Abstract
Autoantibodies to the islet-specific zinc transporter isoform 8 (ZnT8) are detected in the majority of type 1 diabetes patients prior to and at clinical diagnosis. The presence of ZnT8Ab after diagnosis has not been investigated. This study analyzed the autoantibody response to ZnT8 in regard to age at onset and disease duration. Two new onset type 1 diabetes patient cohorts with different age distributions at onset (2–17 and 15–34 years of age at onset), a longitudinal subset of the younger type 1 diabetes patient cohort (n = 32), and a cohort of GAD65Ab-positive LADA patients (n = 47) was analyzed for the presence of autoantibodies directed to the two major isoforms, ZnT8-Arginine (ZnT8R) and ZnT8-Tryptophan (ZnT8W). The majority of type 1 diabetes patients tested positive for ZnT8Ab to both isoforms. ZnT8Ab titers were significantly higher in the younger type 1 diabetes patients as compared with the older cohort (ZnT8RAb at a median of 148 and 29 U/ml, respectively, p < 0.001) (ZnT8WAb at a median of 145 and 58 U/ml, respectively, p < 0.01). ZnT8RAb and ZnT8WAb titers were significantly lower in the LADA patients (ZnT8RAb at a median of 14 U/ml, ZnT8WAb at a median of 25 U/ml) as compared with either type 1 diabetes cohorts. In our longitudinal analysis of type 1 diabetes patients after clinical diagnosis, ZnT8Ab levels to both isoforms declined significantly during the initial year of disease (ZnT8RAb from a median of 320–162 U/ml, p = 0.0001; ZnT8WAb from a median of 128–46 U/ml, p = 0.0011). The antibody titers further declined during the following 4 years (p < 0.0001). We conclude that ZnT8Ab presents a useful marker for type 1 diabetes, especially in younger patients at disease diagnosis.
Keywords: Autoimmune diabetes, ZnT8, autoantibodies, longitudinal, radioligand binding assay
Introduction
The autoimmune response in patients with type 1 diabetes is often accompanied by the presence of circulating autoantibodies to autoantigens expressed in the pancreatic beta cells. Autoantibodies to insulin (IAA), the tyrosine-phosphatase-like protein IA-2 and the smaller isoform of glutamate decarboxylase (GAD65), are routinely used in the evaluation of the autoimmune response and risk assessment of individuals (for reviews see [1,2]).
The islet-specific zinc transporter isoform 8 (ZnT8) is a promising new autoantigen in type 1 diabetes [3,4]. This protein is encoded by SLC30A8 [5] and autoantibodies are detected in new onset type 1 diabetes patients, most importantly in a sizeable percentage (up to 30%) of type 1 diabetes patients who otherwise test negative for autoantibodies [4]. When using all four autoantibodies combined, more than 96% of Caucasian type 1 diabetes patients can be identified at disease onset [3].
Two autoantibody epitopes were identified at the cytosolic C-terminus of the ZnT8 molecule [6]. One of these epitopes critically depends on the amino acid residue at position 325. A common polymorphism at this position results in isoforms of the protein that carry either Arginine or Tryptophan at this position [7]. ZnT8R is the prevalent variant in Europeans and African-Amercians, while ZnT8W can be found in ~25% of Europeans, ~2% of African-Americans, and almost 50% of Asians. A third polymorphism at the same amino acid is caused by a nucleic acid substitution of the second nucleotide of this codon, resulting in ZnT8Q, which is rare in all populations tested (for review see [8]). A correlation between autoantibody specificity and the individual’s polymorphism suggests that the response is directed to self and does not arise from molecular mimicry [9,10].
The use of ZnT8Ab as an independent marker for autoimmune diabetes has been discussed, and the autoantibodies can be detected in the prediabetic period, where they are usually preceded by GAD65Ab and IAA [3,10]. The presence of ZnT8Ab after the clinical diagnosis of diabetes has not been investigated so far.
Our goal was to investigate the humoral response to ZnT8 in regard to age at onset and disease duration. We analyzed two new onset type 1 diabetes patient cohorts with different age distributions at onset and a longitudinal cohort of young type 1 diabetes patients for the presence of ZnT8Ab to both major isoforms ZnT8R and ZnT8W.
Materials and methods
Subjects
Details of the study cohorts are provided in Tables I and II.
Table I.
Characteristics of study cohorts.
| 2–17 type 1 diabetes | 15–34 type 1 diabetes | LADA | |
|---|---|---|---|
| N | 249 | 343 | 47 |
| Gender | 119 (F) 123 (M) | 223 (M) 125 (F) | 39 (M) 8 (F) |
| Age at onset (median and range) (years) | 10 (2–17) | 25 (15–34) | 30–70 |
| Duration (median and range) (months) | Onset | Onset | 3 (1–17) |
F, Female; M, Male. Samples were taken at time of clinical diagnosis.
