 43 (56.6) |
| Hip fracture risk score, median (IQR), % | ||||
| Baseline | 3.5 (1.8–6.3) | 3.3 (1.7–5.8) | 5.8 (3.2–9.9) | 6.8 (3.2–9.9) |
| Second BMD measurec | 4.1 (2.4–7.1) | 3.8 (2.2–6.3) | 6.8 (4.1–11.1) | 7.7 (4.2–11.1) |
| Second BMD measure | ||||
| BMD, mean (SD), g/cm2 | 0.77 (0.15) | 0.79 (0.15) | 0.68 (0.12) | 0.67 (0.13) |
| T score | ||||
| ≥−1.0 | 148 (18.5) | 140 (20.3) | 8 (7.1) | 5 (6.6) |
| −1.01 to −2.49 | 382 (47.6) | 343 (49.8) | 39 (34.5) | 23 (30.3) |
| ≤−2.5 | 272 (33.9) | 206 (29.9) | 66 (58.4) | 48 (63.2) |
| Hip fracture risk score using the second BMD measure, median (IQR), %d | 4.6 (2.6 to 8.3) | 4.2 (2.5 to 7.5) | 8.2 (4.7 to 13.5) | 8.6 (5.0 to 13.8) |
| Change in BMD, mean (SD) per year, % | −0.6 (1.8) | −0.6 (1.8) | −0.9 (1.9) | −1.2 (2.00) |
| Absolute change in BMD, mean (SD) per year, g/cm2 | −0.005 (0.01) | −0.005 (0.01) | −0.006 (0.01) | −0.008 (0.01) |
Abbreviations: BMD, bone mineral density; BMI, body mass index, which is calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range.
Measured at the time of the second BMD measure, unless otherwise specified.
Major osteoporotic fracture includes fractures of the hip, spine, shoulder, and forearm. This category is not mutually exclusive with the hip fracture category.
Risk scores were estimated using updated clinical characteristics at the time of the second BMD test along with historic (baseline) BMD.
Risk scores were estimated using updated clinical characteristics and the second BMD test.
In unadjusted models, every SD decrease in annual percent BMD change was associated with an HR of 1.47 for risk of incident hip fracture (95% CI, 1.18–1.83; Table 2). Results were similar after adjusting for clinical characteristics (HR, 1.33 [95% CI, 1.06–1.68]) and after further adjustment for baseline BMD (HR, 1.43 [95% CI, 1.16–1.78]). At 10 years’ follow-up, the absolute risk of hip fracture was 10.2% among participants with a mean BMD percent change compared with 14.1% among participants with BMD change 1 SD below the mean (risk difference, 3.9%).
Table 2.
Association Between BMD Change per SD Decrease and Risk of Hip Fracture and Major Osteoporotic Fracture
| Risk of Fracture, Hazard Ratio (95% CI) | |||
|---|---|---|---|
| Unadjusted | Adjusteda | Adjusted and Baseline BMDa | |
| Hip fracture | |||
| Annualized percent BMD change | 1.47 (1.18–1.83) | 1.33 (1.06–1.68) | 1.43 (1.16–1.78) |
| Annualized absolute BMD change | 1.34 (1.09–1.66) | 1.25 (0.99–1.58) | 1.47 (1.15–1.86) |
| Major osteoporotic fracture | |||
| Annualized percent BMD change | 1.25 (1.04–1.50) | 1.13 (0.93–1.37) | 1.21 (1.01–1.45) |
| Annualized absolute BMD change | 1.18 (0.99–1.41) | 1.09 (0.90–1.32) | 1.23 (1.00–1.50) |
Abbreviation: BMD, bone mineral density.
Adjusted for age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), weight loss (per pound), and history of fracture as measures at the time of the second BMD test.
For major osteoporotic fractures, every SD decrease in annual percent BMD change was associated with an HR of 1.21 for risk of fracture after adjusting for baseline BMD (95% CI, 1.01 to 1.45). At 10 years’ follow-up, the absolute risk of major osteoporotic fracture was 15.6% among participants with a mean BMD percent change compared with 18.3% among participants with a BMD change 1 SD below the mean (risk difference, 2.7%).
Results for absolute BMD change were similar to percent BMD change. Results were similar when considering the effect of BMD change on fracture rates (eTable 1 in the Supplement).
