Abstract
Pediatric cardiomyopathies, which are rare but serious disorders of the muscles of the heart, affect at least one in every 100,000 children in the USA. Approximately 40% of children with symptomatic cardiomyopathy undergo heart transplantation or die from cardiac complications within 2 years. However, a significant number of children suffering from cardiomyopathy are surviving into adulthood, making it an important chronic illness for both pediatric and adult clinicians to understand. The natural history, risk factors, prevalence and incidence of this pediatric condition were not fully understood before the 1990s. Questions regarding optimal diagnostic, prognostic and treatment methods remain. Children require long-term follow-up into adulthood in order to identify the factors associated with best clinical practice including diagnostic approaches, as well as optimal treatment approaches. In this article, we comprehensively review current research on various presentations of this disease, along with current knowledge about their causes, treatments and clinical outcomes.
Keywords: cardiomyopathy, heart failure, pediatric, risk factors, transplantation, treatment
Pediatric cardiomyopathies are disorders that affect the muscles of the heart. At any given time point, they affect at least 100,000 children worldwide. In the USA, at least one in every 100,000 children under the age of 18 years receives a diagnosis of primary cardiomyopathy [1]. The highest incidence is in children under 1 year of age. Morbidity and mortality of these diseases are high, and they are the most common cause of a heart transplant in children older than 1 year. Nearly 40% of children with a symptomatic cardiomyopathy either undergo heart transplantation or die within 2 years [2]. As a chronic disease, pediatric cardiomyopathy requires a comprehensive treatment approach and a multidisciplinary chronic disease program approach in order to improve health outcomes in a validated, cost-effective manner [3]. The course of pediatric cardiomyopathy is often progressive. The identification of risk factors helps define high-risk populations and leads to early diagnosis and therapy, which may alter the disease course. Understanding the risk factors may also lend to prevention of the disease through targeted strategies (Figure 1). In this review, we summarize the current knowledge about these diseases and their causes and treatments in children.
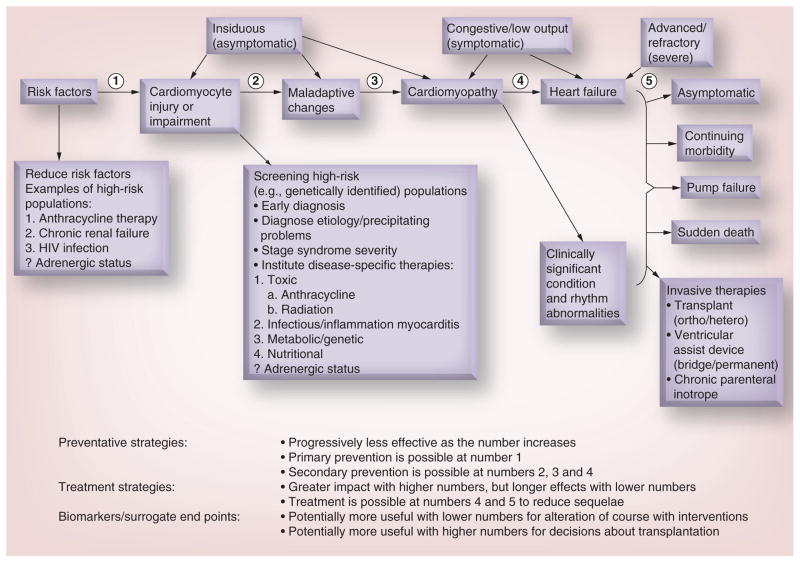
Figure 1. Stages in the course of pediatric ventricular dysfunction.
Review of the stages in the course of pediatric ventricular dysfunction that can be followed by echocardiographic measurements of left ventricular structure and function in conjunction with cardiac biomarkers that have been validated as surrogates for clinically significant cardiac end points. The identification of risk factors and high-risk populations for ventricular dysfunction are highlighted where their use may lead to preventive or early therapeutic strategies, while the determination of etiology may lead to etiology-specific therapies. Points (1–5) indicate stage-related points of intervention for preventive and therapeutic strategies, and where biomarkers and surrogate markers may be used. ? indicates that the role of adrenergic status is unknown.
Reproduced with permission from [21].
Dilated cardiomyopathy in childhood
Dilated cardiomyopathy (DCM) is a rare, but debilitating disease of the heart that can lead to heart failure in both children and adults. One of several phenotypic classifications of cardiomyopathy (the others being hypertrophic cardiomyopathy (HCM) or restrictive cardiomyopathy (RCM), left ventricular (LV) noncompaction and arrhythmogenic right ventricular dysplasia, as well as mixed phenotypic disease [e.g., dilated-hypertrophic cardiomyopathy), DCM is usually progressive and is a leading indication for cardiac transplantation in adults and children [4].
In the USA, the epidemiology of DCM shows that the annual incidence in children younger than 18 years is approximately 0.57 cases per 100,000 person-years [5]. Race, sex, age, and environmental and genetic factors influence the development and outcomes of the disease [6]. African–American children have a higher incidence and worse outcomes compared with white children in the USA. The incidence of DCM has been found to be higher than expected in HIV-1-infected patients, who are one of the most rapidly growing groups with acquired heart disease [7,8]. In addition, childhood cancer survivors tend to present with early DCM that seems to progress to RCM after anthracycline or heart radiotherapy treatments [9,10].
Effective treatment options for DCM in children are limited. Despite advances in the medical management of pediatric heart failure, the ability to restore native cardiac function in this population remains limited. Children are managed with protocols modeled on those for treating adults, and there are few cause- or child-specific therapies.
For children with DCM, the prognosis is generally poor. Approximately 40% of children undergo cardiac transplantation or die within 5 years of being diagnosed with DCM [2]. Cardiac transplantation in children is a successful treatment for end-stage heart failure, but it does carry the burden of life-long immunosuppression and limited graft survival, as well as the potential need for repeated heart transplantation procedures. Furthermore, the availability of heart transplantation as a final treatment option for children with DCM is only available in countries with advanced healthcare systems, which include transplant capabilities.
DCM is primarily diagnosed using echocardiography or, more recently, cardiac magnetic resonance (MR) imaging in symptomatic patients (e.g., unexplained heart failure) or via screening studies in children who have a familial history of DCM, an inborn error of metabolism, a neuromuscular disorder or a malformation syndrome associated with DCM [5]. Some children with acute myocarditis may also progress to chronic DCM [5]. Systolic dysfunction and progressive LV dilation are the hallmarks of DCM. Severity of LV dilation at the time of listing for heart transplant is associated with the risk of death [11,12]. Echocardiography is the primary source of information for diagnosing and monitoring the disease; for example, measures of systolic versus diastolic diameter (fractional shortening) or volume (ejection fraction) are routinely collected to monitor LV systolic function.