Table II.
Characteristics of longitudinal subset for 2–17-year-old type 1 diabetes patients.
| Longitudinal subset 2–17 type 1 diabetes | |
|---|---|
| N | 32 |
| Gender | 13(F) 19(M) |
| Age at onset (median and range) (years) | 10 (2–16) |
F, Female; M, Male.
2–17-year-old type 1 diabetes cohort
Serum samples from newly diagnosed type 1 diabetes patients (n = 249) aged 2–17 years were obtained as part of a study conducted at the St Görans Children Hospital, Stockholm, Sweden, and represented 80% of all children diagnosed in Stockholm during 1992–2002. In a subset of these patients (n = 32), additional blood samples were obtained 1 year and 5 years after the onset of the disease. The diagnosis of type 1 diabetes was based on the World Health Organization criteria (WHO).
15–34-year-old type 1 diabetes cohort
Newly diagnosed 15–34-year-old type 1 diabetes patients were registered in 1992–1993 in the Diabetes Incidence Study in Sweden [11]. The patients were all diagnosed with diabetes mellitus according to WHO criteria. All types of diabetes: type 1 diabetes, type 2 diabetes, unclassifiable diabetes, and secondary diabetes were reported, with gestational diabetes as the only exception. Blood samples together with clinical classification were obtained in 764 patients at diagnosis and these patients were classified as follows: 583 type 1 diabetes patients, 110 type 2 diabetes patients, and 71 patients with unclassifiable diabetes. We analyzed a random subset (n = 343) of the samples obtained from type 1 diabetes patients.
LADA patients
GAD65Ab-positive LADA patients (n = 47) (30–70 years of age, 39 males) were part of a controlled clinical trial with injections of alum-formulated recombinant human GAD65 (rhGAD65) [12]. Patients were eligible for the study if they were aged 30–70 years, diagnosed with type 2 diabetes within the previous 5 years; tested positive for GAD65Ab, controlled their blood glucose levels with diet, oral hypoglycemic agents, or both, but not with insulin. GAD65Ab-positive type 2 diabetes patients with the above clinical parameters are generally classified as LADA patients. Participating females had to be of non-child-bearing potential. Samples used in this study were collected prior to the initiation of the injection protocol.
All subjects or their legal guardians gave informed consent. Local institutional ethics committee approval and subjects’ consent were obtained prior to collection of all serum samples.
All serum samples were continuously stored at −70°C, antibody titers to GAD65 of randomly selected samples were stable during the storage time (data not shown).
Generation of ZnT8 expression constructs
The complementary DNA (cDNA) construct consisting of the C-terminal domain (aa 268–369) from human islet ZnT8 was a kind gift from Dr J. C. Hutton (Barbara Davis Center for Childhood Diabetes, University of Colorado at Denver and Health Sciences Center, Aurora, CO, USA). The C-terminal construct was subcloned into the pTnT™ vector (Promega, Madison, WI, USA) to generate plasmid pThZnT8R. This construct was used for the expression of ZnT8R. This construct was used to generate the ZnT8 isoform ZnT8W using Phusion™ site-directed mutagenesis kit (Finnzymes Oy, Espoo, Finland).
Each construct was confirmed by DNA sequences prior to use.
Radioligand binding assay
GAD65Ab were determined using the radioligand binding assay (RBA) previously described [13]. Briefly, recombinant [35]S-GAD65 was produced in an in vitro-coupled transcription and translation system with SP6 RNA polymerase and nuclease-treated rabbit reticulocyte lysate (Promega). Sera (2.5 μl) were incubated with [35]S-GAD65 (25,000 of TCA-precipitable radioactivity). After an overnight incubation at 4°C, antibody-bound [35]S-GAD65 was separated from unbound antigen by precipitation with Protein A Sepharose (Invitrogen, Carlsbad, CA, USA). The immunoprecipitated radioactivity was counted on a Wallac Microbeta Liquid Scintillation Counter (Perkin Elmer Life and Analytical Sciences, Inc., Boston, MA, USA).
Antibody levels were expressed as a relative index to correct for interassay variation using the WHO standard for GAD65Ab [14]. To determine the relative index, positive and negative control samples were included in all assays. The cut-off for positivity for GAD65Ab (index of 0.04) was taken at the 98th percentile of the GAD65Ab index of 50 healthy control subjects (non-diabetic individuals without known autoimmune disease and no family history of diabetes). This cut-off value was confirmed by displacement assays using recombinant human GAD65 (200 ng/ml) (Diamyd Medical AB, Stockholm, Sweden) competing with radiolabeled recombinant human [35]S-GAD65 as previously described [13,15]. All samples were tested in duplicates and the coefficient of variations was determined for each sample (average 5.6, lowest 0.4, highest 10).
