Abstract
The roles of activated glia and of glial cytokines in the pathogenesis of Alzheimer’s disease are reviewed. Interleukin-1 (IL-1), a microglia-derived acute phase cytokine, activates astrocytes and induces expression of the astrocyte-derived cytokine, S100β, which stimulates neurite growth (and thus has been implicated in neuritic plaque formation) and increases intracellular free calcium levels. Interleukin-1 also upregulates expression and processing of β-amyloid precursor proteins (β-APPs) (thus favoring β-amyloid deposition) and induces expression of α1-antichymotrypsin, thromboplastin, the complement protein C3, and apolipoprotein E, all of which are present in neuritic plaques. These cytokines, and the molecular and cellular events that they engender, form a complex of interactions that may be capable of self-propagation, leading to chronic overexpression of glial cytokines with neurodegenerative consequences. Self-propagation may be facilitated by means of several reinforcing feedback loops. β-Amyloid, for instance, directly activates microglia, thus inducing further IL-1 production, and activates the complement system, which also leads to microglial activation with IL-1 expression. Self-propagation also could result when S100β-induced increases in intraneuronal free calcium levels lead to neuronal injury and death with consequent microglial activation. Such chronic, self-propagating, cytokine-mediated molecular and cellular reactions would explain the progressive neurodegeneration and dementia of Alzheimer’s disease.
Keywords: Alzheimer’s disease, cytokines, S100 protein, interleukin-1, review
Extracellular deposition of β-amyloid, initially in the form of diffuse amyloid deposits not associated with glial, neuronal, neuritic, or synaptic alterations, is widely believed to be an important early event in the progressive neurodegeneration and dementia of Alzheimer’s disease.1 These diffuse deposits, however, neither establish the diagnosis of Alzheimer’s disease nor explain the progressive dementia characteristic of this disease. It is the appearance of the diagnostic neuritic plaques, with associated dystrophic neurites and “condensed” (congophilic) amyloid, and ultimately the loss of neurons and synapses,2–4 that correlate with the intellectual decline of Alzheimer’s disease.3,5,6 Neuritic plaques have been shown to contain, in addition to β-amyloid and dystrophic neurites, various nonamyloid proteins (including α1-antichymotrypsin,7 thromboplastin,8 the complement protein C3,9 and apolipoprotein E10), and activated glial cells (astrocytes11–13 and microglia14) overexpressing neurotrophic cytokines.12,13
The cytokine concept developed from earlier work with leucocyte-derived polypeptide factors (lymphokines, monokines, etc) that orchestrate the multiple cellular interactions involved in immune responses. With the discovery that these regulatory molecules were not limited to cells of the immune system either in origin or in target spectrum, the more general term cytokine was introduced.15 Cytokines are secreted molecules, characteristically expressed constitutively in low amounts, which are promptly upregulated in response to various environmental stimuli (including increased concentrations of other cytokines). They may have effects on their cell of origin (autocrine effects), on adjacent cells of other types (paracrine effects), or systemically (endocrine effects).
There is increasing evidence that plaque-associated activated glia and glia-derived cytokines are important pathogenic factors in the progression of neuropathologic changes, and, by extension, in the progression of dementia in Alzheimer’s disease. This has led to the concept of a chronic, smoldering inflammatory process underlying the pathological progression of this disease. In this article we examine the roles of activated astrocytes and microglia, and of the cytokines they elaborate, in Alzheimer’s disease. We propose that these cytokines are key links in a complex of molecular and cellular interactions that, in the form of limited acute phase responses to various forms of central nervous system injury or insult, assist in repair and recovery from such injury. However, established functions of acute-phase-response cytokines suggest that chronic elevation (eg, in response to cumulative insults) may lead to the characteristic pathological features of Alzheimer’s disease. This concept suggests that Alzheimer’s disease has a multifactorial origin, and may be better conceived as a final common pathway of neurological decline rather than as a distinct disease entity with a single, identifiable origin.
MICROGLIA
Microglia are a resident population of brain cells of uncertain (but probably mesodermal) embryological origin.16 A variety of pathological insults result in microglial activation, a complex of morphological and biochemical alterations that include enlargement and proliferation, expression of novel cell-surface antigens (leukocyte common antigen, immunoglobulin Fc receptors, major histocompatibility complex [MHC] class I and class II glycoproteins, β2-integrins, and the vitronectin receptor), 17,18 and release of various proteinases, cytokines (including interleukin-1 [IL-1], interleukin-6 [IL-6], and tumor necrosis factor-α [TNF-α]),19,20 and reactive oxygen and nitrogen intermediates.21 Activated microglia are active in phagocytosis of necrotic material in a variety of pathological conditions and may be responsible for the alternative pathway cleavage of the β-amyloid precursor protein (β-APP) to yield amyloidogenic fragments in Alzheimer’s disease.22 Perineuronal microglia also may be involved in synaptic remodeling after neuronal injury.23
Activated microglia are constant and prominent components of neuritic plaques in Alzheimer’s disease.24 They also are found, although in lesser numbers, in the diffuse cerebral amyloid deposits that are thought to precede neuritic plaque formation in this disease25 but are not found in significant numbers in the diffuse amyloid plaques of the cerebellum,26 where progression to neuritic plaques is not observed.27–30
ASTROCYTES
Astrocytes, like microglia, become activated in response to various pathological insults, and such astrocytic activation is prominent in Alzheimer’s disease and in many other neurodegenerative conditions. This activation is marked by hypertrophy31 and results in expression of structural proteins (eg, glial fibrillary acidic protein, [GFAP]), adhesion molecules; antigen presenting capabilities including MHC antigens (for reviews see Frohman et al32 and Malhotra et al33) and cytokines, such as S100β.12,34,35 Activated astrocytes respond to IL-1 by producing a variety of cytokines, including TNF-α,36 IL-6, IL-8, colony stimulating factor-1 (CSF-1),37 granulocyte-macrophage CSF, granulocyte-CSF38 and S100β.39 Interleukin-1–stimulated, activated astrocytes also produce the complement protein C340 and apolipoprotein E.41
In Alzheimer’s disease activated astrocytes form a halo around neuritic plaques, and extend processes into the cores of these plaques.12,13,42 As is the case with activated microglia, these cells are found in fewer numbers in diffuse cerebral amyloid deposits (Mrak, Sheng, Griffith; unpublished data) and are not significantly increased in number in the cerebellum of Alzheimer patients.26
INTERLEUKIN-1
Interleukin-1 is an acute phase cytokine that has numerous systemic effects.43 The principal source of both the α and β isoforms are macrophages in the periphery and microglia in the central nervous system.44–46 These two isoforms, encoded by genes on chromosome 2,47 are synthesized as 33 kDa precursors that are cleaved to yield 17 kDa products. For the β isoform only the 17 kDa cleavage product is biologically active. For the α isoform both the 33 kDa and 17 kDa molecules are biologically active, but only the 17 kDa molecule is secreted (for recent reviews of IL-1 functions see Rothwell48 and Dinarello and Wolffe43).