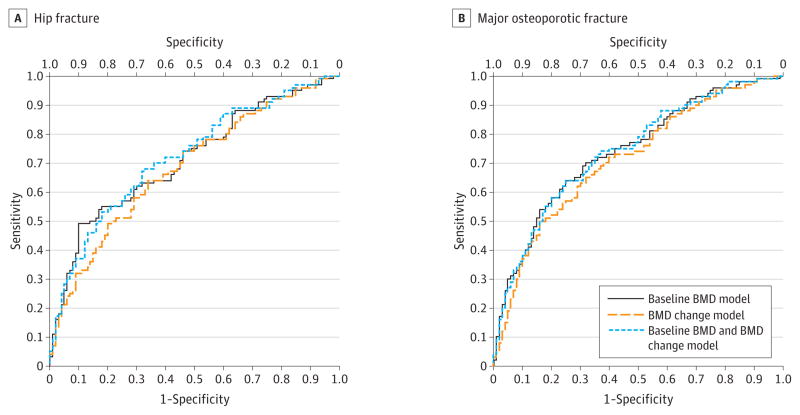
In ROC curve analyses (Figure 1), models associating baseline BMD with hip or major osteoporotic fracture performed somewhat better than models with BMD change. For example, the area under the curve (AUC) was 0.71 (95% CI, 0.65–0.78) for the baseline BMD model compared with 0.68 (95% CI, 0.62–0.75) for the BMD percent change model. Moreover, the addition of BMD change to a model with baseline BMD did not meaningfully improve performance (AUC, 0.72 [95% CI, 0.66–0.79]).
Figure 1. Receiver Operating Characteristic Curves for Models Investigating Fracture in Older Adults From the Framingham Osteoporosis Study.
BMD indicates bone mineral density. All models are adjusted for age, sex, body mass index, weight loss (per pound), and history of fracture measured at the time of the second BMD test. Models are defined in the Methods section.
In analyses stratified by clinical characteristics (eTable 2 in the Supplement), models performed similarly to the overall results. There was no evidence of significant interactions when comparing HRs.
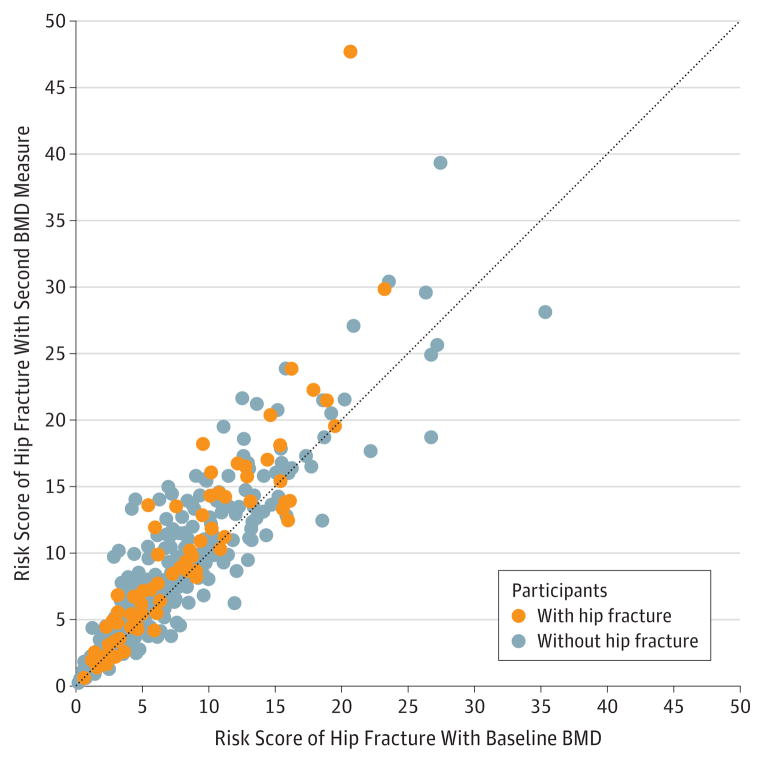
The median absolute change in risk scores of hip fracture was 0.6% after updating clinical characteristics and 0.8% after updating clinical characteristics and the second BMD measure. Individuals with a higher risk of fracture with updated clinical characteristics and baseline BMD had greater changes in fracture risk scores after considering the second BMD measure (Figure 2).
Figure 2. Scatterplot Showing the Distribution of Risk Scores of Hip Fracture With Baseline BMD vs Risk Scores of Hip Fracture and the Second BMD Measure.