Antibodies to the islet cell antigen, IA-2, were measured under identical conditions as described for GAD65Ab. The plasmid containing the cDNA for the cytoplasmic portion of islet antigen 512 (ICA512bdc amino acids 256–556:630–979) was kindly donated by Dr G. Eisenbarth, Barbara Davis Research Center. The same WHO standard serum and control sera as in the GAD65Ab assay were used to correct for interassay variations. The interassay coefficient of variation for each sample was calculated (average 5.4, lowest 0.1, highest 14).
Our laboratory (#775) participated in the Diabetes Antibody Standardization Program workshop [16] and the GAD65Ab assay showed a sensitivity of 86% and specificity of 93%, and the IA2Ab assay showed a sensitivity of 66% and a specificity of 98%.
ZnT8Ab were measured under similar conditions as described for GAD65Ab. The cytosolic segments (aa268–369) encoded the aa325 codon variants, CGG (T) and TGG (W). Results for ZnT8Ab were converted into arbitrary units by extrapolation using a panreactive positive serum from a type 1 diabetes patient with designated 1000 arbitrary units. Cut-off was set at 10 U/ml for autoantibodies to ZnT8R and 18 U/ml for ZnT8W based on the 98th percentile observed in 162 healthy human controls (non-diabetic individuals without known autoimmune disease and no family history of diabetes). All samples were tested in duplicates and the coefficient of variations was determined for each sample (average 6.6, lowest 0.9, highest 14). For measurements of autoantibodies in longitudinal samples, all samples of a given patient were analyzed in one assay to avoid interassay variations.
Statistical analysis
Correlations were calculated using the Spearman rank correlation test. Autoantibody levels between groups were analyzed using the non-parametric analysis of variance (Kruskal–Wallis test) followed by Dunn’s multiple comparisons test. Significance was defined by p < 0.05.
Results
2–17-year-old type 1 diabetes patients
We found that 80% (200/249) of the type 1 diabetes patients’ sera tested positive for ZnT8Ab to at least one of the isoforms. Of the ZnT8Ab-positive samples, 83% (n = 166) were positive for both isoforms, while 10 and 7% reacted with either ZnT8R or ZnT8W, respectively. Antibody titers of ZnT8R and ZnT8W correlated significantly (p < 0.0001; Figure 1(a)).
Figure 1.
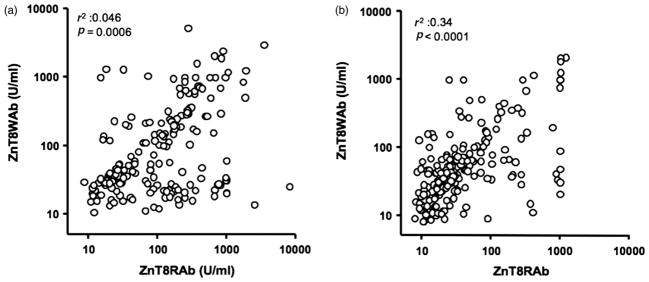
ZnT8Ab titers to ZnT8R and ZnT8W correlate. Serum samples obtained from (a) 2–17-year-old and (b) 15–34-year-old new onset type 1 diabetes patients were tested for their binding to ZnT8R and ZnT8W. Antibody titers are shown as U/ml. R 2 and p-values for r 2 values are shown.
15–34-year-old type 1 diabetes patients
We found 66% (227/343) of the sera in the 15–34-year-old type 1 diabetes patient cohort to contain ZnT8Ab. Of the ZnT8Ab-positive samples, 66% (n = 151) were positive for both isoforms, while 23 and 11% reacted with either ZnT8R or ZnT8W, respectively. Antibody titers of ZnT8R and ZnT8W correlated significantly (p < 0.0001; Figure 1(b)). No correlation between ZnT8Ab titers or frequencies and c-peptide levels or BMI at onset was observed.
ZnT8Ab in LADA patients
We analyzed 47 GAD65Ab-positive LADA patients whose blood were collected within 2 years of diagnosis of diabetes. In this cohort, 42% (n = 20/47) of the patients tested positive for either ZnT8RAb or ZnT8WAb. Of the ZnT8Ab-positive samples, 75% (n = 15) were positive for both isoforms, while 25% reacted with ZnT8R only. No correlation with age and antibody titer or prevalence was found in this cohort.