DCM is characterized by changes at molecular, cellular and interstitial levels following apoptosis, death of cardiomyocytes or collagen deposition, which can have long-term manifestations, such as dilatation of the LV, fibrosis, thinning of the interventricular septum and posterior wall of the LV, and a change in shape such that the LV tends to become more round with decreased contractility. These changes are described as ‘remodeling’ of the heart [13]. Historically, the goal of medical treatment has been the improvement of symptoms. Over the last two decades, the goal of treatment has not been limited to treating the symptoms, but has focused on reversing this remodeling process that is linked to the progression of heart failure. Follow-up of patients with heart failure secondary to DCM focuses on the assessment of symptoms and response to medical treatment, assessment of functional capacity and imaging study (echocardiogram and MRI) to assess the status of the remodeling process. Similar to adult patients, the role of biomarkers such as N-terminal pro-brain natriuretic peptide is gaining a more significant role in children and appears to be helpful in assessing and monitoring heart failure patients [14].
A healthy heart typically becomes hypertrophic in response to ventricular dilation. By contrast, progressive thinning of the LV wall is a classic characteristic of DCM. Thus, LV posterior wall thickness is measured longitudinally to monitor this finding.
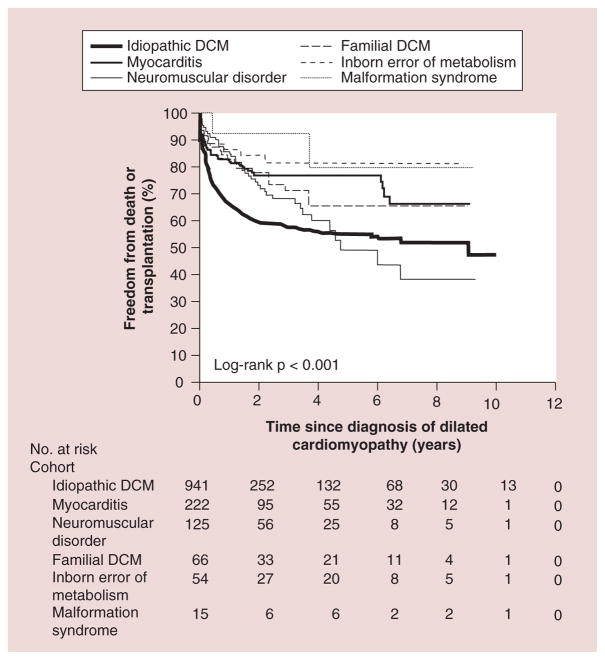
For children, echocardiographic values are usually normalized for age and body surface area by expressing them as Z-scores or the number of standard deviations (SDs) above or below the mean of a normal population. Values greater than two SDs (Z-scores greater than ±2) are generally considered to be rare and may indicate abnormal cardiac structure and function [15]. As DCM progresses, LV walls may thin over time, leading to a gradual reduction in the LV posterior wall thickness Z-score. The North American Pediatric Cardiomyopathy Registry (PCMR) has tracked several thousand children with various types of cardiomyopathies over the past two decades [16]. Data from the PCMR show that children with DCM most often present during the first year of life, a pattern reflecting the genetic causes of clinical cardiac dysfunction. A smaller number presents during adolescence, often showing rapidly progressive disease that leads to cardiac transplantation [5,17]. In children with DCM, considerable differences have been reported in freedom from transplant and survival by cause (Figure 2). Individuals with familial DCM had the best 5-year survival rate at 94%, whereas those with neuromuscular disorders were found to have the worst long-term outcome 5 years after diagnosis, with a 57% survival rate.
Figure 2. Freedom from death or transplantation for patients with pure dilated cardiomyopathy.
DCM: Dilated cardiomyopathy; No.: Number.
Reproduced with permission from [5].
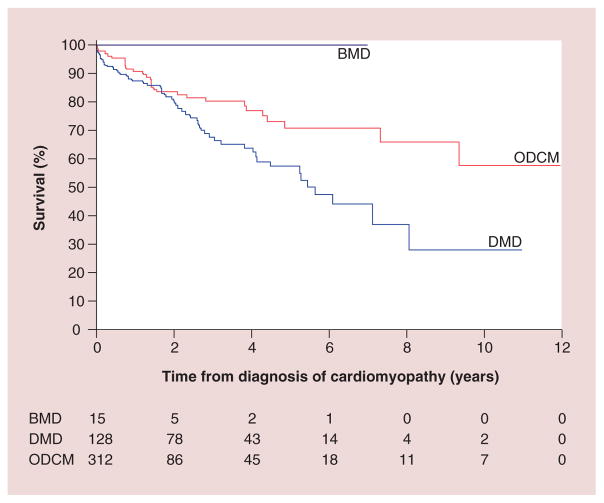
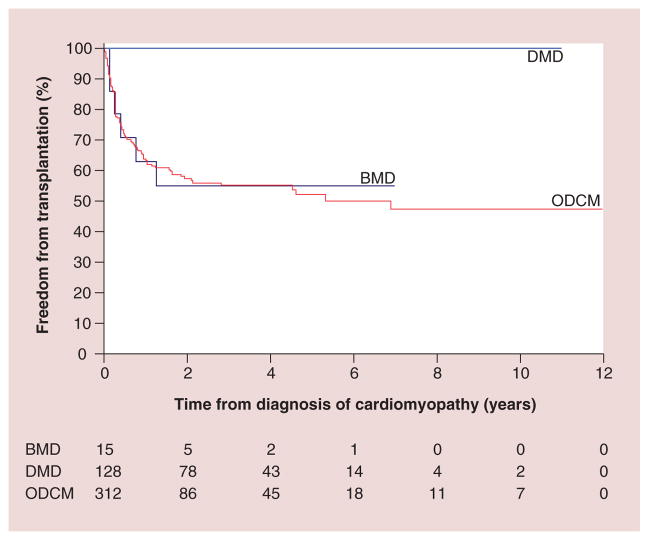
Regarding etiology, DCM is a phenotypic description of pathologic cardiac findings, the causes of which are diverse and often unknown. The PCMR data show that even with concerted diagnostic efforts, a causal diagnosis is not often established. Among 1400 children with DCM, 34% had a specific cause identified, 16% had acute or postmyocarditis, 9% had neuromuscular disorders (mainly Duchenne and Becker muscular dystrophies), 5% had familial cardiomyopathy, 4% had inborn errors of metabolism and 1% had malformation syndromes. The remaining 66% were classified as idiopathic disease. It is posited that most of these idiopathic cases are genetic in etiology, but current studies supporting this proposal are not definitive. Sex and inheritance patterns affected the incidence of some diagnoses; for example, males have a higher incidence of DCM caused by X-linked genetic causes, such as Duchenne muscular dystrophy. Children with Duchenne muscular dystrophy and DCM have a different disease course and a higher mortality [18]. Children with other DCMs had a 5-year survival rate of 71%, whereas the children with Duchenne and Becker muscular dystrophy had a 59% 5-year survival rate (Figure 3). It was also found that 25% of children with Becker muscular dystrophy underwent heart transplantation within 5 months of diagnosis (Figure 4).
