Abstract
Purpose
The purpose of this study is to test the ability of risedronate and estradiol, alone or in combination, to prevent bone loss associated with androgen deprivation therapy in men with prostate cancer.
Materials and methods
This is a randomized placebo-controlled trial of risedronate and estradiol, alone or in combination, in men with prostate cancer receiving androgen deprivation therapy. The primary outcome was change in hip bone mineral density at 1 year.
Results
No statistical difference was found among the groups for bone mineral density changes. The only side effects of note were increased gynecomastia and breast tenderness associated with estrogen therapy. The study was limited by poor accrual and subsequent lack of statistical power.
Conclusions
Men receiving androgen deprivation therapy for prostate cancer are at risk for bone loss and should receive appropriate bone density monitoring and preventive advice about calcium, vitamin D, exercise, and fall prevention. Prescription drugs proven in this patient population should be used when the risk of fracture is high.
Keywords: Prostate cancer, Androgen deprivation therapy, Osteoporosis, Risedronate, Estradiol
Introduction
Androgen deprivation therapy (ADT), most commonly by gonadotropin-releasing hormone (GnRH) agonist administration, is frequently used not only to treat advanced prostate cancer but also to prevent progression of earlier-stage disease. Cross-sectional and longitudinal studies report that men receiving chronic ADT have lower bone mass [1] and that acute bone loss occurs with institution of ADT [2]. An increase in risk of fracture reported in men with prostate cancer treated with ADT is likely the consequence of bone loss [3, 4].
The bone loss that is now recognized as an adverse consequence of the hormonal changes produced by GnRH agonist therapy appears to be related to the suppression of sex steroids. Testosterone is reduced to castrate levels and as a consequence estrogen, produced from aromatization of testosterone, is also reduced. Multiple lines of evidence suggest that estrogen is the predominant sex steroid for bone health in men. Reports range from case studies of men with mutations in the estrogen receptor [5] or aromatase genes [6] to epidemiologic studies correlating bone mass [7-9] and fracture [10, 11] in men with estradiol levels to interventional studies of short-term estrogen administration effects on bone turnover in men [12]. The relationship between changes in estrogen levels and bone loss with ADT therapy has not been examined. However, it seems likely that the GnRH-caused reduction in estrogen from ADT is a major driver of bone loss.
The recognition of bone loss as a consequence of ADT and of the likely role of estrogen as a mediator has resulted in several therapeutic trials of bone antiresorptive agents in men with prostate cancer receiving ADT. The intravenous bisphosphonates zolendronic acid [13] and pamidronate [14] and the oral bisphosphonate alendronate [15] have recently been shown to be efficacious in preventing bone loss in this patient population. In addition, the selective estrogen receptor modulators toremifene [16] and raloxifene [17] have also been demonstrated to prevent the bone loss associated with ADT. Reported here are the results of a randomized placebo-controlled trial of the oral bisphosphonate risedronate compared to estradiol, alone or in combination, to prevent bone loss in men receiving ADT.
Materials and methods
This clinical trial was conducted by the North Central Cancer Treatment Group (NCCTG). It was approved by the National Cancer Institute prior to study initiation and by the local Institutional Review Boards. All patients provided informed consent per federal guidelines. This study was funded by the National Cancer Institute with supplemental funding from Proctor and Gamble.
Patients eligible for this study were greater than 18 years of age, had a history of prostate cancer, and were being treated with androgen ablation therapy in the adjuvant setting or for rising prostate-specific antigen values without evidence of bony metastatic disease. The androgen ablation therapy needed to be planned to be given for at least 6 months after study initiation. When this protocol was initiated, men could not have had prior androgen ablation therapy. However, because of slow accrual, the protocol was modified so that subsequent patients could have had androgen ablation therapy prior to initiation of this study. Alkaline phosphate levels needed to be normal or less than 1.5 times the upper limit of normal with a normal bone scan. Liver enzyme and creatinine testing had to be less than 1.5 times the upper limit of normal. Triglycerides needed to be less than 250 mg/dl.