Interleukin-1 also seems to function as an acute phase cytokine in the brain. For instance, IL-1 is expressed in microglia within hours following head injury in humans, and this expression is accompanied by dystrophic, swollen β-APP immunoreactive (β-APP+) neurites.49 Interleukin-1 upregulates expression of β-APP50–52 through stimulation of the β-APP promoter,53 and it also stimulates β-APP processing.54 Interleukin-1 has autocrine effects on microglia, with promotion of microglial proliferation in mixed neural cell cultures55 and upregulation of microglial expression of IL-6, TNF-α, and IL-1 itself.19,20 Interleukin-1 paracrine effects include activation of astrocytes44,56 with upregulation of expression of astrocytic cytokines, including CSF-1,57,58 IL-6, TNF-α,20,36 and S100β39 as well as expression of astrocyte-derived apolipoprotein E.41 In vitro, low levels of IL-1 promote survival of cultured neurons,59 but higher levels are toxic.60 Interleukin-1 also upregulates expression of tissue factor (thromboplastin) by vascular endothelial cells61 and of α1-antichymotrypsin (an acute phase inflammatory protein62) in cultured cells.63 Both of these proteins, like β-amyloid1 and apolipoprotein E,10 are components of neuritic plaque cores in Alzheimer’s disease.7,8,64
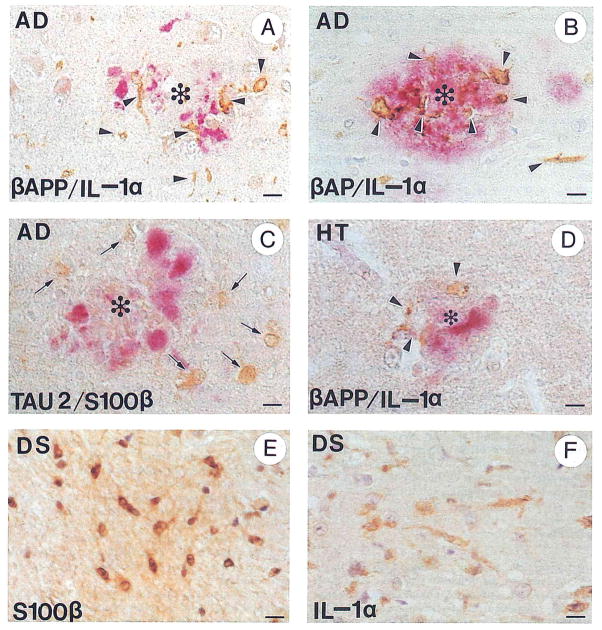
Interleukin-1 is elevated in tissue homogenates of temporal lobe12 and in cerebrospinal fluid65 from patients with Alzheimer’s disease. The α isoform of IL-1 accounts for most of the excessive expression in temporal lobe.66 In Alzheimer patients activated immunoreactive IL-1 microglia are increased sixfold over those present in age matched controls,12 and these microglia show distinct patterns of distribution in these brains. Interleukin-1α immunoreactivity in various brain regions (hippocampus, cortex of the various cerebral lobes, and cerebellum) correlates both with generally recognized patterns of regional susceptibility in Alzheimer’s disease and with concomitant involvement of activated astrocytes in these regions.26 Furthermore, IL-1α immunoreactivity is intimately associated with neuritic plaques in Alzheimer’s disease (Fig 1A and B).25,66 This evidence suggests a seminal role for these cells and this cytokine in the progression of Alzheimer’s disease.
FIGURE 1.