Overlap with other autoantibodies
A random subset of the younger patient cohort (n = 163) and all samples of the older cohort were tested for overlap of ZnT8Ab, GAD65Ab, and IA-2Ab (Figure 2). IAA were not included in the analysis as for some patients insulin therapy was initiated prior to sample collection. In the younger type 1 diabetes patient cohort (n = 163), we found that 50% of the analyzed samples were positive for ZnT8Ab (either specificity), GAD65Ab and IA-2Ab (Figure 2(a)). The inclusion of ZnT8Ab allowed the identification of an additional four patients, which otherwise would have been described as autoantibody-negative. In this cohort, the combined analysis with all three autoanti-bodies allowed the identification of 98% of the patients.
Figure 2.
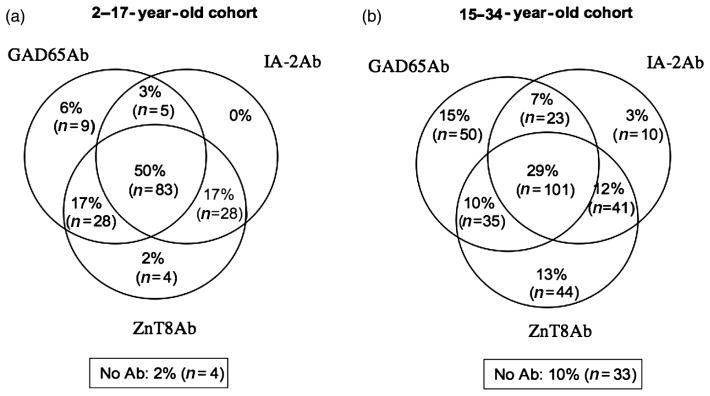
Overlapping prevalence of ZnT8Ab, GAD65Ab, and IA-2Ab at onset. Serum samples obtained from (a) 2–17-year-old and (b) 15–34-year-old new onset type 1 diabetes patients were tested for autoantibodies to ZnT8R and/or W (combined), GAD65, and IA-2. Frequencies of antibodies and their overlap with other autoantibodies are shown as percentage and total number of samples.
In the older type 1 diabetes patient group, 23% (n = 80) of the patients showed reactivity to all three autoantigens (GAD65, IA-2, and ZnT8) (Figure 2(b)). While the analysis for IA-2Ab and GAD65Ab identified 77% of the patients, an additional 13% tested positive for ZnT8Ab. Only 10% of the patients tested negative for all three autoantibodies.
The prevalence of GAD65Ab in the younger cohort and in the older cohort was similar (71 and 77%, respectively), while both IA-2Ab and ZnT8Ab prevalences were lower in the older patient group as compared with the younger patients (IA-2Ab: 48 versus 78%, ZnT8Ab: 63 versus 80%).
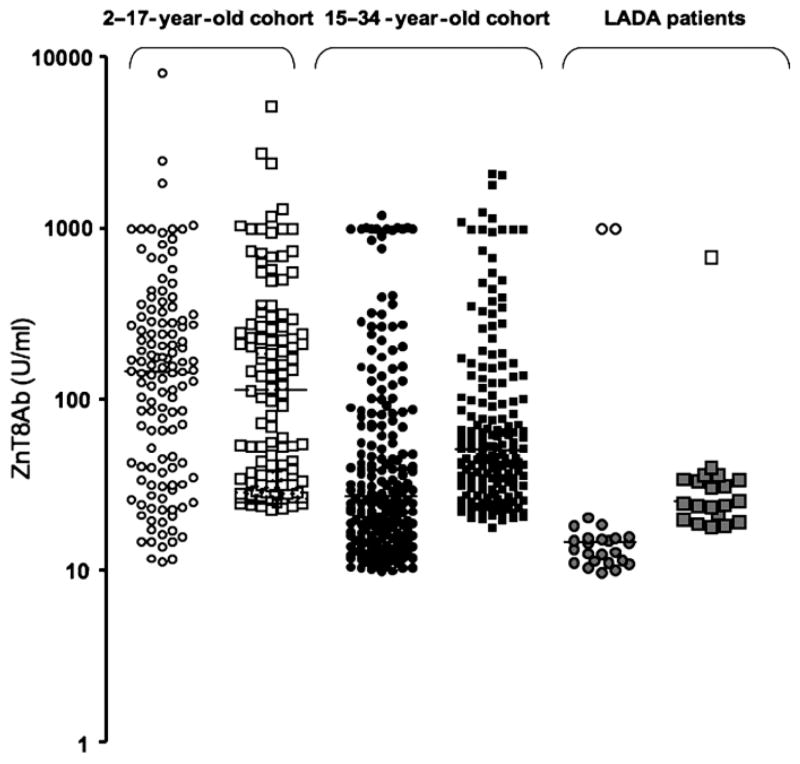
ZnT8Ab titers are significantly higher in the younger type 1 diabetes patient cohort
We compared the ZnT8Ab titers in antibody-positive samples in both type 1 diabetes patient cohorts and LADA patients (Figure 3). Median binding to ZnT8R in the young type 1 diabetes cohort was significantly higher compared with the binding in the older cohort (median 148 and 29 U/ml, respectively; p < 0.001). The same observation was made in regard to ZnT8Ab binding to the ZnT8W isoform (median 145 and 58 U/ml, respectively; p < 0.01). When comparing the ZnT8Ab titers at the age overlap between the two cohorts (15–17 years), no significant difference was observed, indicating that the observed differences are due to age, not cohort (data not shown). No significant difference between the two cohorts was observed in GAD65Ab levels (median index of 0.3 and 0.33), while the IA2-Ab levels in the younger cohort were significantly higher as compared with the older type 1 diabetes patients (median index of 0.4 and 0.05, respectively; p < 0.0001). ZnT8Ab titers to both isoforms were significantly lower in the LADA patients as compared with either type 1 diabetes cohorts (p < 0.0001).