Men were excluded from this protocol if they had any of the following: congestive heart failure under active treatment, coronary artery disease requiring treatment within the last 5 years, thromboembolic problems, systemic steroid use, osteoporosis, past use of a bisphosphonate, sarcoidosis, parathyroid dysfunction, history of symptomatic hypocalcemia or hypercalcemia, current chemotherapy, difficulty swallowing pills, or dental work less than 3 months prior to registration or planned during the study treatment.
Patients were stratified by the duration of prior androgen deprivation therapy (less than 30 vs. 30-150 vs. 151-365 days vs. greater than 365 days). Patients were randomized using the Pocock-Simon dynamic allocation procedure [18]. In a double-blind manner, they were randomly assigned to receive risedronate, 30 mg orally once per week; low-dose estrogen, 0.5 mg orally once per day; both risedronate and estrogen at the same doses; or two placebos looking like risedronate and estrogen, respectively. The patients randomized to receive one active agent alone also received a placebo looking like the alternative agent. All patients were also given calcium, 600 mg to take orally once per day, and vitamin D, 400 international units to take orally once per day. The calcium was to be taken at a different time than the risedronate, so that it did not interfere with absorption of the risedronate. The risedronate was given 30 min before breakfast with a full glass of water with patients instructed not to be lying down for 30 min after receiving the pill.
If patients developed side effects attributed to either estrogen or risedronate, the presumed particular offending medication (or placebo) was to be discontinued.
Risedronate (Actonel) and placebo were supplied by Proctor and Gamble. Estradiol was manufactured by Mylan Pharmaceuticals and was overencapsulated to look similar to a matching placebo.
Potential adverse events were evaluated at baseline and at 2-month intervals when nurses or clinical research associates called patients every other month or talked to the patient at these times in the clinic. These queried adverse events included: cardiac ischemia/infarction (including angina/chest pain), thrombosis/thromboembolism, edema, dyspepsia/heartburn, nausea, vomiting, dysphagia, gastric ulcer, gallstones, rash, headache, abdominal pain or cramping, and gynecomastia. These toxicities were graded by NCI Common Terminology Criteria version 2.0 with the exception of gallstones, which were graded as present or absent.
Tests to judge efficacy and toxicity consisted of bone mineral density (BMD) tests at baseline, 6 months, and 1 and 2 years. Bone mineral density was assessed by dual-energy X-ray absorptiometry devices of the lumbar spine, femoral neck, and total hip. The bone mineral density was to be measured at the same facility each time.
In addition, patients completed a symptom experience diary (SED) at the same four time points. The SED consisted of 18 individual symptom questions each of which were rated on a 0-10 numeric analog scale where 10 represented symptoms as bad as they can be. Patients judged appetite loss, headache, nausea/vomiting, back pain, abdominal pain/cramps, fatigue, heartburn, fluid retention, constipation, visual changes, sleeping trouble, rash, mood changes, breast tenderness or swelling, chest pain, shortness of breath, hot flashes, and quality of life.
Statistical analysis
The primary end point for this study was the average absolute intrapatient change in hip BMD observed in patients from baseline versus 1 year poststudy entry. Secondary end points for this study were numerous. They included: the BMD change from baseline at 6 months; designation of a 5% difference in BMD scores from baseline as indicative of an important loss of bone density; osteoporosis incidence as defined by a standardized BMD score of at least 2.5 standard deviations below the age and gender-specific norm; the incidence of bone fractures, gynecomastia, thrombosis, and heart conditions; the incidence and severity of adverse events; and changes from baseline in patient-reported symptoms as measured by the SED.
Analysis of the primary end point was carried out by comparing average values of the intrapatient change in hip BMD between the control treatment arm and each other treatment arm in turn using standard two-sample or Wilcoxon rank sum methodology [19] dependent upon the normality of the data tested using the Shapiro-Wilk [20] procedures. An intent-to-treat analysis was carried out by categorizing patients as success (less than 5% bone loss from baseline) or failure (5% or more decrease in bone loss from baseline) where any patient who failed to provide complete data was considered treatment failure. The percentage of treatment failures was compared across the treatment groups via chi-squared testing. The analytical procedures detailed for the primary end point was applied to the secondary BMD-related and quality-of-life-related end points. Further analysis examined the clinical significance for changes over time by calculating the percentage of patients in each treatment group that reported an improvement of more than 1 point on the 0-10-point scale for any patient-reported end point. These percentages were compared via chi-squared testing, as were toxicity data and other incidence-rate-based end points.