Immunohistochemical demonstration of IL-1α+ activated microglia and S100β+ activated astrocytes in Alzheimer’s disease and Down’s syndrome, and following head trauma. (A) Alzheimer’s disease: activated IL-1α+ microglia (brown, arrowheads) associated with β-APP+ dystrophic neurites (red) in a neuritic plaque (*). (B) Alzheimer’s disease: activated IL-1α+ microglia (brown, arrowheads) associated with β-amyloid (β-AP) immunopositive deposits (red) in a neuritic plaque (*). (C) Alzheimer’s disease: activated S100β+ astrocytes (brown, arrows) associated with Tau 2+ dystrophic neurites (red) in a neuritic plaque (*). (D) Head trauma: activated IL-1α+ microglia (brown, arrowheads) associated with β-APP+ dystrophic neurites in a neuritic plaque-like structure in a patient surviving 48 hours after head trauma. (E) Down’s syndrome: activated S100β+ astrocytes (brown) in a trisomy 21 fetus at 23 weeks’ gestation; (F) Down’s syndrome: activated IL-1α+ microglia in a 2-year-old patient with Down’s syndrome. Bars represent 15 μm. Immunohistochemical technique122: Paraffin blocks of formalin-fixed mesial temporal lobe tissue were sectioned at 10-μm thickness. Sections were deparaffinized in xylene, rehydrated in a graded series of ethanol solutions, and permeabilized in 0.05% Triton-X 100 for 10 minutes followed by 0.2 N HCl for 20 minutes. Endogenous peroxidase was blocked by incubation with 3% H2O2 in 97% methanol for 30 minutes. Sections for β-APP immunoreaction were pretreated with 99% formic acid for 5 minutes and washed with phosphate-buffered saline. Antibodies employed were polyclonal anti-IL-1α (Cistron, Pine Brook, NJ), diluted 1:20; polyclonal anti-β-amyloid (Boehringer-Mannheim Biochemica, Indianapolis, IN), diluted 1:10; monoclonal anti-β-amyloid (a gift from G.W. Roberts, SmithKline Beecham Pharmaceuticals, Essex, UK), diluted 1:1,000; monoclonal anti-β-APP (clone 22C11; Boehringer-Mannheim Biochemica), diluted 1:10; polyclonal antibovine S100β (a gift from L.J. Van Eldik, Northwestern University, Chicago, IL), diluted 1:300; and monoclonal Tau 2 antibody (Sigma Chemical Company, St Louis, MO), diluted 1:100. For dual immunoreaction sections were further processed according to the manufacturer’s protocol in DAKO’s (Glostrup, Denmark) double immunolabeling kit (K-665).
Further evidence of a role for IL-1 in Alzheimer’s disease is the differential distribution of activated IL-1α+ microglia among different plaque types.25 This differential distribution suggests that activated IL-1α+ microglia are involved in the evolution of these plaques—from the clinically silent, diffuse plaque through the diagnostic neuritic plaque to the inert, end-stage dense core, nonneuritic plaque. Interleukin-1α+ microglia are found in small numbers in most cerebral cortical diffuse plaques in Alzheimer’s disease, suggesting an early involvement of these cytokine-expressing cells in plaque evolution.25 This is in contrast to their absence in the diffuse plaques found in the cerebellum in Alzheimer’s disease,26 where progression to neuritic forms is not observed.27–30 Interleukin-1α+ microglia are larger and most abundant in neuritic plaques without dense β-amyloid cores, again suggesting an important role in plaque evolution. In contrast, fewer of these cells are found in “mature” neuritic plaques (those with a dense β-amyloid core), and no IL-1α+ microglia are found in “burnt-out” plaques (β-amyloid dense core plaques that do not have associated β-APP+ neurites),25 suggesting a waning of microglia-associated inflammatory activity as the dystrophic neurites disappear and an end stage of plaque evolution is reached.
Patients with Down’s syndrome invariably develop striking Alzheimer’s-like neuropathological changes in middle age.67 As Down’s syndrome patients (unlike Alzheimer’s patients) can be recognized at (or even before) birth, these patients provide a model for studying early (predementia) neuropathological changes in Alzheimer’s disease. In Down’s syndrome there is striking, early overexpression of IL-112 by activated microglia (Fig 1F), which increases progressively in fetal, young (0 to 9 years), and adult Down’s syndrome patients.68 In addition to Down’s syndrome, head trauma recently has been recognized as a significant risk factor for the later development of Alzheimer’s disease.69–76 Such acute cerebral trauma has been shown to elicit “acute phase” cellular and molecular responses: β-APP expression is increased within days of a single episode of head injury,77 and this elevation is associated with elevated microglial IL-1α expression49 (Fig 1D). These findings suggest that the mechanism underlying the elevated risk of Alzheimer’s disease observed in head injury patients may involve cytokine-mediated initiation of neurodegenerative processes, which, in concert with advancing age and additional risk factors, may become self-sustaining and terminate in Alzheimer’s disease.
S100β
S100 is most familiar to pathologists as an immunohistochemical marker for tumors of neuroectodermal origin. There are two 10 kDa isoforms, α and β, that are encoded by single genes on chromosomes 1 and 21,78 respectively. In the brain the β isoform is most abundant and is synthesized by and released from astrocytes, whereas the α isoform is expressed in small quantities by neurons.79,80 Activated (“reactive”) astrocytes in Alzheimer’s disease express greatly elevated levels of S100β,42 and both astrocyte activation56 and increased expression of S100β39 are induced in vivo by IL-1.
Several lines of evidence suggest an important intercellular regulatory (cytokine) function for S100β released by activated astrocytes. S100β increases cytoplasmic free calcium levels in neurons,81 stimulates neurite outgrowth in vitro,42 and promotes neuronal survival in vivo.82 During fetal neurological development S100β seems to be an important neurotrophic agent82,83 with effects on neuroblasts and glia during this period.83 S100β also has autocrine effects, including elevation of astrocyte intracellular free calcium levels81 and stimulation of astrocytic proliferation and hypertrophy.84
Mapping of the S100β gene to the Down’s (q22) region of chromosome 21 suggests a signal pathogenic role for S100β in Down’s syndrome and, by analogy, in Alzheimer’s disease.85 This suggestion is supported by the finding of elevated S100β expression (ie, in excess of the 1.5-fold increase expected from gene loading) in activated astrocytes in Down’s syndrome at all ages12 and by the elevated levels of biologically active S100β and S100β mRNA in Alzheimer’s disease.42
Biologically active (homodimeric) S100β levels42 and the numbers of activated S100β+ astrocytes12 are dramatically increased in brains of patients with Alzheimer’s disease, and the distribution of this increase across different brain regions parallels the pattern of involvement of these regions in Alzheimer’s disease.86 These observations, together with the spatial orientation of these activated cells to neuritic (Tau 2+) plaques13 (Fig 1C) and the ability of S100β to promote neurite extension,42 suggest an important participatory (and not merely reactive) role for these astrocytes and for this cytokine in the evolution of neuritic plaques in Alzheimer’s disease.