Figure 3. Survival from time of diagnosis of cardiomyopathy, by diagnosis.
BMD: Becker muscular dystrophy; DMD: Duchenne muscular dystrophy; ODCM: Other dilated cardiomyopathy.
Reproduced with permission from [18].
Figure 4. Freedom from transplantation after diagnosis of cardiomyopathy, by diagnosis.
BMD: Becker muscular dystrophy; DMD: Duchenne muscular dystrophy; ODCM: Other dilated cardiomyopathy.
Reproduced with permission from [18].
At the time of diagnosis, 71% of children present with clinical signs of heart failure and marked LV dysfunction [19]. Although some children present with acute and severely decompensated disease, most are treated as outpatients with oral medications. One review of the PCMR data found that only 16% of children with idiopathic disease were treated with intravenous inotropic agents within a month of diagnosis [15].
With few exceptions, the medical treatment of heart failure aims to counter innate chronic and maladaptive physiologic responses. Data from the adult literature support the routine use of diuretics, angiotensin-converting-enzyme inhibitors, β-adrenergic blockers and aldosterone antagonists for the long-term treatment of heart failure. Because the pathophysiologic characteristics of heart failure in children are similar to those in adults, therapies used in adults have been used to treat children, although with variable degrees of success. One study found that there was a downregulation of β1- and β2-adrenergic receptors in explanted hearts of children with idiopathic DCM, yet β-adrenergic receptor expression was maintained in adults [20]. It is essential to understand pediatric heart failure adrenergic adaptation in addition to complex inheritance patterns in order to determine the function and importance of these pathways [21].
In DCM, children differ from adults in the causes, pharmacodynamics, expected duration of treatment and therapeutic goals in terms of life expectancy and quality of life. In this regard, routine medical therapy often does not improve cardiac function or life expectancy, and cardiac transplantation then becomes the final option.
The prognosis of pediatric DCM depends on age at presentation and heart failure status. Older age, worse LV fractional shortening and more advanced heart failure are associated with worse outcomes [5,19]. Idiopathic DCM diagnosed after 6 years of age has an incidence of death or transplantation that is three- to four-times that of children receiving a diagnosis before the age of 6 years [17].
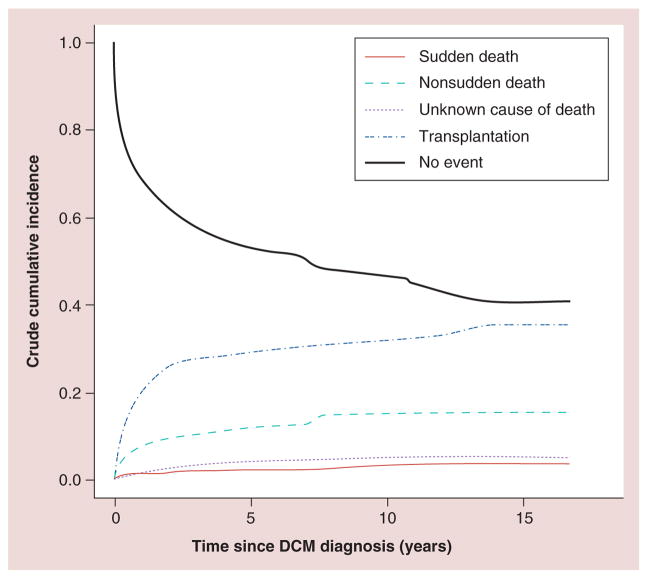
Preventing sudden cardiac death is an important goal in managing advanced heart failure. It is relatively common in adults with heart failure, but a recent study of 1800 children with DCM showed a 5-year sudden cardiac death risk of 2.4% [22]. The same study reported that 1-, 3- and 5-year cumulative incidence rates for non-sudden cardiac death were 8.1, 10.8 and 12.1%, respectively. The 1-, 3- and 5-year incidence for heart transplant was found to be 22, 27 and 29%, respectively (Figure 5). Internal cardiac defibrillator placement is recommended for adults with severely decreased LV function. Echocardiographic predictors, such as LV end-diastolic posterior wall thickness and LV end-diastolic dimension, have been suggested to help stratify this risk in children and to help guide internal cardiac defibrillator placement [22].
Figure 5. Competing risk analysis: outcomes in children with dilated cardiomyopathy.
Competing risk analysis for sudden cardiac death, nonsudden cardiac death, unknown cause of death and cardiac transplantation among 1803 children with dilated cardiomyopathy listed in the Pediatric Cardiomyopathy Registry. The 3-, 5- and 10-year cumulative incidence rates (95% CI) of sudden cardiac death are estimated to be 2.0% (1.4–2.8%), 2.4% (1.7–3.4%) and 2.7% (1.8–3.9%), respectively; of nonsudden cardiac death, 10.8% (9.3–12.4%), 12.1% (10.4–13.9%) and 14.9% (12.6–17.3%), respectively; and of heart transplantation, 27% (25–29%), 29% (27–32%) and 31% (28–34%), respectively. The rate of death of unknown cause (95% CI) was 3.4% (2.6–4.5%), 4.0% (3.0–5.2%) and 5.2 (3.6–7.3%), respectively.
DCM: Dilated cardiomyopathy.
Reproduced with permission from [22].