All hypothesis testing was carried out using a two-sided alternative hypothesis and a 5% type I error rate. While the layout of the experiment had the appearance of a 2×2 factorial design, we chose to analyze the study as three separate treatment arms relative to a reference arm. This was done because of the lack of knowledge regarding the presence of interaction in the design. Factorial designs are a useful and efficient method only if interaction is not present. Powering the study for a factorial structure would introduce the potential for underpowering the primary end point analysis. Hence, the conservative approach was taken of using power calculations for individual treatment arm comparison. No adjustment for multiple testing was undertaken as there was a single a priori designated test of hypothesis for the primary end point. Analysis for the secondary end points is based more on effect size observed rather than the statistical significance, so adjusting for multiple testing is irrelevant.
A two-sample t test with 64 patients per group, splitting the alpha would have provided 80% power to detect a difference of 60% times the standard deviation, which is considered a moderate effect size and clinically significant. An estimate of the standard deviation of the change in hip BMD was roughly 10% of the baseline value in a sample of androgen ablated men [21]. Hence, this sample size would have been able to detect a difference of 5% (50% of the standard deviation) in the change between treatment groups.
Results
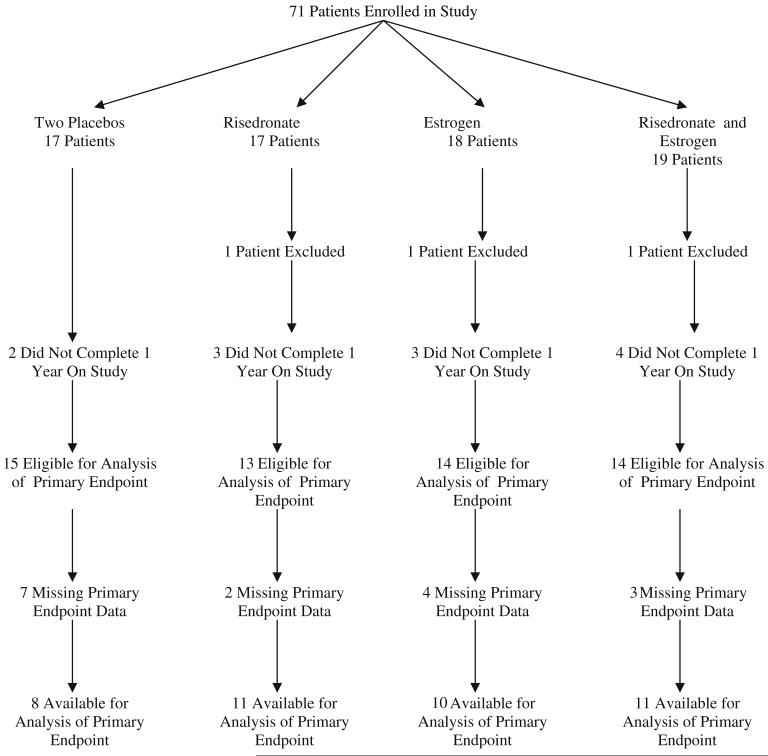
From ten sites, 71 patients were enrolled between March 21, 2003 and July 15, 2005. The study was closed prematurely due to slow accrual. A consort diagram depicts the flow of data (Fig. 1). Baseline patient characteristics are illustrated in Table 1.
Fig. 1.
Patient consort diagram
Table 1.