BMD indicates bone mineral density. Risk scores of hip fracture are calculated with updated clinical characteristics. The dotted line indicates no change between the 2 risk assessment estimates (ie, BMD is unchanged).
Of the 48 participants reclassified as being at high risk of hip fracture with a second BMD measure, 4 participants (8.3%) fractured a hip, whereas 1 of 29 participants (3.4%) reclassified as low risk experienced a fracture. Among the 76 participants with hip fracture, 4 were reclassified as high risk using the second BMD measure, whereas 1 participant was reclassified as low risk (Table 3). The net gain in the percentage of participants with a hip fracture reclassified with a second BMD measure was 3.9% (95% CI, −2.2% to 9.9%). Among the 726 participants without hip fracture, 28 were reclassified as low risk using a second BMD measure, whereas 44 were reclassified as high risk. The net loss in the percentage of participants without hip fracture reclassified as low risk by a second BMD measure was −2.2% (95% CI, −4.5% to 0.1%).
Table 3.
Distribution of Participants Classified as High and Low Risk According to Whether a Fracture Occurred During Follow-up
| Risk Classification Based on Baseline BMDb | Risk Classification Based on
Second BMD Measurea
|
|||
|---|---|---|---|---|
| Fracture |
No Fracture |
|||
| Low Risk | High Risk | Low Risk | High Risk | |
| Hip fracturec | ||||
|
| ||||
| Low risk | 8 | 4 | 212 | 44 |
|
| ||||
| High risk | 1 | 63 | 28 | 442 |
|
| ||||
| Net reclassification index, % (95% CI) | 3.9 (−2.2 to 9.9) | −2.2 (−4.5 to 0.1) | ||
|
| ||||
| Major osteoporotic fractured | ||||
|
| ||||
| Low risk | 46 | 12 | 495 | 43 |
|
| ||||
| High risk | 1 | 54 | 11 | 140 |
|
| ||||
| Net reclassification index, % (95% CI) | 9.7 (3.4 to 15.7) | −4.6 (−6.7 to −2.6) | ||
Abbreviation: BMD, bone mineral density.
FRAX risk scores were calculated using updated clinical characteristics and the second BMD measure.
FRAX risk scores were calculated using updated clinical characteristics and a historic (baseline) measure of BMD.
Participants classified as high risk (≥3% fracture risk score) and low risk (<3% fracture risk score) of hip fracture.
Participants classified as high risk (≥20% fracture risk score) and low risk (<20% fracture risk score) of major osteoporotic fracture.
Of the 55 participants (21.8%) reclassified as being at high risk of major osteoporotic fracture with a second BMD measure, 12 participants (21.8%) experienced a fracture, whereas 1 of 12 participants (8.3%) who were reclassified as low risk experienced a fracture. The net gain in the percentage of participants with a major osteoporotic fracture reclassified as high risk with a second BMD measure was 9.7% (95% CI, 3.4% to 15.7%; Table 3). The net loss in the percentage of participants without major osteoporotic fracture reclassified as low risk by a second BMD measure was −4.6% (95% CI, −6.7% to −2.6%).
Discussion
In this population-based study of men and women, BMD change of the femoral neck over an average of 3.7 years was independently associated with hip and major osteoporotic fracture. At 10 years’ follow-up, 1 SD decrease in annual percent BMD change compared with mean BMD change resulted in 3.9 excess hip fractures per 100 persons. However, BMD change provided little additional information beyond baseline BMD for the clinical management of osteoporosis. The second BMD measure resulted in a small proportion of individuals reclassified as high risk of hip or major osteoporotic fracture, and it is unclear whether this reclassification justifies the current US practice of performing serial BMD tests at 2.2-year intervals.13
Prior studies examining the contribution of BMD change to the prediction of fracture have yielded mixed results. Studies with a strong association between BMD loss and fracture have typically focused on individuals with accelerated bone loss,7,8,20 whereas studies that have examined the effect of BMD change continuously have found baseline BMD is more strongly associated with fracture risk than BMD change.7,9–11 In a young Canadian clinical cohort (mean age, 64.9 years), no association between fracture and BMD change existed after adjusting for baseline BMD. The relatively older age of our cohort may explain why we found a statistical association between BMD change and fracture. Bone mineral density change may be less useful in a younger population at low risk of fracture.
