Summary
A device capable of detecting seizures and alerting caregivers would be a major advance for epilepsy management, and could be used to guide early intervention and prevent seizure-related injuries. The objective of this work was to evaluate a seizure advisory system (SAS) that alerts caregivers of seizures in canines with naturally occurring epilepsy. Four dogs with epilepsy were implanted with a SAS that wirelessly transmits continuous intracranial EEG (iEEG) to an external device embedded with a seizure detection algorithm and the capability to alert caregivers. In this study a veterinarian was alerted by automated text message if prolonged or repetitive seizures occurred, and a rescue therapy protocol was implemented. The performance of the SAS caregiver alert was evaluated over the course of 8 weeks. Following discontinuation of antiepileptic drugs, the dogs experienced spontaneous unprovoked partial seizures that secondarily generalized. Three prolonged or repetitive seizure episodes occurred in 2 of the dogs. On each occasion, the SAS caregiver alert successfully alerted an on call veterinarian who confirmed the seizure activity via remote video-monitoring. A rescue medication was then administered and the seizures were aborted. This study demonstrates the feasibility of a SAS caregiver alert for prolonged or repetitive seizures, and enabling rescue medications to be delivered in a timely manner. The SAS may improve the management of human epilepsy by alerting caregivers of seizures, enabling early interventions, and potentially improving outcomes and quality of life of patients and caregivers.
Keywords: epilepsy, seizure management, seizure advisory, caregiver alert, EEG, device
1. Introduction
Poorly- controlled epilepsy represents a significant risk for injury and death as well as large economic burden (Manjunath et al 2012, Sperling 2004). Sudden unexplained death in epilepsy (SUDEP) may be a consequence of unwitnessed repetitive or prolonged seizures in vulnerable patients (Ficker 2000, Sperling et al 1999, Tomson et al 2005). The ability to accurately monitor and quantify the occurrence, duration, and intensity of seizures should improve the management of patients, help prevent seizure-related injuries, and may provide a strategy to prevent SUDEP. In addition, a device capable of accurately detecting seizures should prove useful for evaluation of AED and other therapies, since patient reporting of seizures and their diaries are known to be inaccurate (Hoppe et al 2007). Moreover, non-convulsive seizures are often unrecognized by patients but may contribute to complications arriving from epilepsy (Dunne et al 1987).
A seizure advisory system (SAS) capable of alerting patients and caregivers about seizures has been developed. The SAS is an implantable device that wirelessly transmits intracranial EEG (iEEG) from 16 implanted electrodes to an externally worn processing unit for storing continuous iEEG, analysis, and communicating results to patient or caregiver via text messaging (Figure 1). A previous study evaluated the performance of the seizure detection algorithm embedded in the SAS device in dogs with naturally occurring partial epilepsy (Davis et al 2011). In this study, we use the SAS to deliver automated caregiver alerts via text-messaging to an on-call veterinarian for acute repetitive or prolonged seizures.
Figure 1.
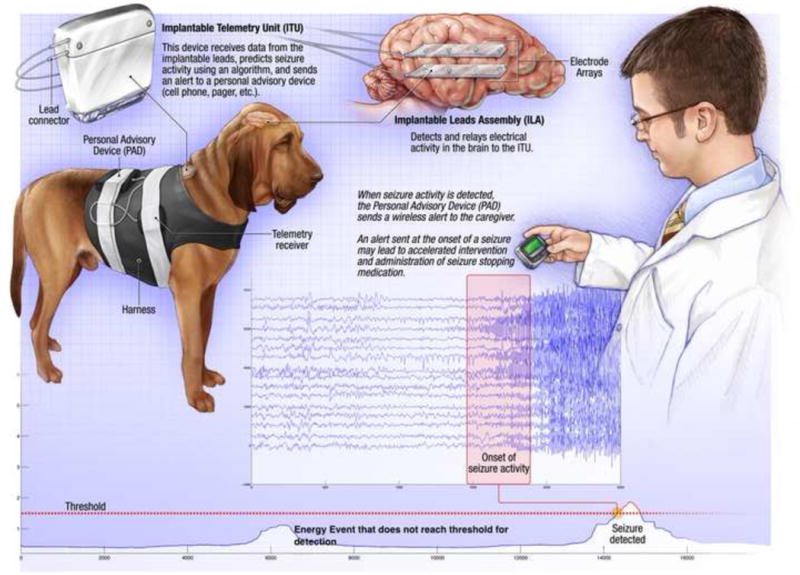
NeuroVista Seizure Advisory System (SAS). Schematic of SAS components implanted in dog. The SAS system includes: implantable lead assembly (ILA) placed in the subdural space (right), Implantable telemetry unit (ITU), Personal Advisory Device (PAD). The paging mode can deliver information to remote user via a wireless computer link (Davis et al 2011). Bottom) A representative sample of energy calculated from 16 channel intracranial EEG. The detection threshold (dashed line) is set to achieve 100% sensitivity for seizure detection. The initial increase in EEG energy at ~5000 seconds was not a seizure and did not pass threshold for detection. The 2nd energy increase was a seizure and passed threshold for detection.