In Down’s syndrome overexpression of S100β is apparent as early as 18 to 19 weeks in utero,12,68 an effect that may be interpreted as a direct genetic consequence of trisomy 21. This increase in S100β expression in Down’s syndrome fetuses (Fig 1E) is not accompanied by elevated expression of GFAP.68 Like the early expression of IL-1 observed in Down’s syndrome, S100β expression increases progressively in fetal, young, and adult Down’s syndrome patients.68 In contrast, deposition of extracellular β-amyloid and formation of neuritic plaques are not detectable before adolescence.68,87 These findings strongly suggest that overexpression of IL-1 and S100β are important early events in the genesis of Alzheimer’s-like neuropathological changes in Down’s syndrome.
OTHER CYTOKINES
Interleukin-6 has been shown to be elevated in temporal lobe tissue from patients with Alzheimer’s disease,88 and IL-6 immunoreactivity is demonstrable in and around some senile plaques in Alzheimer’s disease.89,90 Unlike IL-1, IL-6 is not elevated in serum or cerebrospinal fluid from patients with Alzheimer’s disease.89,91 The activated microglia in Alzheimer’s brain express interferon-α.92 Interleukin-2 immunoreactivity is profuse in brain tissue from both control and Alzheimer patients.93 Serum TNF-α levels have been reported to be both elevated94 and depressed95 in Alzheimer’s disease.
CYTOKINE-β-AMYLOID RELATIONSHIPS
The involvement of β-amyloid and its precursor, β-APP, in Alzheimer’s disease is, of course, well established.1 β-Amyloid is an important and prominent constituent of the characteristic plaques of Alzheimer’s disease, and β-APP was first identified in the course of a search for the origin of these amyloid deposits.96 Dystrophic neurites within plaques react intensely with anti-βAPP antibodies25,49 (Fig 1A). These structures are intimately associated with activated IL-1α+ microglia25 and with classical complement pathway proteins,9,97 suggesting a cytolytic attack by this system against neuronal membranes. Upregulation of a membrane inhibitor of reactive lysis (that protects host cells against complement-mediated lysis) in dystrophic neurites and neurofibrillary tangle-containing neurons in Alzheimer’s disease has been reported.98
The β-amyloid precursor proteins are a closely related group of integral membrane proteins.96,99 These proteins are synthesized in cell somas and undergo fast anterograde axonal transport.100 They are present at synapses,96,101 interact with second messenger systems,102 and appear to be important in memory formation.103 Like S100β, these proteins are encoded by a gene mapped to chromosome 21 but, unlike S100β,85 this gene lies outside the Down’s syndrome region.104,105 The major degradation pathway for β-APP does not yield amyloidogenic fragments.99 However, cleavage of these proteins within their hydrophobic intramembranous domains (normally a minor degradation pathway) yields an amyloidogenic peptide that accumulates in both the diffuse and neuritic plaques of Alzheimer’s disease.1,106 β-Amyloid precursor protein is not a cytokine but does enhance survival of cultured neurons107 and regulate the effects of nerve growth factor on neurite outgrowth108 and cell adhesion.109 Furthermore, two of the three β-APP isoforms (751 kDa and 770 kDa) contain a Kunitz type protease inhibitor110,111 and have regulatory effects on neuronal growth.102 Neuronal β-APP expression also has been associated with neurofibrillary tangle formation in Alzheimer’s disease.112
β-Amyloid, the amyloidogenic cleavage product of β-APP, was once thought to be inert, but now seems to participate intimately in the neurodegenerative events of Alzheimer’s disease. β-Amyloid stimulates microglial activation and microglial expression of IL-1,113 and directly activates (ie, without the intervention of immunoglobulins) the classical, but not the alternative, complement pathway leading to cell lysis.97 Purified β-amyloid also has been shown to form calcium channels in artificial phosphatidylserine bilayers114; in this way β-amyloid might act synergistically with S100β to effect neuronal cell injury and death through increases in intracellular calcium concentrations.
PATHOGENIC IMPLICATIONS
It is evident from the foregoing discussion that activated glia and their cytokines, β-amyloid and its precursor proteins, and the complement proteins are components of a complex system of cellular and molecular interactions in Alzheimer’s disease. The potential positive feedback and autocrine activities within such a system suggest that these interactions might become chronically self-propagating. Such a self-propagating system, shown diagrammatically in Fig 2, would be capable of explaining many of the characteristic neuropathological features of Alzheimer’s disease, including formation of neuritic plaques containing activated astrocytes, activated microglia, β-APP+ 115 and Tau 2+ 116 overgrown dystrophic neurites as well as extracellular deposits containing β-amyloid, α1antichymotrypsin, thromboplastin, the complement protein C3, and apolipoprotein E.
FIGURE 2.
Diagram of proposed pathogenic interactions between glial cytokines and molecular and cellular events in Alzheimer’s disease. Micro, microglial; Astro, astroglial; PN-1, protease nexin 1; α1-ACT, α1 antichymotrypsin; (Ca)i, intracellular free calcium concentration; P-tau, excessively phosphorylated tau; PHF, paired helical filaments in neurofibrillary tangles; PolyA+ mRNA, polyadenylated mRNA.
This self-propagating pathogenic process might result from any of several events capable of initiating chronic elevation of IL-1 or from the cumulative effects of several such events. Interleukin-1, in turn, would stimulate neuronal and endothelial cell synthesis and the processing of β-APP; upregulate expression of plaque components, such as α1-antichymotrypsin, thromboplastin, the complement protein C3, and apolipoprotein E; and activate microglia and astrocytes with induction of S100β expression.
S100β, in turn, would stimulate neurite growth, promote astrocyte activation, and increase intracellular free calcium levels in neurons and astrocytes. Chronic elevation of intracellular free calcium would favor calcium-mediated events, such as excessive phosphorylation, possibly including abnormal phosphorylation of the tau present in the neurofibrillary tangles117 in neurons and neurites, and could ultimately result in the neuronal cell death characteristic of Alzheimer’s disease.