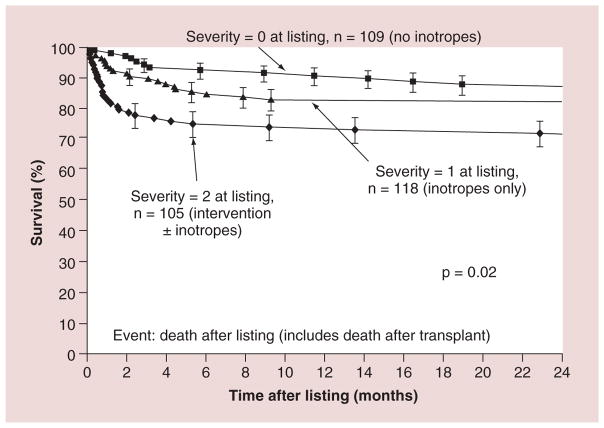
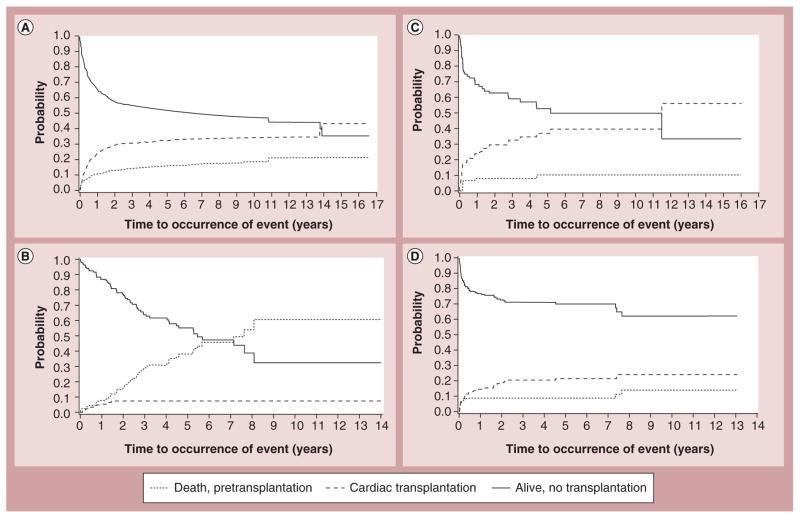
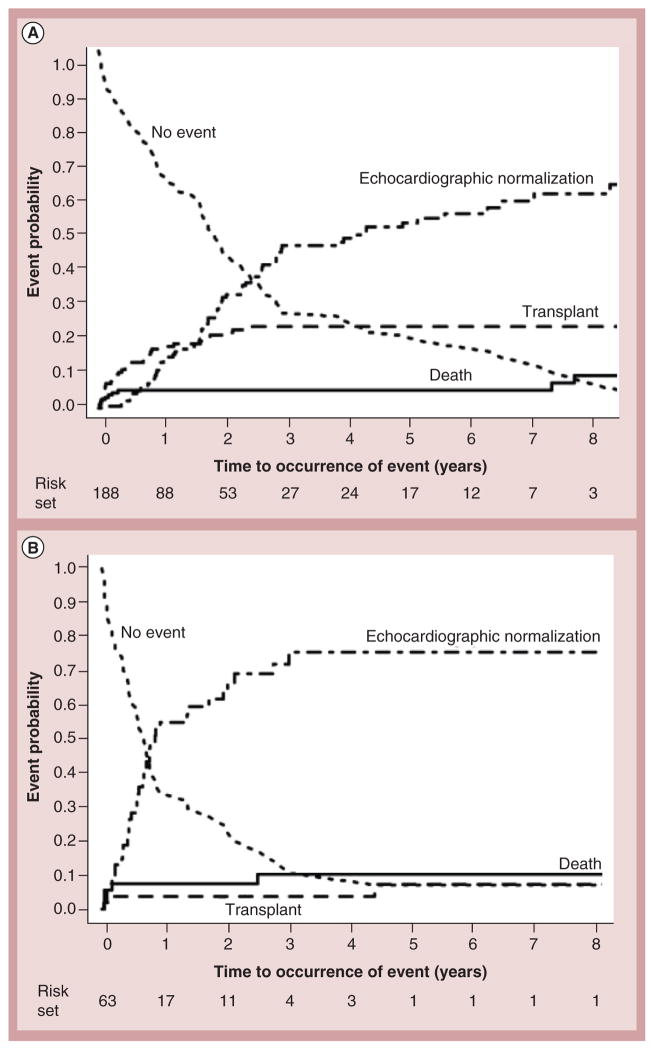
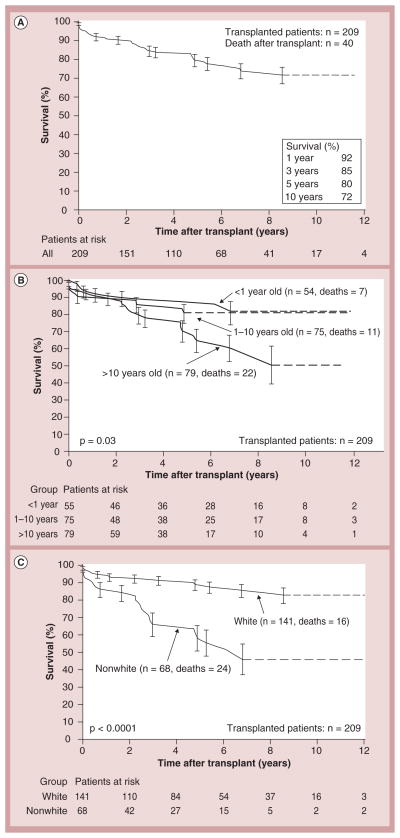
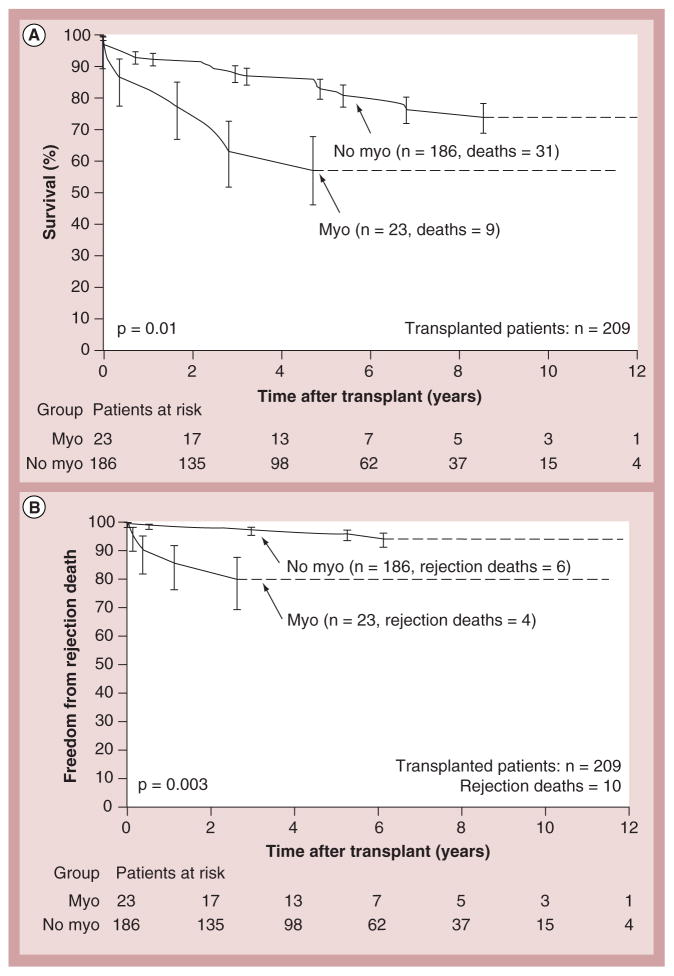
Cardiac transplantation remains the only and definitive treatment for children progressing to end-stage heart failure. Results are generally good, with 1- and 5-year survival rates of 92 and 80%, respectively [23,24]. Larsen et al. found that at 12 months after listing for transplantation, mortality was 26% in children with mechanical ventilation or circulatory support, 16% in children on intravenous inotropic support and 9% in children with neither (Figure 6) [23]. Increased LV end-diastolic dimension is associated with an increased risk of transplantation – but not of death – in children with idiopathic DCM (Figure 7) [17]. In addition, short stature is related to risk of death, but not to transplantation [17]. However, it has been recently reported that children with myocarditis have better outcomes and similar proportions of death and transplantation as children with DCM 3 years after presentation (Figure 8) [25]. The frequency of death while waiting for transplant reached 11% in the PCMR cohort (Figure 9) [26], emphasizing the importance of timing in the decision to list for and receive a transplant. Among children who underwent transplantation, 1-, 3- and 5-year survival rates were 92, 80 and 72%, respectively (Figure 10A). Nonwhite race, older age at transplant, worse pretransplant LV function and myocarditis are associated with a higher risk of death after transplantation (Figure 10B & C). The 1- and 3-year survival rates after transplantation of children with myocarditis were 83 and 65%, respectively, whereas those without myocarditis had 93 and 88%, respectively (Figure 11A & B). Children who were mechanically ventilated at listing for transplant experienced a lower survival rate. The higher risk of death after transplantation for children with myocarditis may be the result of residual infectious or immune effects from the primary disease, which are associated with more frequent and severe rejection episodes [26].
Figure 6. Survival after listing for heart transplantation among children with cardiomyopathy by heart failure severity score.
2: Children on mechanical ventilatory or circulatory support; 1: Children on intravenous inotropic support without mechanical support; 0: Children on neither intravenous inotropic nor mechanical support.
Reproduced with permission from [23].
Figure 7. Competing risk estimates of death, cardiac transplantation and survival for children with dilated cardiomyopathy.
Caused by (A) idiopathic dilated cardiomyopathy (n = 1192), (B) neuromuscular disease (n = 139), (C) familial isolated dilated cardiomyopathy (n = 79) and (D) myocarditis (n = 272).
Reproduced with permission from [17].
Figure 8. Crude cumulative incidences of echocardiographic normalization, cardiac transplantation and death among children with myocarditis (combined biopsy-confirmed myocarditis and probable myocarditis groups), and abnormal function at presentation.