While there are many animal models of epilepsy (Pitkanen & McIntosh 2006, Sarkisian 2001) the majority require the use of a chemical or physical insults resulting in seizures which may significantly differ from human epilepsy (Sarkisian 2001). In contrast, naturally occurring canine partial epilepsy is an excellent model for human epilepsy because of the clinical (Berendt et al 1999, Chandler 2006, Jeserevics et al 2007, Pellegrino & Sica 2004) and pharmacological (Leppik et al 2009, Volk et al 2008) similarity to human focal epilepsy. Importantly, dogs are large enough to accommodate the implantable SAS device, designed for humans, tested in this study (8).
The same principles of AED therapy apply to dogs and humans, although the altered metabolism and rapid elimination of some AEDs dictates the use of a subset of drugs in dogs. Approximately 25% of epileptic dogs remain uncontrolled, which is a rate comparable to humans (11–15). Based on these observations, dogs with naturally occurring epilepsy are good candidates for evaluating a seizure advisory system. The purpose of this study was to evaluate the SAS for alerting a veterinarian to acute repetitive or prolonged seizures in dogs with naturally occurring epilepsy. This work is a critical step toward evaluating the ability of a seizure monitoring system to alert patients/caregivers and initiate an intervention. In the future, a similar system implementing seizure forecasting algorithms may allow medications to be given to prevent seizure occurrence.
2. Methods
Five dogs with naturally occurring idiopathic epilepsy were implanted with SAS devices as previously described, Figure 1 (8). This study was approved by the University of Minnesota Institutional Animal Care and Use Committee. The dogs were housed in a canine epilepsy monitoring unit (EMU) with continuous recorded video. Phenobarbital therapy was withheld which resulted in naturally occurring seizures. Over an 8 week period, seizure activity was documented by review of the SAS device output, and validated by expert visual review of iEEG data and video recordings.
The SAS was programmed to alert an on-call veterinarian (NP) via an automated text-message when prolonged (single seizure lasting longer than 5 minutes) or repetitive seizures (2 or more seizures within 1 hour, or 3 or more seizures within 4 hours) were detected. The on-call veterinarian (NP) confirmed the seizure activity via remote video-monitoring when alerted to prolonged or repetitive seizures. In the event of prolonged or repetitive seizures, a rescue therapy protocol was initiated consisting of diazepam (0.5 mg/kg) or phenobarbital (6 mg/kg) administered as single IV dose via an indwelling catheter or vascular access port. Blood samples (2–5 mL) were collected at 30, 60, and 120 minutes following dosing. All blood samples were placed on ice immediately and centrifuged to separate plasma. Plasma samples were frozen at −20°C for later analysis of serum drug concentration.
Analysis of AEDs in plasma
Drug concentrations of the rescue therapy (phenobarbital and diazepam) were measured in the plasma samples using validated HPLC-UV methods. Phenobarbital and diazepam were extracted from 0.25 ml of plasma via liquid-liquid extraction. Phenytoin and nordiazepam were used as internal standards for phenobarbital and diazepam, respectively. A standard curve using 8 concentrations (run in triplicate) and twelve quality % control samples (low, med and high) was included with the study samples. The calibration curve for quantitation ranged from 0.1 to 5 μg/mL for phenobarbital, and from 50 to 1000 ng/ml for diazepam, with acceptable linearity (r2> 0.995).