The combined effects of β-APP upregulation and processing would favor increased β-amyloidogenic cleavage products. This, together with increased production of α1-antichymotrypsin, thromboplastin, and apolipoprotein E, could result in extracellular deposits (plaques) containing these proteins. The β-amyloid in these plaques, in turn, could have effects that would feed back to propagate the system. These include (1) direct activation of microglia with microglial IL-1 production and consequent astrocyte activation with increased S100β expression, and (2) activation of the classical complement pathway that, in turn, leads to microglial activation with IL-1 production.
Neuronal cell injury and cell death might result from the combined effects of increased neuronal intra-cytoplasmic calcium levels (attributable to S100β-mediated increases in neuronal free calcium levels and possibly to β-amyloid-mediated formation of neuronal cell membrane calcium channels), accumulation of neurofibrillary tangles (caused by calcium-mediated phosphorylation of tau), and complement-mediated cell damage (resulting from β-amyloid binding to and activation of complement). Such cellular injury would, in turn, activate microglia and provide yet another feedback mechanism for propagation of the cytokine cycle.
CONCLUSIONS
Central nervous system injury provokes a limited acute phase cellular and molecular response, including elaboration of the glial cytokines IL-1 and S100β, which is important in healing and repair. However, chronic or repeated stimulation of this response may produce a self-sustaining cycle that gives rise to the characteristic neuropathological changes of Alzheimer’s disease. Thus, Alzheimer’s disease may not be a distinct disease entity but rather a syndrome with multiple origins, all leading to chronic self-propagation of a vicious circle of glial acute phase responses, formation of plaques, neuronal cell death, and synaptic loss. As such, it may be impossible to identify a single initiating molecular or cellular event as the cause of Alzheimer’s disease. Recognized risk factors for Alzheimer’s disease include age,118 trisomy 21,67 certain mutations in the β-APP gene,119 the type 4 allele of apolipoprotein E,120 and a history of head trauma.69–76 However, regardless of initiating or promoting factors, chronic elevation of IL-1 would seem to be a key factor in the pathogenic progression observed in Alzheimer’s disease. This hypothesis, together with our finding of elevated levels of IL-1 in Alzheimer’s disease, has led to the suggestion of a novel treatment strategy based on pharmacological intervention in IL-1 production or actions.121
Acknowledgments
Supported in part by National Institutes of Health grants AG10208, MH45729, NS27414, and AG12411.
We thank Sue Woodward for technical assistance and Pam Free for secretarial support.
Abbreviations
- IL
interleukin
- APP
amyloid precursor protein
- MHC
major histocompatibility complex
- TNF
tumor necrosis factor
- GFAP
glial fibrillary acidic protein
- CSF
colony stimulating factor
References
- 1.Selkoe DJ. Alzheimer’s disease: A central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Davies CA, Mann DMA, Sumpter PQ, et al. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 4.Masliah E, Terry RD, Alford M, et al. Cortical and subcortical patterns of synatophysin-like immunoreactivity in Alzheimer’s disease. Am J Pathol. 1991;138:235–246. [PMC free article] [PubMed] [Google Scholar]
- 5.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 6.Samuel W, Terry RD, DeTeresa R, et al. Clinical correlates of cortical and nucleus basalis pathology in Alzheimer dementia. Arch Neurol. 1994;51:772–778. doi: 10.1001/archneur.1994.00540200048015. [DOI] [PubMed] [Google Scholar]
- 7.Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- 8.McComb RD, Miller KA, Carson SD. Tissue factor antigen in senile plaques of Alzheimer’s disease. Am J Pathol. 1991;139:491–494. [PMC free article] [PubMed] [Google Scholar]
- 9.McGeer PL, Akiyama H, Itagaki S, et al. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 10.Namba Y, Tomonaga M, Kawasaki H, et al. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutz-feldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 11.Mandybur TI, Chuirazzi BA. Astrocytes and the plaques of Alzheimer’s disease. Neurology. 1990;40:635–639. doi: 10.1212/wnl.40.4.635. [DOI] [PubMed] [Google Scholar]
- 12.Griffin WST, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng JG, Mrak RE, Griffin WST. S100β protein expression in Alzheimer’s disease: Potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 14.Rozemuller JM, Eikelenboom P, Stam FC. Role of microglia in plaque formation in senile dementia of the Alzheimer’s type. Vir-chows Arch B Cell Pathol Incl Mol Pathol. 1986;51:247–254. doi: 10.1007/BF02899034. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S. Cell mediated immunity and the inflammatory system. Hum Pathol. 1976;7:249–264. doi: 10.1016/s0046-8177(76)80036-6. [DOI] [PubMed] [Google Scholar]
- 16.Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: A historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 17.Thomas WE. Brain macrophages: Evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- 18.McGeer PL, Kawamata T, Walker DG, et al. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 19.Sebire G, Emilie D, Wallon C, et al. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J Immunol. 1993;150:1517–1523. [PubMed] [Google Scholar]
- 20.Lee SC, Liu W, Dickson DW, et al. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 21.Banati RB, Gehrmann J, Schubert P, et al. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer LM, Shein DS, Brannaman CA, et al. Processing of neuronally-generated βAPP by macrophages and microglia. J Neuropathol Exp Neurol. 1993;52:296. [Google Scholar]