Figure 3.
ZnT8Ab titers to both ZnT8 isoforms are significantly higher in the younger type 1 diabetes patient cohort. Serum samples obtained from 2–17-year-old (open symbols) and 15–34-year-old (black symbols) new onset type 1 diabetes patients, and LADA patients (gray symbols) were tested for their binding to ZnT8R (circles) and ZnT8W (squares). Median binding is indicated. Note that the ZnT8Ab titer is displayed on a logarithmic scale.
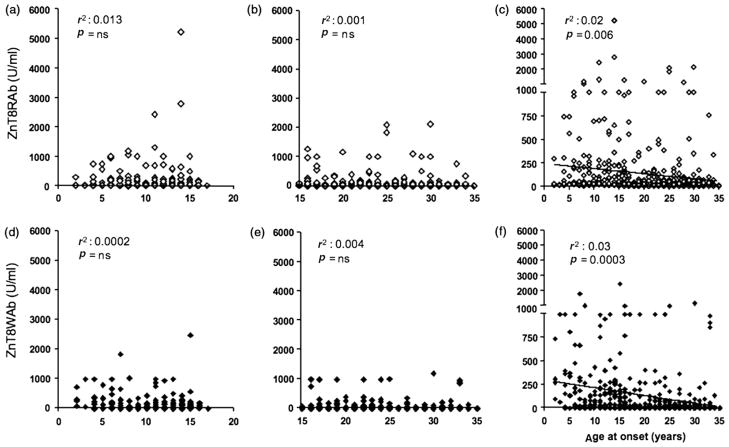
Correlation of ZnT8Ab titers and age at onset
When analyzing the individual cohorts for a correlation between age at onset and ZnT8Ab titers, we found no correlation (Figure 4(a),(b),(d),(e)). However, after combining both cohorts, we observed a significant inverse correlation between age at onset and ZnT8Ab titers for ZnT8RAb and ZnT8WAb (p = 0.006 and 0.0003, respectively; Figure 4(c),(f)).
Figure 4.
ZnT8Ab titers correlated with age at onset. Correlation between ZnT8RAb titers (a, b, c) and ZnT8WAb titers (d, e, f) with age at onset was tested in the 2–17-year-old cohort (a, d), the 15–34-year-old cohort (b, e), and the combined cohort (c, f). R 2 and p-values for r2 values are shown.
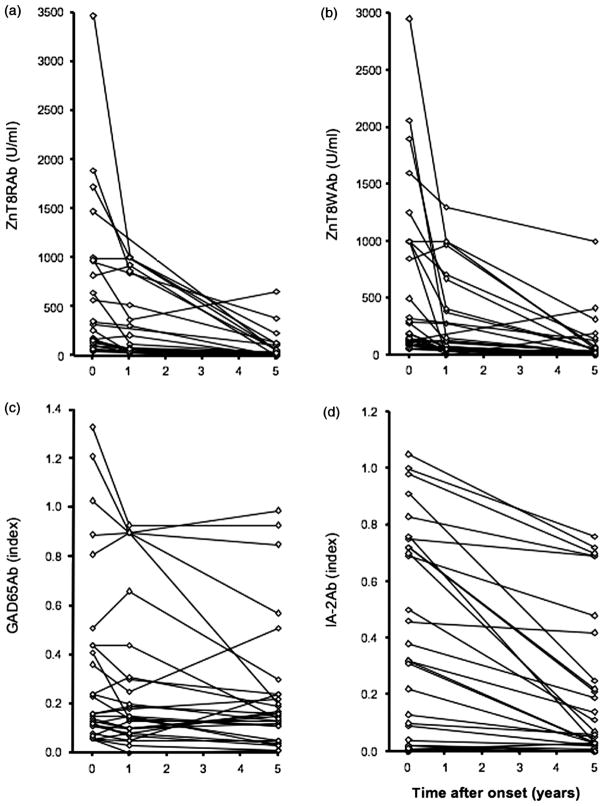
ZnT8Ab titers decline significantly after onset
We analyzed ZnT8Ab-positive longitudinal samples obtained from a subgroup of the 2–17-year-old type 1 diabetes patients (n = 32). For these patients, blood samples were collected at onset, 1 year, and 5 years after onset. These samples were analyzed for ZnT8Ab to both isoforms, GAD65Ab, and IA2-Ab (for IA2-Ab only samples taken at onset and at 5 years of disease duration were analyzed; Figure 5).