(A) With or (B) without left ventricular end-diastolic dilation at diagnosis. The two groups differed in the incidence of cardiac transplant (p = 0.02) and echocardiographic normalization rates (p < 0.001), but not mortality (p = 0.45). Curves are truncated at 8 years. Reproduced with permission from [25].
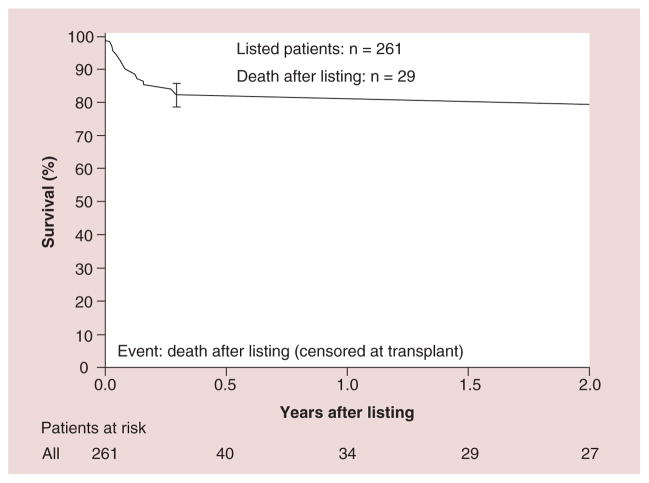
Figure 9. Kaplan–Meier survival curve for the first 2 years after listing (censored at transplantation) for children with dilated cardiomyopathy.
n = 261; 26 deaths by 2 years after listing. Error bar represents 70% confidence limits.
Reproduced with permission from [26].
Figure 10. Kaplan–Meier post-transplantation survival curve. (A).
Children with dilated cardiomyopathy (n = 209); (B) children <1, 1–10 and >10 years of age at transplantation; and (C) nonwhite versus white children. Error bars represent 70% confidence limits. Dashes are included where there is an insufficient sample size to continue the Kaplan–Meier curves.
Reproduced with permission from [26].
Figure 11. Kaplan–Meier post-transplant survival and freedom from rejection curves for children with myocarditis versus no myocarditis.
(A) Survival curves for children with the diagnosis of myocarditis versus no myocarditis (at presentation). (B) Compares freedom from rejection death after transplantation for children with the diagnosis of myocarditis versus no myocarditis. Error bars represent 70% confidence limits. Dashes are included where there is an insufficient sample size to continue the Kaplan–Meier curves.
Myo: Myocarditis.
Reproduced with permission from [26].
HCM in childhood
From a diagnostic perspective, HCM is a heterogeneous group of disorders characterized by “unexplained LV hypertrophy associated with nondilated ventricular chambers in the absence of another cardiac or systemic disease that itself would be capable of producing the magnitude of hypertrophy evident in a given patient” [27]. In children, ventricular hypertrophy is considered clinically present when septal wall thickness is above at least two- to three-times the age- and sex-adjusted SDs of the mean of a normative population.
Generally considered to be asymmetric, HCM affects multiple segments of the heart, including the interventricular septum [28]. Histopathologic analysis shows increased early myocardial apoptosis and the resulting fibrosis. A recent study reported that more histologic disarray in cardiac biopsy specimens was associated with younger age at death, ventricular arrhythmia and ischemia [29]. The HCM phenotype can be obstructive or nonobstructive, which is an important distinction, because each phenotype requires different therapy. HCM is associated with symptomatic heart failure, pain, arrhythmia and sudden cardiac death [30]. Heart failure as a presenting symptom in children with HCM is most likely in the first year of life when mortality rates are highest [31].