- 23.Schoen SW, Graeber MB, Kreutzberg GW. 5′-Nucleotidase immunoreactivity of perineuronal microglia responding to rat facial nerve axotomy. Glia. 1992;6:314–317. doi: 10.1002/glia.440060410. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski HM, Vorbrodt AW, Weigel J, et al. Ultrastructure of the cells forming amyloid fibers in Alzheimer disease and scrapie. Am J Med Genet Suppl. 1990;7:287–297. doi: 10.1002/ajmg.1320370757. [DOI] [PubMed] [Google Scholar]
- 25.Griffin WST, Sheng JG, Roberts GW, et al. Interleukin-1 expression in different plaque types in Alzheimer’s disease: Significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Sheng JG, Mrak RE, Griffin WST. Interleukin-1α expression in brain regions in Alzheimer’s disease: Correlation with neuritic plaque distribution. J Neuropathol Exp Neurol. 1995;54:435. doi: 10.1111/j.1365-2990.1995.tb01063.x. (abstr) [DOI] [PubMed] [Google Scholar]
- 27.Kemper T. Neuroanatomical and neuropathological changes in normal aging and in dementia. In: Albert ML, editor. Clinical Neurobiology of Aging. New York; NY: Oxford University Press; 1984. pp. 9–52. [Google Scholar]
- 28.Ikeda SI, Allsop D, Glenner GG. Morphology and distribution of plaque and related deposits in the brain of Alzheimer’s disease and control cases. Lab Invest. 1989;60:113–122. [PubMed] [Google Scholar]
- 29.Joachim CL, Morris JH, Selkoe DJ. Diffuse senile plaques occur commonly in cerebellum in Alzheimer’s disease. Am J Pathol. 1989;435:309–319. [PMC free article] [PubMed] [Google Scholar]
- 30.Price JL, Davis PB, Morris JC, et al. The distribution of tangles, plaques, and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 31.da Cunha A, Jefferson JJ, Tyor WR, et al. Gliosis in human brain: Relationship to size but not other properties of astrocytes. Brain Res. 1993;600:161–165. doi: 10.1016/0006-8993(93)90415-j. [DOI] [PubMed] [Google Scholar]
- 32.Frohman EM, van den Noort S, Gupta S. Astrocytes and intracerebral immune responses. J Clin Immunol. 1989;9:1–9. doi: 10.1007/BF00917121. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra SK, Shnitka TK, Elbrink J. Reactive astrocytes-A review. Cytobios. 1990;61:133–160. [PubMed] [Google Scholar]
- 34.Mossakowski MJ, Weinrauder H. Glial fibrillary acidic protein and S100 protein in abnormal astrocytes in Wilson’s disease. Neuropatol Pol. 1986;24:365–376. [PubMed] [Google Scholar]
- 35.Kimura T, Budka H. Glial fibrillary acidic protein and S-100 protein in human hepatic encephalopathy: Immunocytochemical demonstration of dissociation of two glia-associated proteins. Acta Neuropathol (Berl) 1986;70:17–21. doi: 10.1007/BF00689509. [DOI] [PubMed] [Google Scholar]
- 36.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 37.Hao C, Guilbert LJ, Fedoroff S. Production of colony-stimulating factor-1 (CSF-1) by mouse astroglia in vitro. J Neurosci Res. 1990;27:314–323. doi: 10.1002/jnr.490270310. [DOI] [PubMed] [Google Scholar]
- 38.Aloisi F, Care A, Borsellino G, et al. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 39.Wu JH, Sheng JG, Skinner RA, et al. Interleukin-1 (IL-1) induced expression of Alzheimer disease-related proteins in rat brain. Soc Neurosci Abstr. 1993;19:1037. (abstr) [Google Scholar]
- 40.Rus HG, Kim LM, Niculescu FI, et al. Induction of C3 expression in astrocytes is regulated by cytokines and Newcastle disease virus. J Immunol. 1992;148:928–933. [PubMed] [Google Scholar]
- 41.Das S, Geller L, Niethammer M, et al. Expression of the Alzheimer amyloid-promoting factors α1-antichymotrypsin and apolipoprotein E is induced in astrocytes by IL-1. Neurobiol Aging. 1994;15:17. (suppl) [Google Scholar]
- 42.Marshak DR, Pesce SA, Stanley LC, et al. Increased S100β neurotrophic activity in Alzheimer’s disease temporal lobe. Neurobiol Aging. 1992;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- 43.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 44.Giulian D, Lachman LB. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985;228:497–499. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- 45.Hetier E, Ayala J, Denefle P, et al. Brain macrophages synthesize interleukin-1 and interleukin-1 mRNAs in vitro. J Neurosci Res. 1988;21:391–397. doi: 10.1002/jnr.490210230. [DOI] [PubMed] [Google Scholar]
- 46.Righi M, Mori L, DeLibero G, et al. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- 47.Boultwood J, Breckon G, Birch D, et al. Chromosomal localization of murine interleukin-1 alpha and beta genes. Genomics. 1989;5:481–485. doi: 10.1016/0888-7543(89)90013-x. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- 49.Griffin WST, Sheng JG, Gentleman SM, et al. Microglial interleukin-1α expression in human head injury: Correlations with neuronal and neuritic β-amyloid precursor protein expression. Neurosci Lett. 1994;176:133–136. doi: 10.1016/0304-3940(94)90066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldgaber D, Harris HW, Hla T, et al. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altstiel LD, Sperber K. Cytokines in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:481–495. doi: 10.1016/0278-5846(91)90023-t. [DOI] [PubMed] [Google Scholar]
- 52.Forloni G, Demicheli F, Giorgi S, et al. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells. Modulation by interleukin-1. Brain Res Mol Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly RJ, Freidhoff AJ, Beer B, et al. Interleukin-1 stimulates the beta-amyloid precursor protein promoter. Cell Mol Neurobiol. 1990;10:485–495. doi: 10.1007/BF00712843. [DOI] [PubMed] [Google Scholar]
- 54.Buxbaum JD, Oishi M, Chen HI, et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganter S, Northoff H, Mannel D, et al. Growth control of cultured microglia. J Neurosci Res. 1992;33:218–230. doi: 10.1002/jnr.490330205. [DOI] [PubMed] [Google Scholar]
- 56.Giulian D, Woodward J, Young DG, et al. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SC, Liu W, Roth P, et al. Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. J Immunol. 1993;150:594–604. [PubMed] [Google Scholar]