Figure 5.
Longitudinal titers of ZnT8Ab decline shortly after disease onset. Longitudinal samples from type 1 diabetes patients were obtained at onset, 1 and 5 years after onset. Samples were analyzed for (a) ZnT8RAb, (b) ZnT8WAb, (c) GAD65Ab, and (d) IA2Ab. Antibody-positive samples were analyzed for their respective antibody titer in respect to disease duration.
We found that ZnT8RAb levels decreased significantly from 320 (28–3472 U/ml) to 162 U/ml (9–1000 U/ml) (p = 0.0001) during the initial year after onset of disease. Further decline was observed during the following 4 years (to 27, 0–817 U/ml) (p < 0.0001). Of the 31 patients that tested positive for ZnT8RAb at onset, 13 (42%) showed a decline below the cut-off for positivity.
Similarly, autoantibody levels to ZnT8W decreased significantly from 128 (28–2960 U/ml) to 46 U/ml (0–1300 U/ml) (p = 0.0011) in the initial year after onset of diabetes. Further decline was observed during the following 4 years (to 24, 0–1000 U/ml) (p < 0.0001). Of the 29 patients that tested positive for ZnT8WAb at onset, 12 (41%) declined below the cut-off for positivity during the first 5 years of disease duration.
Of the samples that were initially positive for both antibodies (n = 28), 32% (n = 9) declined below cutoff, 17% (n = 5) lost only one antibody and 50% (n = 14) remained positive for both autoantibodies. No significant change in GAD65Ab titer in the GAD65Ab-positive samples (n = 28; Figure 5(c)) was observed during the entire followup period, while the IA2Ab titer declined significantly (Figure 5(d); p < 0.0001).
Discussion
Our analysis of ZnT8Ab in type 1 diabetes patients and LADA patients showed the presence of this novel autoantibody in the majority of type 1 diabetes patients, while fewer LADA patients tested ZnT8Ab positive. As in previous studies, positivity for ZnT8RAb and ZnT8WAb strongly correlated [8,10]. ZnT8Ab binding to ZnT8 R325 and ZnT8 W325 isoforms is somewhat regulated by the individual’s polymorphism at position 325 [8]. ZnT8Ab obtained from individuals with homozygous SLC30A8 genotypes are often restricted to their respective ZnT8 isoform, indicating that amino acid 325 greatly affects a major antibody epitope [8]. However, this restriction is not absolute, suggesting that ZnT8Ab recognize also other epitopes [8,10]. We conclude that either the majority of the here analyzed patients were heterozygote for the SLC30A8 polymorphism encoding ZnT8R or ZnT8W, or that a large proportion of ZnT8Ab binds to an epitope independent from amino acid residue 325. SLC30A8 genotypes of the patients were not available to address this question.
Previously a correlation of age at onset and ZnT8Ab prevalence in <1–18-year-old type 1 diabetes patients was demonstrated, followed by a decline of ZnT8Ab prevalence in 23–30-year-old type 1 diabetes patients [3]. While we could not observe a direct correlation between age at onset and ZnT8Ab prevalence in the younger patient cohort, we found a significant inverse correlation between age and ZnT8Ab when combining both cohorts, reflecting the decrease of ZnT8Ab prevalence in older patients [3]. Moreover, the ZnT8Ab frequency and titer in type 1 diabetes patients with an older age at onset was significantly lower. The lack of a direct correlation of ZnT8 prevalence and age at onset in the younger patient cohort may be due to a different age distribution between our patient cohort and the earlier study [3]. It is of interest that IAA and IA-2Ab show an inverse correlation with age at onset, while GAD65Ab show no and in some studies, even a positive correlation with age at onset [17]. We found similar prevalences of GAD65Ab in the younger cohort and in the older cohort, while both IA-2Ab and ZnT8Ab prevalences were lower in the older patient group, supporting the understanding of the latter antibodies to be negatively correlated with age at onset. It remains to be determined what causes these differences in age-antibody correlations. Our observation of low frequency and titers of ZnT8Ab in LADA patients may be due to the advanced age of these patients or the relatively long disease duration.