Our ROC curves suggest that BMD change provides little clinical value beyond baseline BMD, yet there are limitations to ROC curve analyses. When regression models are reasonably good at predicting an outcome, large associations between a new marker and outcome are required to increase the ROC meaningfully.18 The NRI was developed to address this concern when there are established clinical cut points used to determine treatment.18 A potential value of NRI analysis is that a new marker could be clinically useful if adding the new marker to an established tool results in a greater proportion of individuals with an event reclassified at high risk without substantial misclassification of individuals without the event.
We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture. Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on historic BMD and updated clinical characteristics. For major osteoporotic fractures, a second BMD measure appeared somewhat more useful. The differences in our results for hip and major osteoporotic fracture may be due to chance or because non–hip fractures were not confirmed by medical records, resulting in some potential misclassification.
In a post hoc analysis of 2 randomized clinical trials, investigators found women who lose the most BMD during the first year of follow-up were most likely to gain in the next year. This phenomenon of regression to the mean suggests that participants in our study with unusual BMD loss may be outliers with more typical BMD change in subsequent years. This further supports our conclusions that repeating BMD testing through a narrow time window may not improve fracture risk stratification.
Some experts suggest that shorter BMD rescreening intervals are warranted in individuals at high risk of fracture.4 However, previous studies found no difference in the association of BMD change and fracture according to baseline T score or BMD. We also found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score. Framingham participants excluded from our study were older. It remains possible, then, that older and frailer individuals will benefit from serial BMD screening, but we were unable to detect trends due to limited numbers of fractures in stratified analyses.
Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture. For these individuals a repeat screening test provides the opportunity for clinicians to intervene with osteoporosis medications that reduce the risk of fracture, even among persons 75 years or older.21–24 We found no obvious differences among individuals with improvement in risk classification after the second BMD measure with the exception of baseline T score; mean T scores were less in participants with improved risk classification than in participants with worsened reclassification (−1.6 improved reclassification vs −1.2 worsened reclassification). Data from the Study of Osteoporotic Fractures (SOF) confirm that less than 10% of postmenopausal women with normal BMD or mild osteopenia will lose bone to osteoporotic levels within 15 years.25 Our study adds evidence that repeating a BMD screening test throughout a short time interval is unlikely to change clinical practice, particularly among individuals with normal or mild bone loss at baseline. We suggest that further clinical investigation is needed to predict which adults are likely to transition from low to high risk of fracture and may benefit from repeat BMD screening.
The strengths of this study include a large, population-based sample with 2 BMD measures approximately 4 years apart. Our longitudinal data allowed us to examine a variety of models. We considered updated clinical characteristics and historic BMD in our models, a circumstance that resembles what might occur in clinical practice. Furthermore, we used the NRI as an alternative method to describe the clinical contribution of a second BMD measure.
Limitations
There are limitations of this study. First, most participants had BMD measured on 2 different machines. Adjustments were made using previously published calibrations,15 but intermachine and intramachine precision error could result in misclassification. Second, we did not have information on osteoporosis medications with the exception of estrogen. Excluding women using estrogen (n = 26) from the models did not change our results. We do not expect that bisphosphonate use was common given the timing and location of this study. Importantly, our findings are applicable to an untreated, screened population, and they may not generalize to individuals using osteoporosis medications. Third, we did not have information on parental history of hip fracture when calculating risk probabilities. Accounting for family history would have increased the proportion of individuals classified as high risk at baseline; thus, the effect of repeating BMD may have been less if we included this characteristic. Fourth, our results can only be generalized through 12 years of follow-up. Finally, our study comprised white participants, and the significance of repeating a BMD measure may differ in other racial and ethnic populations. It may be useful to validate our findings in a separate population.
Conclusion
Repeating a BMD test in 4 years provided little additional value beyond baseline BMD when assessing fracture risk. Furthermore, a second BMD measure resulted in little change in risk classification used in clinical management. Although the appropriate time interval between BMD screening tests remains unknown, the current clinical practice of repeating a BMD test every 2 years to improve fracture risk stratification may not be necessary in all adults 75 years or older untreated for osteoporosis. Further study is needed to determine an appropriate rescreening interval and to identify individuals who might benefit from more frequent rescreening intervals.
Supplementary Material
Acknowledgments
Funding/Support: This work was funded in part through grants R01-AR/AG 41398 and NIA K23 AG033204 from the National Institutes of Health, N01-HC-25195 from the National Heart, Lung, and Blood Institute’s Framingham Heart Study, and contributions from the Friends of Hebrew SeniorLife.