- 58.Thery C, Stanley ER, Mallat M. Interleukin 1 and tumor necrosis factor-alpha stimulate the production of colony-stimulating factor 1 by murine astrocytes. J Neurochem. 1992;59:1183–1186. doi: 10.1111/j.1471-4159.1992.tb08366.x. [DOI] [PubMed] [Google Scholar]
- 59.Brenneman DE, Schultzberg M, Bartfai T, et al. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 60.Brenneman DE, Page SW, Schultzberg M, et al. A decomposition product of a contaminant implicated in L-tryptophan eosinophilia myalgia syndrome affects spinal cord neuronal cell death and survival through stereospecific, maturation and partly interleukin-1-dependent mechanisms. J Pharmacol Exp Ther. 1993;266:1029–1035. [PubMed] [Google Scholar]
- 61.Bevilacqua MP, Pober JS, Majeau GR, et al. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Ann Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 63.Mackiewicz A, Speroff T, Ganapathi MK, et al. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol. 1991;146:3032–3037. [PubMed] [Google Scholar]
- 64.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cacabelos R, Barquero M, Garcia P, et al. Cerebrospinal fluid interleukin-1 beta (IL-1 beta) in Alzheimer’s disease and neurological disorders. Methods Find Exp Clin Pharmacol. 1991;13:455–458. [PubMed] [Google Scholar]
- 66.Stanley LC, Griffin WST. Localization of IL-1α and IL-1β in diseases with gliosis, dementia and immune suppression. Soc Neurosci Abstr. 1990;16:1345. (abstr) [Google Scholar]
- 67.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 68.Baggott PJ, Sheng JG, Cork L, et al. Expression of Alzheimer’s disease (AD)-related proteins during development in Down’s syndrome (DS) Soc Neurosci Abstr. 1993;19:182. (abstr) [Google Scholar]
- 69.Gautrin D, Gauthier S. Alzheimer’s disease. Environmental factors and etiologic hypotheses. Can J Neurol Sci. 1989;16:375–387. doi: 10.1017/s0317167100029425. [DOI] [PubMed] [Google Scholar]
- 70.Graves AB, White E, Koepsell TD, et al. The association between head trauma and Alzheimer’s disease. Am J Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 71.Edwards JK, Larson EB, Hughes JP, et al. Are there clinical and epidemiological differences between familial and non-familial Alzheimer’s disease? J Am Geriatr Soc. 1991;39:477–483. doi: 10.1111/j.1532-5415.1991.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 72.Mortimer JA, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer’s disease: A collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20:28–35. doi: 10.1093/ije/20.supplement_2.s28. (suppl) [DOI] [PubMed] [Google Scholar]
- 73.Williams DB, Annegers JF, Kokmen E, et al. Brain injury and neurologic sequelae: A cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41:1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 74.Gentleman S, Roberts G. Risk factors in Alzheimer’s disease. BMJ. 1992;304:118–119. doi: 10.1136/bmj.304.6819.118-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Duijn CM, Tanja TA, Haaxma R, et al. Head trauma and the risk of Alzheimer’s disease. Am J Epidemiol. 1992;135:775–782. doi: 10.1093/oxfordjournals.aje.a116364. [DOI] [PubMed] [Google Scholar]
- 76.Mayeux R, Ottman R, Tang MX, et al. Genetic susceptibility and head injury as risk factors for Alzheimer’s disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 77.Roberts GW, Gentleman SM, Lynch A, et al. βA-4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 78.Morii K, Tanaka R, Takahashi Y, et al. Structure and chromosome assignment of human S100 alpha and beta subunit genes. Biochem Biophys Res Commun. 1991;175:185–191. doi: 10.1016/s0006-291x(05)81218-5. [DOI] [PubMed] [Google Scholar]
- 79.Shashoua VE, Hesse GW, Moore BW. Proteins of the brain extracellular fluid: Evidence for release of S-100 protein. J Neurochem. 1984;42:1536–1541. doi: 10.1111/j.1471-4159.1984.tb12739.x. [DOI] [PubMed] [Google Scholar]
- 80.Van Eldik LJ, Zimmer DB. Secretion of S-100 from rat C6 glioma cells. Brain Res. 1987;436:367–370. doi: 10.1016/0006-8993(87)91681-7. [DOI] [PubMed] [Google Scholar]
- 81.Barger SW, Van Eldik LJ. S100 β stimulates calcium fluxes in glial and neuronal cells. J Biol Chem. 1992;267:9689–9694. [PubMed] [Google Scholar]
- 82.Bhattacharyya A, Oppenheim RW, Prevette D, et al. S100 is present in developing chicken neurons and Schwann cells and promotes neuron survival in vivo. J Neurobiol. 1992;23:451–466. doi: 10.1002/neu.480230410. [DOI] [PubMed] [Google Scholar]
- 83.Sarnat HB. Regional differentiation of the human fetal ependyma: Immunocytochemical markers. J Neuropathol Exp Neurol. 1992;51:58–75. doi: 10.1097/00005072-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Selinfreund RH, Barger SW, Pledger WJ, et al. Neurotrophic protein S100β stimulates glial cell proliferation. Proc Natl Acad Sci U S A. 1991;88:3554–3558. doi: 10.1073/pnas.88.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allore R, O’Hanlon D, Price R, et al. Gene encoding the β subunit of S100 protein is on chromosome 21: Implications for Down syndrome. Science. 1988;239:1311–1313. doi: 10.1126/science.2964086. [DOI] [PubMed] [Google Scholar]
- 86.Van Eldik LJ, Griffin WST. S100β expression in Alzheimer’s disease: Relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223:398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 87.Rumble B, Retallack R, Hilbich C, et al. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer’s disease. N Engl J Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- 88.Wood JA, Wood PL, Ryan R, et al. Cytokine indices in Alzheimer’s temporal cortex: No changes in mature IL-1 beta or IL-1 RA but increases in the associated acute phase proteins IL-6, alpha 2-macroglobulin and C-reactive protein. Brain Res. 1993;629:245–252. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 89.Bauer J, Strauss S, Schreiter-Gasser U, et al. Interleukin-6 and α-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 90.Strauss S, Bauer J, Ganter U, et al. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest. 1992;66:223–230. [PubMed] [Google Scholar]