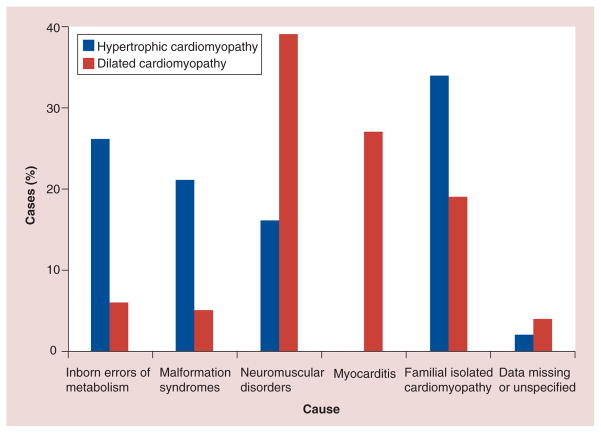
Epidemiologically, the prevalence of HCM is estimated to be approximately 0.2% worldwide [27]. As noted above, given the heterogeneity of HCM, understanding its cause may yield important information, not only about prognosis, but also about which treatments may offer the most promise. Sarcomere mutations, Noonan syndrome, inborn errors of metabolism and other genetic syndromes are the most common known causes of HCM (Figure 12), but as noted above, many cases in the PCMR are classified as idiopathic [31,32].
Figure 12. Primary causes of 61 cases of hypertrophic cardiomyopathy and 77 cases of dilated cardiomyopathy in patients diagnosed from 1996 to 1999.
The primary causes of the remaining 135 cases of hypertrophic cardiomyopathy and 162 cases of dilated cardiomyopathy were unknown at diagnosis.
Reproduced with permission from [2].
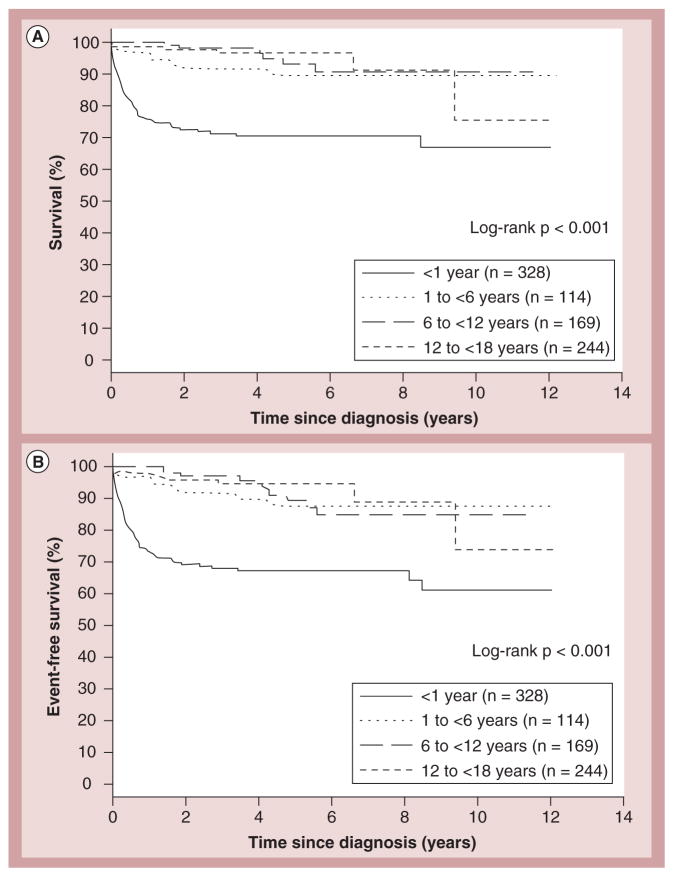
In children with HCM who survive infancy, sudden cardiac death is a well-known complication. The incidence of sudden cardiac death in children and adolescents is estimated at up to 6.2 cases per 100,000 population [33–37]. Overall, 36% of cases of pediatric sudden cardiac death are attributable to HCM compared with 3% in children with DCM [22]. Risk factors for poorer outcomes include a diagnosis before 12 months of age, malformation syndromes and certain inborn errors of metabolism (Figure 13) [29,31,38]. The probability of death or heart transplantation 2 years after diagnosis of HCM increases when there are multiple risk factors present at diagnosis compared with patients with none/one risk factor [38]. Because sudden cardiac death in children is rare, data from adults are used to justify secondary prevention techniques.
Figure 13. Survival rates for the end points of death and death or transplant for children with hypertrophic cardiomyopathy.
Survival rates from diagnosis of cardiomyopathy to (A) death (log-rank p < 0.001) and (B) death or transplantation (log-rank p < 0.001) in the combined prospective and retrospective cohorts (n = 855) by age at diagnosis (<1, 1 to <6, 6 to <12, and 12 to <18 years).
Reproduced with permission from [31].
The common pathophysiology of the array of disorders termed HCM includes thickening of the LV wall without dilation of the LV itself. The resulting impairment in cardiac output is sometimes attributed to the septal wall physically obstructing LV outflow, but other anatomic abnormalities have been implicated. Echocardiography can detect these anatomic changes and is helpful not only in making the diagnosis, but in monitoring disease progression [39]. Furthermore, hemodynamic measurements taken during cardiac catheterization show that the Valsalva maneuver increases the intraventricular gradient.
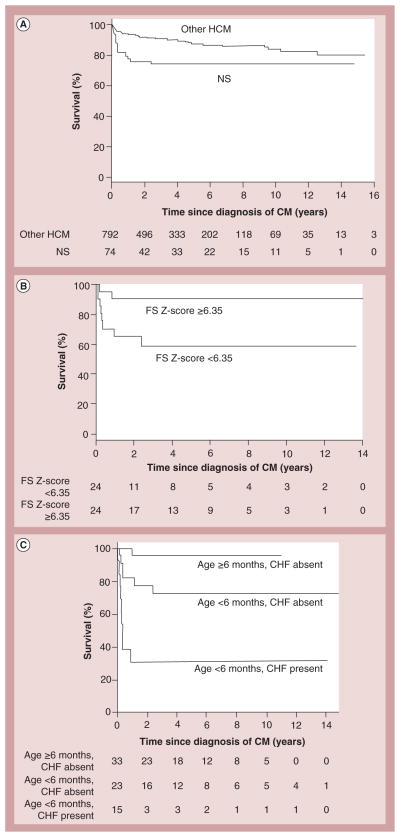
With impaired components of sarcomere function, a murine genetic study demonstrated that preclinical pharmacotherapy may preclude or at least delay the need for invasive procedures [32,40]. However, when associated with inborn errors of metabolism, pharmacotherapy alone is often insufficient, leading to earlier consideration of transplantation. When Noonan syndrome is compared with other causes of HCM, data from the PCMR show the average age at the time of HCM diagnosis is 0.4 years as opposed to 8.0 years, which is associated with poorer outcomes [41]. Patients with Noonan syndrome were also found to have a higher mortality than those with other varieties of HCM (Figure 14A) [41]. Higher survival rates were found in patients with LV fractional shortening Z-scores above 6.35 (Figure 14B). The presence of heart failure and HCM diagnosis prior to 6 months of age were found to be independent predictors of death (Figure 14C). As is the case with inborn errors of metabolism, in Noonan syndrome, more aggressive treatments, including transplantation, may need to be considered earlier in the course of the disease.