Baseline patient characteristics
| 2 Placebos (N=17) |
Risedronate (N=16) |
Estrogen (N=17) |
Risedronate plus estrogen (N=18) |
Total (N=68) | |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 67.9 (8.05) | 71.9 (9.64) | 73.1 (7.29) | 66.9 (9.85) | 69.9 (8.97) |
| Range | 54.0–78.0 | 51.0–85.0 | 60.0–82.0 | 51.0–82.0 | 51.0–85.0 |
| Race | |||||
| White | 14 (82%) | 15 (94%) | 16 (94%) | 15 (83%) | 60 (88%) |
| Black or African American | 2 (12%) | 1 (6%) | 0 (0%) | 2 (11%) | 5 (7%) |
| American Indian or Alaska Native | 0 (0%) | 0 (0%) | 1 (6%) | 1 (6%) | 2 (3%) |
| Not reported: patient refused or not available | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Pelvic RT | |||||
| Yes | 12 (71%) | 6 (38%) | 9 (53%) | 9 (50%) | 36 (53%) |
| No | 5 (29%) | 10 (63%) | 8 (47%) | 9 (50%) | 32 (47%) |
| Treatment | |||||
| LHRH agonist given for rising PSA | 10 (59%) | 8 (50%) | 8 (47%) | 9 (50%) | 35 (52%) |
| Adjuvant setting | 7 (41%) | 8 (50%) | 9 (53%) | 9 (50%) | 33 (49%) |
| Duration of LHRH | |||||
| ≤30 days | 0 (0%) | 2 (13%) | 2 (112%) | 2 (11%) | 6 (9%) |
| >30–150 days | 5 (29%) | 5 (31%) | 3 (18%) | 4 (22%) | 17 (25%) |
| 151–365 days | 5 (29%) | 4 (25%) | 5 (29%) | 5 (28%) | 19 (28%) |
| >365 days | 7 (41%) | 5 (31%) | 7 (41%) | 7 (39%) | 26 (38%) |
| Femoral neck BMD at baseline (gm/cm2) | |||||
| N | 14 | 15 | 13 | 16 | 58 |
| Mean (SD) | 0.9 (0.14) | 0.9 (0.15) | 0.9 (0.15) | 0.9 (0.12) | 0.9 (0.14) |
| Median | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Range | (0.7–1.1) | (0.7–1.2) | (0.7–1.2) | (0.6–1.2) | (0.6–1.2) |
| Femoral neck T score baseline | |||||
| N | 16 | 15 | 14 | 16 | 61 |
| Mean (SD) | −0.6 (1.39) | −0.8 (0.88) | −0.4 (1.36) | −0.9 (0.94) | −0.7 (1.15) |
| Median | −0.9 | −1.3 | −0.8 | −1.0 | −0.9 |
| Range | (−2.5–2.3) | (−1.9–1.1) | (−2.0–2.1) | (−2.1–1.3) | (−2.5–2.3) |
Treatment effectiveness was measured using the change from baseline femoral neck BMD at 1 year. A Kruskal-Wallis test between arms revealed that there was no statistically significant difference in the change from baseline (Table 2). Further, there was no significant change at the 6-month time point either.
Table 2.
Femoral neck BMD (gm/cm2) difference from baseline
| Arm |
|||||
|---|---|---|---|---|---|
| 2 placebos | Risedronate | Estrogen | Risedronate plus estrogen | ||
| 6 months (p=0.65) | N | 10 | 11 | 11 | 11 |
| Mean | −0.0011 | −0.0127 | 0.0053 | −0.0081 | |
| SD | 0.0431 | 0.0540 | 0.0347 | 0.0285 | |
| Median | −0.003 | 0.000 | 0.010 | −0.010 | |
| 1 year (p=0.74) | N | 8 | 11 | 10 | 11 |
| Mean | 0.0096 | −0.0104 | 0.0068 | 0.0190 | |
| SD | 0.0366 | 0.0528 | 0.0302 | 0.0376 | |
| Median | 0.001 | 0.010 | 0.002 | 0.020 | |
Tables 3 and 4 illustrate the femoral neck data regarding whether patients developed osteoporosis (defined by score of at least 2.5 standard deviations below the age and gender-specific norm) or had a 5% or greater decrease in BMD. Similar nonsignificant data were seen for lumbar spine BMD. There were no reported bone fractures.
Table 3.
Incidence of osteoporosis per arm
| Arm |
|||||
|---|---|---|---|---|---|
| 2 Placebos | Risedronate | Estrogen | Risedronate plus estrogen | ||
| 6 months (p=0.98) | Frequency | 5 | 4 | 5 | 6 |
| Percent | 29.41 | 25.00 | 29.41 | 33.33 | |
| 1 year (p=0.96) | Frequency | 7 | 5 | 6 | 7 |
| Percent | 41.18 | 31.25 | 35.29 | 38.89 | |
Table 4.