Role of the Sponsor: The National Institutes of Health, National Heart, Lung, and Blood Institute, and Friends of Hebrew SeniorLife had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Berry reports receiving royalties for her author contributions to UpToDate. Dr Kiel reports receiving grants from Amgen, Eli Lilly, Hologic, Merck Sharp & Dohme, and Roche; receiving consulting fees for serving on scientific advisory boards from Amgen, Ammonett Pharma, Eli Lilly, Merck Sharp & Dohme, and Novartis; and receiving royalties for his author contributions to UpToDate and Springer. No other disclosures were reported.
Author Contributions: Dr Berry had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Berry, Samelson, Kiel.
Acquisition of data: McLean, Broe, Kiel.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Berry, Samelson.
Critical revision of the manuscript for important intellectual content: Samelson, Pencina, McLean, Cupples, Broe, Kiel.
Statistical analysis: Berry, Samelson, Pencina, Cupples.
Obtained funding: Kiel.
Administrative, technical, or material support: Broe, Kiel.
Study supervision: Kiel.
References
- 1.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Osteoporosis Foundation. [Accessed August 28, 2013];Clinician’s guide to prevention and treatment of osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
- 3.Black DM, Rosen CJ. Following the bone density trail: a clinical perspective. J Clin Endocrinol Metab. 2012;97(4):1176–1178. doi: 10.1210/jc.2012-1528. [DOI] [PubMed] [Google Scholar]
- 4.Yu EW, Finkelstein JS. Bone density screening intervals for osteoporosis: 1 size does not fit all. JAMA. 2012;307(24):2591–2592. doi: 10.1001/jama.2012.6977. [DOI] [PubMed] [Google Scholar]
- 5.Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742. doi: 10.1002/jbmr.1580. [DOI] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, Langsetmo L, Joseph L, et al. CaMos Research Group. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24(2):361–370. doi: 10.1359/jbmr.081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthon PM, Ewing SK, Mackey DC, et al. Osteoporotic Fractures in Men (MrOS) Research Group. Change in hip bone mineral density and risk of subsequent fractures in older men. J Bone Miner Res. 2012;27(10):2179–2188. doi: 10.1002/jbmr.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie WD, Morin SN, Lix LM. Manitoba Bone Density Program. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab. 2012;97(4):1211–1218. doi: 10.1210/jc.2011-2871. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TV, Center JR, Eisman JA. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res. 2005;20(7):1195–1201. doi: 10.1359/JBMR.050215. [DOI] [PubMed] [Google Scholar]
- 11.Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women. Arch Intern Med. 2007;167(2):155–160. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Delzell E, Zhao H, et al. Central DXA utilization shifts from office-based to hospital-based settings among Medicare beneficiaries in the wake of reimbursement changes. J Bone Miner Res. 2012;27(4):858–864. doi: 10.1002/jbmr.1534. [DOI] [PubMed] [Google Scholar]
- 13.McAdam-Marx C, Unni S, Ye X, Nelson S, Nickman NA. Effect of Medicare reimbursement reduction for imaging services on osteoporosis screening rates. J Am Geriatr Soc. 2012;60(3):511–516. doi: 10.1111/j.1532-5415.2011.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiel DP, Mercier CA, Dawson-Hughes B, Cali C, Hannan MT, Anderson JJ. The effects of analytic software and scan analysis technique on the comparison of dual x-ray absorptiometry with dual photon absorptiometry of the hip in the elderly. J Bone Miner Res. 1995;10(7):1130–1136. doi: 10.1002/jbmr.5650100719. [DOI] [PubMed] [Google Scholar]
- 16.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women: the Framingham Study. N Engl J Med. 1987;317(19):1169–1174. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63(1):46–55. doi: 10.1016/j.jclinepi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605–615. [PubMed] [Google Scholar]
- 20.Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture. Bone. 1996;19(1):9–12. doi: 10.1016/8756-3282(96)00102-0. [DOI] [PubMed] [Google Scholar]
- 21.Boonen S, Black DM, Colón-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58(2):292–299. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonen S, Klemes AB, Zhou X, Lindsay R. Assessment of the relationship between age and the effect of risedronate treatment in women with postmenopausal osteoporosis: a pooled analysis of 4 studies. J Am Geriatr Soc. 2010;58(4):658–663. doi: 10.1111/j.1532-5415.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- 23.Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54(5):782–789. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 24.Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96(6):1727–1736. doi: 10.1210/jc.2010-2784. [DOI] [PubMed] [Google Scholar]
- 25.Gourlay ML, Fine JP, Preisser JS, et al. Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.