Figure 14. Survival rates for children with hypertrophic cardiomyopathy with and without Noonan syndrome, and by risk factors at diagnosis for those with Noonan syndrome.
(A) Estimated survival since diagnosis of HCM in children with (n = 74) and without (n = 792) NS; log-rank p = 0.03. The size of the risk set is shown below the x-axis. (B) Survival by left ventricular FS Z-score in children with NS and HCM. Estimated survival since diagnosis of HCM in 48 children with NS by LV FS Z-score at the time of HCM diagnosis (<6.35 vs ≥6.35 to where 6.35 is the median). Log-rank p = 0.02. The 5-year survival is 59% for children with a Z-score <6.35 and 90% for children with a Z-score ≥6.35. The size of the risk set is shown below the x-axis. (C) Survival by age and CHF in children with NS and HCM. Estimated survival since diagnosis of HCM in 74 children with NS by age and CHF status at the time of HCM diagnosis (log-rank p < 0.001). The size of the risk set is shown below the x-axis. The subgroup of three cases with CHF who were diagnosed at age ≥6 months is not shown (one known to survive 5.5 months postdiagnosis and two were not seen after diagnosis).
CHF: Congestive heart failure; CM: Cardiomyopathy; FS: Fractional shortening of left ventricle; HCM: Hypertrophic cardiomyopathy; NS: Noonan syndrome.
Reproduced with permission from [41].
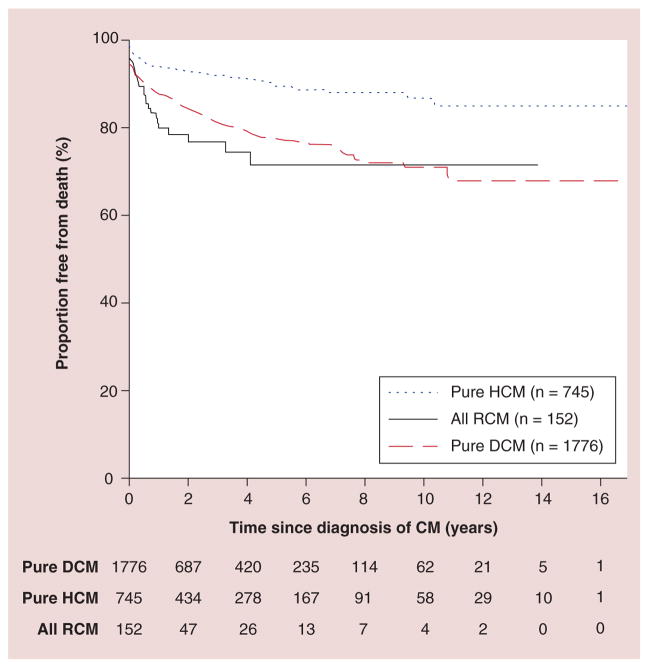
In terms of overall survival, children with HCM have better overall and transplant-free survival than patients with other types of cardiomyopathy (Figure 15) [42]. However, as in other types of cardiomyopathy, therapy may need to be escalated rapidly as heart failure worsens. Cardiac transplantation is beneficial, although it has complications, as previously noted for children with DCM, and donor hearts are scarce.
Figure 15. Probability of freedom from death (censored at transplantation) among 3375 children diagnosed with cardiomyopathy in the Pediatric Cardiomyopathy Registry, stratified by type of cardiomyopathy.
CM: Cardiomyopathy; DCM: Dilated cardiomyopathy; HCM: Hypertrophic cardiomyopathy; RCM: Restrictive cardiomyopathy.
Reproduced with permission from [42].
Treatments for patients with HCM are diverse. In non-obstructive conditions, pharmacotherapy may not be necessary. However, in symptomatic states, such as in obstructive HCM, treatments include pharmacotherapy, sometimes implanting automatic cardioverter–defibrillators, and rarely transplantation [27].
In those patients for whom medications are indicated, β-blockers and calcium channel blockers may be beneficial. Both drugs prolong diastole and permit more LV filling, thus decreasing LV outflow obstruction. β-blockers can prevent sudden cardiac death in asymptomatic patients and have been associated with better survival rates [43,44]. While calcium channel blockers are used in adults, verapamil may be associated with an increased risk of death in children with advanced LV hypertrophy [45]. Disopyramide is sometimes added to β-blockers and, in one retrospective study, reduced both symptoms and subaortic gradients [45]. Septal myectomy may be considered in patients with suitable anatomy and severe symptoms. In one retrospective case series of 25 patients, a trans-aortic septal myectomy was safe, had minimal complications, improved survival and reduced LV outflow tract gradients [46].
Data from adults indicate that automatic cardioverter–defibrillation offers secondary prevention against sudden cardiac death, but there is little data or consensus in pediatric patients [47,48]. Recent unexplained syncope was associated with an increased risk of sudden cardiac death [49], but indications for internal cardioverter–defibrillator insertion as both primary and secondary prophylaxis remain undefined [45].
Guidelines for screening family members of patients diagnosed with HCM have not been established. Nonetheless, while the modality and frequency of screening remain uncertain, it is common to review the medical histories of first-degree relatives and to assess their cardiac structure and function when the patient receives the diagnosis [19,50]. In addition, although the general population is not screened for HCM in the USA, ECG has a negative predictive value greater than 99% [51]. The Bethesda guidelines advocate screening children before participating in competitive athletics [52], although the details of how and when to screen are debated.
The prognosis for children with HCM is largely better than it is for children with other forms of cardiomyopathy. In many children, HCM is asymptomatic and undiagnosed until adulthood. Children with symptomatic HCM have relatively poorer survival, especially if symptoms appear earlier in life.