Incidence of 5% decrease in femoral neck BMD per arm
| Arm |
|||||
|---|---|---|---|---|---|
| 2 placebos | Risedronate | Estrogen | Risedronate plus estrogen | ||
| 6 months | Frequency | 8 | 8 | 7 | 8 |
| Percent | 47.06 | 50.00 | 41.18 | 44.44 | |
| 1 year | Frequency | 9 | 7 | 7 | 7 |
| Percent | 52.94 | 43.75 | 41.18 | 38.89 | |
There were no serious adverse events in any participant. Of all the queried toxicities, the only one that looked different between arms was gynecomastia (Table 5). Likewise, on the symptom experience diary, the only item of note was that the men reported significantly more breast tenderness changes (from their baseline values) in the estrogen-containing study arms (Table 6).
Table 5.
Incidence of gynecomastia per arm
| Arm |
|||||
|---|---|---|---|---|---|
| 2 Placebos | Risedronate | Estrogen | Risedronate plus estrogen | ||
| 6 months (p=0.059) | Frequency | 2 | 4 | 9 | 5 |
| Percent | 13% | 25% | 56% | 28% | |
| 1 year (p=0.050) | Frequency | 0 | 3 | 5 | 6 |
| Percent | 0 | 19% | 31% | 33% | |
Table 6.
Patient-reported breast tenderness/swelling
| 2 Placebos | Risedronate | Estrogen | Risedronate plus estrogen | Total | p value | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| N | 16 | 16 | 17 | 18 | 67 | 0.58 |
| Mean (SD) | 93.8 (11.47) | 97.5 (7.75) | 89.4 (20.76) | 93.9 (12.90) | 93.6 (14.11) | |
| Change from baseline at 6 months | ||||||
| N | 13 | 13 | 15 | 14 | 55 | 0.05 |
| Mean (SD) | 1.5 (20.35) | −0.8 (4.94) | −10.7 (31.95) | −14.3 (21.38) | −6.4 (22.72) | |
| Median | 0.0 | 0.0 | −10.0 | 0.0 | 0.0 | |
| Change from baseline at 1 year | ||||||
| N | 13 | 13 | 10 | 13 | 49 | 0.01 |
| Mean (SD) | −5.4 (23.67) | −2.3 (14.81) | −19.0 (35.42) | −30.8 (33.28) | −14.1 (29.08) | |
| Median | 0.0 | 0.0 | −25.0 | −20.0 | 0.0 | |
Scale as 0–100 where 0 is tenderness/swelling as bad as it can be. A positive change from baseline indicates an improvement
Discussion
Since the conception of this current study, the association between ADT and bone loss and fractures in men with prostate cancer has become widely recognized. Studies have demonstrated the ability of an intravenous bisphosphonate [13] and a selective estrogen receptor modulator to attenuate the bone loss [16].
There are a number of potential reasons why the current study was unable to report positive study results. First, this study suffers from a gross lack of statistical power, given the low numbers of patients. Enrollment may have been low due to several factors. One is that, at the time the study opened for enrollment, the association between ADT and bone loss was only an emerging concern. Therefore, patients and physicians may not have recognized the importance of the question being addressed. Second is that the study opened relatively soon after the results of the Women’s Health Initiative study was announced with its conclusion that risks of hormone replacement (including estrogen) exceeded benefits for postmenopausal women [22]. Since this study included an estrogen, it may have deterred physicians and patients from considering participation. In addition, many men receiving ADT are managed by urologists; recruitment of these patients by the medical oncology majority physicians in the NCCTG was hampered.
Another potential reason for the negative study results is that this study included men on ADT for any length of time prior to enrollment, as opposed to just at the time of ADT initiation. Men were allowed to be entered at any time after ADT had been initiated because accrual was poor when it was attempted to enter them only at the time of ADT initiation. Other investigators have shown that bone loss with ADT is most rapid soon after it is initiated [2]. Therefore, including men beyond that initial phase of rapid bone loss may have limited the power of the study to detect a change.