3. Results
The performance of the SAS seizure detection algorithm was previously demonstrated to have high sensitivity (100%) and specificity (91%) (8). The average latency of detection based on expert visual review of seizure onset was 6.75 seconds (average from 9 seizures recorded during one month). Four of the five dogs had seizures during the 8 week study. The 5th dog did not have any seizure activity during the 8 week observation period. The four dogs with seizures exhibited partial onset, secondarily generalized seizures that electrographically were similar to human seizures (8). All seizures were spontaneous, unprovoked partial onset seizures that secondarily generalized lasting 1–2 minutes. Two of the dogs experienced a combined total of three prolonged or repetitive seizures during the study (Table 1). Alerts of repetitive or prolonged seizures were sent to a veterinarian by a text message who confirmed the seizure activity via video. Rescue medication was administered within 15 min (range 2–15 min) of alert. Diazepam and phenobarbital were administered on two and one occasion(s), respectively. Seizures were aborted in each instance as determined by visual observation of motor seizures and confirmed by cessation of electrical seizure activity on iEEG. There were no additional seizures between the alert and drug administration. A representative iEEG of seizure onset and emergency therapy with phenobarbital (6 mg/kg) is shown in Figure 2. Phenobarbital concentrations at 30 min were 9.95 and 10.6 μg/mL. Diazepam concentrations were 0.8 and 1.1 ug/mL at 30 mins. On one occasion, the dog had another seizure 1.5 hours following diazepam administration. At the time of the seizure, the diazepam plasma concentration was approximately 0.35 μg/mL. Phenobarbital was then administered and the seizure was aborted.
Table 1.
Summary of Rescue Therapy in Dogs with Epilepsy.
| Event | Seizure Emergency (# of seizures) | Druga | Dose (mg/kg) | Time of dose after alert | Number of seizures within 4 hrs post-dose |
|---|---|---|---|---|---|
| 1 | 3 within 1 hr | DZP | 0.5 | 15 min | 0 |
| 2 | 3 within 3 hr | DZP | 0.5 | 15 min | 1 (1.5 hr) |
| 3 | 3 within 4 hr | PB | 6 | 2 min | 0 |
| 4b | 2 additional | PB | 6 | 19 min | 0 |
DZP, diazepam; PB, phenobarbital
two additional seizures occurred in one dog within 4 hours
Figure 2.

Representative canine iEEG (only 4 channels shown) of seizure emergency therapy with phenobarbital (6 mg/kg). A cluster of seizures occurred and triggered a series of device detections resulting in an automated alert to the on call veterinarian. Phenobarbital was given within 5 minutes of the seizure shown and no subsequent seizures occurred.
4. Discussion
Over the 8 week caregiver alert period, all 3 episodes of repetitive or prolonged seizure activity resulted in successful automated alerts and interventions. Based on review of video and IEEG, there were no missed seizures. These results build on the previously reported performance of this system to reliably detect seizures (Davis et al 2011). In the current study, we focused on secondarily generalized seizures and seizure clusters. Given the limited spatial coverage with strip electrodes in dogs, there may be focal seizures that did not spread and were not detected by the device.
This pilot study demonstrates the feasibility of a SAS to deliver an automated caregiver alert of prolonged or repetitive seizures, enabling administration of rescue medications in a timely manner. Alerting patients and their caregivers of seizures may prevent or reduce the risk of physical harm, and possibly even death due to SUDEP. While intravenous administration of AEDs following an alert is not practical, there are several non-intravenous routes of administration with rapid onset of action which may be useful with the SAS. For example, intranasal and intramuscular administrations of midazolam have been shown to be as effective as an intravenous dose of diazepam and lorazepam, respectively (Lahat et al 2000, Mahmoudian & Zadeh 2004, Silbergleit et al 2012).
Another benefit of such a system is improved management of epilepsy by providing information to clinicians on onset, duration, and frequency of recognized and unrecognized seizures. While other systems have been employed to alert individuals or caregivers of seizures including motion detectors and seizure response dogs, this is the first report to our knowledge of a caregiver alert based on continuous monitoring iEEG in naturally occurring epilepsy.
The long-term goal of our research, and application of this system, is to predict seizure occurrence allowing a caregiver or the patient to administer a medication such as a benzodiazepine either intranasally or intramuscularly to prevent the seizure. Future work with the SAS device in canines with naturally occurring epilepsy will investigate the possibility of using continuous iEEG for seizure forecasting and the development of responsive therapies that might prevent seizure occurrence.
We evaluated the performance of the seizure advisory system in dogs with epilepsy
We demonstrated the feasibility of an alert system of prolonged or repetitive seizures.
All seizures resulted in successful automated alerts and interventions.