In our longitudinal cohort of young type 1 diabetes patients, we found that ZnT8Ab titers and IA2Ab titers declined rapidly after onset of disease, while GAD65Ab levels appeared stable. These findings are in agreement with earlier studies suggesting that GAD65Ab persist over many years after clinical onset [17]. Previous studies showed a decline in ICA and IA-2Ab levels after clinical onset of type 1 diabetes [18,19], while others reported stable IA-2Ab frequencies [20,21]. The persistence of autoantibodies, despite the loss of beta cells as the antigen source after disease onset, has been attributed to continuous beta cell regeneration, protein mimicry, incomplete destruction of the beta cells, release of autoantigen from other sources, cross-reactivity [22], or the existence of an antigen-independent memory through autoantibody-specific anti-idiotypic antibodies [23,24].
While IA-2 and GAD65 are expressed in small quantities in tissues other than pancreatic beta cells [25,26], ZnT8 is expressed in beta cells and alpha cells only [27,28]. A tight correlation of declining ZnT8Ab titers and autoimmune destruction of pancreatic beta cells may suggest the antibody as a potential marker for islet destruction as has been discussed [3]. Such a marker would indeed be of extreme interest, because current assessments of beta cell mass by insulin or C-peptide levels do not only necessarily reflect the number of beta cells, but also their functional state [29]. However, a previous study reported no change in ZnT8Ab levels during the prediabetic period, when the beta cell mass is likely to decline [3].
In conclusion, our study confirms ZnT8Ab as promising markers for type 1 diabetes, especially in younger patients. This new marker allows the detection of type 1 diabetes with increased sensitivity, as also evident from our study. Screening protocols for the identification of individuals at risk for type 1 diabetes and characterization of autoimmune diabetes should include ZnT8Ab, while the significant decline in autoantibody titer after clinical onset of type 1 diabetes may limit its use after diagnosis. Whether the ZnT8 antigen may be used in tolerance induction for the prevention of type 1 diabetes, as demonstrated for insulin and GAD65 in NOD mice (for review see [30]) and to some extent in humans [12,31,32], will need to be established.
Footnotes
Declaration of interest: This study was performed as independent research sponsored by the National Institutes of Health(DK53456, DK53004, DK26190, and DK17047, a Basic Science Award from the American Diabetes Association to CSH, the EU 7th Framework Programme DIAPREPP (grant agreement 202013), the Swedish Research Council (2007–2266), and the Swedish Diabetes Association. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Sherr J, Sosenko J, Skyler JS, Herold KC. Prevention of type 1 diabetes: The time has come. Nat Clin Pract Endocrinol Metab. 2008;4:334–343. doi: 10.1038/ncpendmet0832. [DOI] [PubMed] [Google Scholar]
- 2.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41:11–18. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 3.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenzlau JM, Moua O, Sarkar SA, Yu L, Rewers M, Eisenbarth GS, Davidson HW, Hutton JC. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann NY Acad Sci. 2008;1150:256–259. doi: 10.1196/annals.1447.029. [DOI] [PubMed] [Google Scholar]
- 5.Seve M, Chimienti F, Devergnas S, Favier A. In silico identification and expression of SLC30 family genes: An expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genomics. 2004;5:32. doi: 10.1186/1471-2164-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzlau JM, Moua O, Liu Y, Eisenbarth GS, Hutton JC, Davidson HW. Identification of a major humoral epitope in Slc30A8 (ZnT8) Ann NY Acad Sci. 2008;1150:252–255. doi: 10.1196/annals.1447.028. [DOI] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 8.Wenzlau JM, Frisch LM, Gardner TJ, Sarkar S, Hutton JC, Davidson HW. Novel antigens in type 1 diabetes: The importance of ZnT8. Curr Diab Rep. 2009;9:105–112. doi: 10.1007/s11892-009-0019-4. [DOI] [PubMed] [Google Scholar]
- 9.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A common non-synonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achenbach P, Lampasona V, Landherr U, Koczwara K, Krause S, Grallert H, Winkler C, Pfluger M, Illig T, Bonifacio E, Ziegler AG. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia. 2009;52:1881–1888. doi: 10.1007/s00125-009-1438-0. [DOI] [PubMed] [Google Scholar]