- 91.van Duijn CM, Hofman A, Nagelkerken L. Serum levels of interleukin-6 are not elevated in patients with Alzheimer’s disease. Neurosci Lett. 1990;108:350–354. doi: 10.1016/0304-3940(90)90666-w. [DOI] [PubMed] [Google Scholar]
- 92.Akiyama H, Ikeda K, Katoh M, et al. Expression of MRP14, 27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J Neuroimmunol. 1994;50:195–201. doi: 10.1016/0165-5728(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 93.Luber-Narod J, Rogers J. Immune system associated antigens expressed by cells of the human central nervous system. Neurosci Lett. 1988;94:17–22. doi: 10.1016/0304-3940(88)90263-7. [DOI] [PubMed] [Google Scholar]
- 94.Fillit H, Ding WH, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- 95.Cacabelos R, Alvarez XA, Franco-Maside A, et al. Serum tumor necrosis factor (TNF) in Alzheimer’s disease and multi-infarct dementia. Methods Find Exp Clin Pharmacol. 1994;16:29–35. [PubMed] [Google Scholar]
- 96.Kang J, Lemaire HG, Unterbeck A, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 97.Rogers J, Cooper NR, Webster S, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGeer PL, Walker DG, Akiyama H, et al. Detection of the membrane inhibitor of reactive lysis (CD59) in diseased neurons of Alzheimer brain. Brain Res. 1991;544:315–319. doi: 10.1016/0006-8993(91)90071-3. [DOI] [PubMed] [Google Scholar]
- 99.Selkoe DJ, Berman-Podlisny M, Joachim CL, et al. β-amyloid precursor protein of Alzheimer’s disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and non-neural tissues. Proc Natl Acad Sci U S A. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koo EH, Sisodia SS, Archer DR, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schubert W, Prior R, Weidemann A, et al. Localization of Alzheimer βA4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563:184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- 102.Araki W, Kitaguchi N, Tokushima Y, et al. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- 103.Huber G, Martin JR, Loffler J, et al. Involvement of amyloid precursor protein in memory formation in the rat: An indirect antibody approach. Brain Res. 1993;603:348–352. doi: 10.1016/0006-8993(93)91261-p. [DOI] [PubMed] [Google Scholar]
- 104.Tanzi RE, Gusella JF, Watkins PC, et al. Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 105.Goldgaber D, Lerman MI, McBride OW, et al. Characterization and chromosomal localization of cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 106.Joachim CL, Selkoe DJ. The seminal role of β-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992;6:7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 107.Whitson JS, Selkoe DJ, Cotman CW. Amyloid β protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- 108.Milward EA, Papadopoulos R, Fuller SJ, et al. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 109.Schubert D, Jin LW, Saitoh T, et al. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 110.Kitaguchi N, Takahashi Y, Tokushima Y, et al. Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitor activity. Nature. 1988;331:530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- 111.Oltersdorf T, Fritz LC, Schenk DB, et al. The secreted form of the Alzheimer’s amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989;341:144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- 112.Schmechel DE, Goldgaber D, Burkhart DS, et al. Cellular localization of messenger RNA encoding amyloid-β-protein in normal tissue and in Alzheimer disease. Alzheimer Dis Assoc Disord. 1988;2:96–111. doi: 10.1097/00002093-198802020-00002. [DOI] [PubMed] [Google Scholar]
- 113.Araujo DM, Cotman CW. Beta-amyloid stimulates glial cells in vitro to produce growth factors that accumulate in senile plaques in Alzheimer’s disease. Brain Res. 1992;569:141–145. doi: 10.1016/0006-8993(92)90380-r. [DOI] [PubMed] [Google Scholar]
- 114.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by trimediamine and aluminum. Proc Natl Acad Sci U S A. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cork LC, Masters C, Beyreuther K, et al. Development of senile plaques: Relationships of neuronal abnormalities and amyloid deposits. Am J Pathol. 1990;137:1383–1392. [PMC free article] [PubMed] [Google Scholar]
- 116.Papasozomenos SC. Tau protein immunoreactivity in dementia of the Alzheimer type. 1. Morphology, evolution, distribution and pathogenetic implications. Lab Invest. 1989;60:123–137. [PubMed] [Google Scholar]
- 117.Papasozomenos SC, Binder LI. Phosphorylation determines two distinct species of tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8:210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- 118.Evans DA, Funkenstein H, Albert MA, et al. Prevalence of Alzheimer’s disease in a community population of older persons. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 119.Murrell J, Farlow M, Ghetti B, et al. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 120.Strittmatter WJ, Saunders AM, Schmeckel D, et al. Apolipoprotein E: High avidity binding to β-amyloid and increased frequency of type 4 allele in late onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roberts GW, Gentleman SM, Stefan MD, et al. Alzheimer’s disease: Prospects for treatment. Lancet. 1993;341:432. doi: 10.1016/0140-6736(93)93022-s. [DOI] [PubMed] [Google Scholar]
- 122.Griffin WST, Stanley LC, Yeralan O, et al. Methods for the study of cytokines in human neurodegenerative disease. In: DeSouza EB, editor. Neurobiology of Cytokines. Vol. 17. New York: Academic Press, Methods in Neuroscience; 1993. pp. 268–287. [Google Scholar]