This system may improve the management of human epilepsy.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) NS073557 (U01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
Dr. Worrell has served as a paid consultant for Neurovista and Medtronic. Dr. Cloyd has served as a paid consultant for Allergan, Lundbeck, Upsher-Smith Laboratories, Sunovian Pharmaceuticals, Pfizer, and UCB. He also has been involved in product development with Lundbeck and CyDex Pharmaceuticals. Drs. Patterson, Coles, and Cloyd have received support from NeuroVista through grants to their respective institutions during part of the period of the research activity. Drs. Sheffield and Mavoori, John Higgins, Michael Bland, and Kent Leyde served as employees of NeuroVista during the period of the research activity. Dr. Litt has served as a paid consultant for Neurovista. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Contributor Information
Lisa D Coles, Email: durh0016@umn.edu.
Edward E Patterson, Email: patte037@umn.edu.
Warren D Sheffield, Email: wdsheffield@gmail.com.
Jaideep Mavoori, Email: jmavoori@hotmail.com.
Jason Higgins, Email: jason.higgins11@gmail.com.
Mike Bland, Email: mike.r.bland@gmail.com.
Kent Leyde, Email: kent.leyde@gmail.com.
James C Cloyd, Email: cloyd001@umn.edu.
Brian Litt, Email: littb@mail.med.upenn.edu.
Gregory Worrell, Email: Worrell.Gregory@mayo.edu.
References
- Berendt M, Hogenhaven H, Flagstad A, Dam M. Electroencephalography in dogs with epilepsy: similarities between human and canine findings. Acta Neurol Scand. 1999;99:276–83. doi: 10.1111/j.1600-0404.1999.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Chandler K. Canine epilepsy: what can we learn from human seizure disorders? Vet J. 2006;172:207–17. doi: 10.1016/j.tvjl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Davis KA, Sturges BK, Vite CH, Ruedebusch V, Worrell G, et al. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res. 2011;96:116–22. doi: 10.1016/j.eplepsyres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne JW, Summers QA, Stewart-Wynne EG. Non-convulsive status epilepticus: a prospective study in an adult general hospital. Q J Med. 1987;62:117–26. [PubMed] [Google Scholar]
- Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia. 2000;41(Suppl 2):S7–12. doi: 10.1111/j.1528-1157.2000.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64:1595–9. doi: 10.1001/archneur.64.11.1595. [DOI] [PubMed] [Google Scholar]
- Jeserevics J, Viitmaa R, Cizinauskas S, Sainio K, Jokinen TS, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med. 2007;21:1299–306. doi: 10.1892/06-285.1. [DOI] [PubMed] [Google Scholar]
- Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. Bmj. 2000;321:83–6. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik IE, Patterson E, Hardy B, Cloyd JC. Canine status epilepticus: Proof of principle studies. Epilepsia. 2009;50(Suppl 12):14–5. doi: 10.1111/j.1528-1167.2009.02362.x. [DOI] [PubMed] [Google Scholar]
- Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5:253–5. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Manjunath R, Paradis PE, Parise H, Lafeuille MH, Bowers B, et al. Burden of uncontrolled epilepsy in patients requiring an emergency room visit or hospitalization. Neurology. 2012;79:1908–16. doi: 10.1212/WNL.0b013e318271f77e. [DOI] [PubMed] [Google Scholar]
- Pellegrino FC, Sica RE. Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol. 2004;115:477–87. doi: 10.1016/s1388-2457(03)00347-x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23:241–61. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR. Overview of the Current Animal Models for Human Seizure and Epileptic Disorders. Epilepsy Behav. 2001;2:201–16. doi: 10.1006/ebeh.2001.0193. [DOI] [PubMed] [Google Scholar]
- Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr. 2004;9:98–101. doi: 10.1017/s1092852900008464. 06–9. [DOI] [PubMed] [Google Scholar]
- Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46:45–50. doi: 10.1002/1531-8249(199907)46:1<45::aid-ana8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Tomson T, Walczak T, Sillanpaa M, Sander JW. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. 2005;46(Suppl 11):54–61. doi: 10.1111/j.1528-1167.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- Volk HA, Matiasek LA, Lujan Feliu-Pascual A, Platt SR, Chandler KE. The efficacy and tolerability of levetiracetam in pharmacoresistant epileptic dogs. Vet J. 2008;176:310–9. doi: 10.1016/j.tvjl.2007.03.002. [DOI] [PubMed] [Google Scholar]