A further reason for negative study results, as opposed to positive study results with another bisphosphonate, is because the bisphosphonate that was studied in this current trial is not as potent as the intravenous zoledronate studied in the positive study [13].
In addition, the relatively short study period for which evaluable data were available in this study may have hampered the ability to see a difference between the study arms.
Of note, at the time that this current study was developed, another almost identical study design (using intravenous zoledronate as the bisphosphonate of study) was independently developed into a clinical protocol. This study also suffered from poor study accrual and did not generate any substantial data (personal communication, Dawn Hershman 2008).
No other studies using estrogens have been reported in this patient population, and only one other has been reported using oral bisphosphonates [15]. Studies with fracture as the primary end point rather than change in BMD have not yet been reported in men with prostate cancer receiving ADT but are needed. Until such studies are available, it seems prudent to monitor men receiving ADT for bone loss by measuring BMD and to initiate preventive measures with adequate calcium, vitamin D, and weight-bearing exercise. Prescription therapies shown to have benefit in this group should be considered for those with low BMD.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35195, CA-63844, CA-35267, CA-35272, CA-35119, CA 124477, and CA-35431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional participating institutions: Upstate Carolina CCOP, Spartanburg, SC (James D. Bearden, III, M.D.); Hawaii Minority-Based CCOP, Honolulu, HI 96813 (William S. Loui, M.D.); Mayo Clinic Florida, Jacksonville, FL 32224 (Edith A. Perez, M.D.)
Contributor Information
Ann E. Kearns, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Donald W. Northfelt, Mayo Clinic Arizona, Scottsdale, AZ 85259-5404, USA
Amylou C. Dueck, Mayo Clinic Arizona, Scottsdale, AZ 85259-5404, USA
Pamela J. Atherton, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Shaker R. Dakhil, Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882, USA
Kendrith M. Rowland, Jr, Carle Cancer Center CCOP, Urbana, IL 61801, USA.
Jyotsna Fuloria, Ochsner CCOP, New Orleans, LA 70121, USA.
Patrick J. Flynn, Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416, USA
Todor Dentchev, Altru Health Systems, Grand Forks, ND 58201, USA.
Charles L. Loprinzi, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
References
- 1.Stoch SA, Parker RA, Chen L, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–2791. doi: 10.1210/jcem.86.6.7558. doi:10.1210/jc.86.6.2787. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. doi:10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. doi:10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. doi:10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 5.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. doi:10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 6.Morishima A, Grumbach MM, Simpson ER, et al. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. doi:10.1210/jc.80.12.3689. [DOI] [PubMed] [Google Scholar]
- 7.Gennari L, Merlotti D, Martini G, et al. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab. 2003;88:5327–5333. doi: 10.1210/jc.2003-030736. doi:10.1210/jc.2003-030736. [DOI] [PubMed] [Google Scholar]
- 8.Khosla S, Melton LJ, 3rd, Atkinson EJ, et al. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. doi:10.1210/jc.86.8.3555. [DOI] [PubMed] [Google Scholar]
- 9.Szulc P, Munoz F, Claustrat B, et al. Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. J Clin Endocrinol Metab. 2001;86:192–199. doi: 10.1210/jcem.86.1.7126. doi:10.1210/jc.86.1.192. [DOI] [PubMed] [Google Scholar]
- 10.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168:47–54. doi: 10.1001/archinternmed.2007.2. doi:10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 11.Mellstrom D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23:1552–1560. doi: 10.1359/jbmr.080518. doi:10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 12.Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. doi:10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. doi:10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. doi:10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 16.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–155. doi: 10.1016/j.juro.2007.08.137. doi:10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. doi:10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trials. Biometrics. 1975;31:103–115. doi:10.2307/2529712. [PubMed] [Google Scholar]
- 19.Hilton JF, Mehta CR. Power and sample size calculations for exact conditional tests with ordered categorical data. Biometrics. 1993;49:609–616. doi:10.2307/2532573. [PubMed] [Google Scholar]
- 20.Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika. 196552:591–611. [Google Scholar]
- 21.Kiralti BJ. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–132. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 22.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. doi:10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]