- 11.Törn C, Landin-Olsson M, Lernmark A, Palmer JP, Arnqvist HJ, Blohme G, Lithner F, Littorin B, Nystrom L, Schersten B, Sundkvist G, Wibell L, Östman J. Prognostic factors for the course of beta cell function in autoimmune diabetes. J Clin Endocrinol Metab. 2000;85:4619–4623. doi: 10.1210/jcem.85.12.7065. [DOI] [PubMed] [Google Scholar]
- 12.Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, Harris RA, Robertson JA, Lernmark A. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Compl. 2005;19:238–246. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Hampe CS, Hammerle LP, Bekris L, Ortqvist E, Kockum I, Rolandsson O, Landin-Olsson M, Torn C, Persson B, Lernmark A. Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab. 2000;85:4671–4679. doi: 10.1210/jcem.85.12.7070. [DOI] [PubMed] [Google Scholar]
- 14.Mire-Sluis AR, Das RG, Lernmark Å. The World Health Organization international collaborative study for islet cell antibodies. Diabetologia. 2000;43:1282–1292. doi: 10.1007/s001250051524. [DOI] [PubMed] [Google Scholar]
- 15.Oak S, Phan TH, Gilliam LK, Hirsch IB, Hampe CS. Animal insulin therapy induces a biased insulin antibody response that persists for years after introduction of human insulin. Acta Diabetol. 2009 doi: 10.1007/s00592-009-0135-2. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: First assay proficiency evaluation. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 17.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, Schaefer JB, Zarghami M, Day HL, Landin-Olsson M, Palmer JP, Janer-Villanueva M, Hood L, Sundkvist G, Lernmark A, Breslow N, Dahlquist G, Blohmé G Diabetes Incidence in Sweden Study Group; Swedish Childhood Diabetes Study Group. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 18.Borg H, Gottsater A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51:1754–1762. doi: 10.2337/diabetes.51.6.1754. [DOI] [PubMed] [Google Scholar]
- 19.Decochez K, Tits J, Coolens JL, Van Gaal L, Krzentowski G, Winnock F, Anckaert E, Weets I, Pipeleers DG, Gorus FK. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care. 2000;23:838–844. doi: 10.2337/diacare.23.6.838. [DOI] [PubMed] [Google Scholar]
- 20.Jensen RA, Gilliam LK, Törn C, Landin-Olsson M, Karlsson FA, Palmer JP, Kockum I, Akesson K, Lernmark B, Lynch K, Breslow N, Lernmark A Diabetes Incidence Study in Sweden (DISS) group. Multiple factors affect the loss of measurable Cpeptide over 6 years in newly diagnosed 15- to 35-year-old diabetic subjects. J Diabetes Compl. 2007;21:205–213. doi: 10.1016/j.jdiacomp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Borg H, Marcus C, Sjoblad S, Fernlund P, Sundkvist G. Islet cell antibody frequency differs from that of glutamic acid decarboxylase antibodies/IA2 antibodies after diagnosis of diabetes. Acta Paediatr. 2000;89:46–51. doi: 10.1080/080352500750029059. [DOI] [PubMed] [Google Scholar]
- 22.Savola K, Sabbah E, Kulmala P, Vahasalo P, Ilonen J, Knip M. Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–1297. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 23.Oak S, Gilliam LK, Landin-Olsson M, Torn C, Kockum I, Pennington CR, Rowley MJ, Christie MR, Banga JP, Hampe CS. The lack of anti-idiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci USA. 2008;105:5471–5476. doi: 10.1073/pnas.0800578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayak R, Mitra-Kaushik S, Shaila MS. Perpetuation of immunological memory: A relay hypothesis. Immunology. 2001;102:387–395. doi: 10.1046/j.1365-2567.2001.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tillakaratne NJ, Erlander MG, Collard MW, Greif KF, Robin AJ. Glutamate decarboxylases in nonneural cells of rat testis and oviduct: Differential expression of GAD65 and GAD67. J Neurochem. 1992;58:618–627. doi: 10.1111/j.1471-4159.1992.tb09763.x. [DOI] [PubMed] [Google Scholar]
- 26.Takeyama N, Ano Y, Wu G, Kubota N, Saeki K, Sakudo A, Momotani E, Sugiura K, Yukawa M, Onodera T. Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci. 2009;84:678–687. doi: 10.1016/j.lfs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283:10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 28.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 29.Gillard P, Vandemeulebroucke E, Keymeulen B, Pirenne J, Maes B, De Pauw P, Vanrenterghem Y, Pipeleers D, Mathieu C. Functional beta-cell mass and insulin sensitivity is decreased in insulin-independent pancreas-kidney recipients. Transplantation. 2009;87:402–407. doi: 10.1097/TP.0b013e3181928a1c. [DOI] [PubMed] [Google Scholar]
- 30.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–161. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 31.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 32.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The diabetes prevention trial-type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]