RCM in childhood
RCM is the least common of the major pediatric cardiomyopathy phenotypes and has the poorest prognosis. In the PCMR database, RCM only accounts for 4.5% of the cases. However, this relatively small proportion of pediatric cardiomyopathy cases has a disproportionately high morbidity and mortality. In a recent study of one of the largest cohorts of children with RCM, Webber et al. found that 25% of the children with RCM in the PCMR had a family history of cardiomyopathy [42]. Pathophysiologically, patients with RCM have cardiac muscle disease, leading to diastolic dysfunction of either or both ventricles, marked by decreased LV compliance and relaxation, with marked left atrial enlargement [53]. Impairment occurs when most of the ventricle fills rapidly in early diastole, permitting little to no increase in ventricular volume after the end of the early filling period. This impairment in LV diastolic function increases the LV end-diastolic pressure (LVEDP) and decreases cardiac output. The higher LVEDP results in pulmonary artery hypertension and, subsequently, right-sided heart failure.
Regarding etiology, several systemic and myocardial diseases are associated with RCM [54–56]. While idiopathic RCM is the most common form, it may also occur in patients with amyloidosis, inborn errors of metabolism, sarcoidosis and scleroderma [57]. A linkage analysis for selected sarcomere contractile protein genes identified troponin I (TNNI3) as the gene most likely to be responsible for the development of RCM [58], suggesting that RCM is part of the clinical expression of hereditary sarcomeric contractile protein disease. Familial evaluation should be considered when RCM has been diagnosed in a family member.
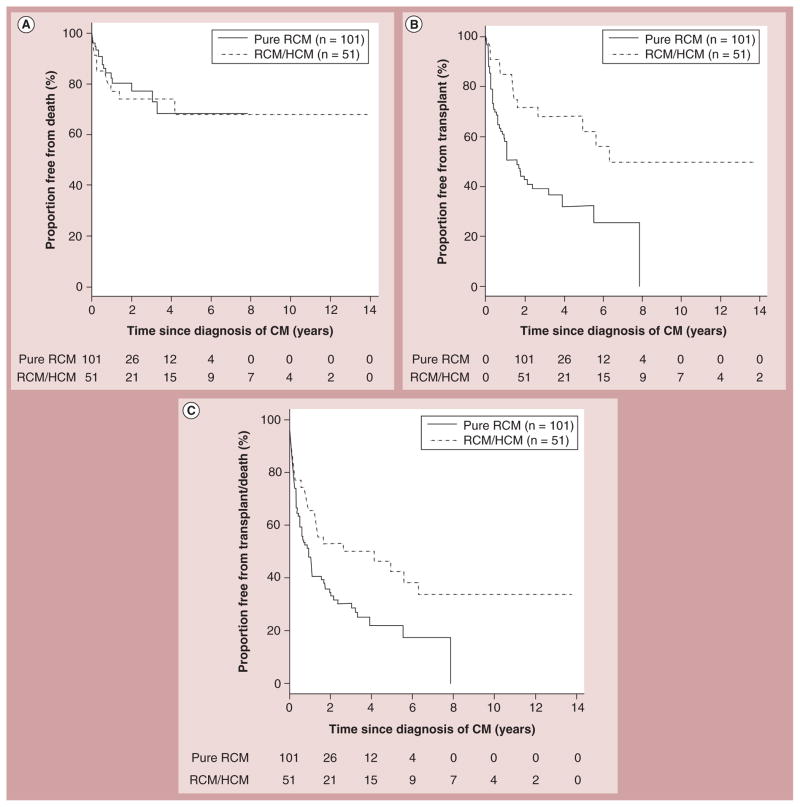
Angelini et al. suggested that RCM and HCM might represent two different phenotypes of the same basic sarcomeric disease [59]. Myocyte disarray is indicative of HCM and has been seen in patients with RCM [60]. In addition to the pure RCM phenotype, a mixed phenotype has been recently reported in the PCMR database [42]. In this report, Webber et al. found that survival did not differ between patients with pure RCM and those with mixed RCM and HCM, although transplant-free survival was lower in the pure RCM group (Figure 16) [42].
Figure 16. Survival curves for children with pure restrictive cardiomyopathy compared with those with a mixed restrictive and hypertrophic cardiomyopathy phenotype.
(A) Probability of freedom from death (censored at transplantation), (B) transplantation and (C) death or transplantation among 152 children with RCM stratified by phenotype (pure RCM vs mixed/overlapping phenotype RCM/HCM).
CM: Cardiomyopathy; HCM: Hypertrophic cardiomyopathy; RCM: Restrictive cardiomyopathy.
Reproduced with permission from [42].
Desmin-related myopathies are rare cardiac and skeletal muscle disorders [61–64]. Their diagnosis requires identification of desmin deposits in biopsies of cardiac or skeletal muscle through ultrastructural and immunohistochemical analyses. Mutations in the genes encoding desmin and αB-crystallin cause desmin accumulation in the cytoplasm. One study links RCM to mutations of the desmin gene [64], while other published cases describe RCM associated with isolated cardiac accumulation of desmin [65]. While rare, desmin accumulation may explain the occurrence of RCM and atrioventricular block in some subpopulations [42].
Secondary RCM may occur due to systemic disease and can be broadly categorized as infiltrative, iatrogenic and oncologic. Infiltrative RCM is seen in amyloidosis, hemochromatosis and storage disorders. Deposits of amyloid in the heart can occur in some diseases caused by mutations in the genes for transthyretin and apolipoprotein [66,67]. In addition to impaired diastolic function in RCM, ventricular wall thickness is often increased, with normal or slightly impaired systolic function [42]. An ECG may show low voltage in the standard leads, and echocardiography reveals amyloid infiltration of the myocardium and valves. Depolarization abnormalities, nonspecific ST segment and T-wave abnormalities, and pathologic Q-waves are sometimes seen on the ECG, and ventricular hypertrophy can sometimes be diagnosed. A cardiac biopsy shows typical features of amyloid deposits in the myocardium. In hemochromatosis, iron deposition occurs in the myocardium and in multiple other organs. Hemochromatosis is an autosomal recessive disorder and rarely develops as an isolated cardiac manifestation of RCM [66]. Anderson-Fabry’s disease is an X-linked lysosomal storage disorder caused by mutations in the gene for α-galactosidase A, which, in some patients, may also be associated with RCM.
RCM can also occur in hypereosinophilic syndromes and can be induced by exposure to various drugs and parasitic infections. It also occurs in patients with systemic diseases [66,67], including scleroderma and sarcoidosis. In addition, patients who have had chest radiotherapy may sustain myocardial and endocardial injury, leading to fibrosis, which many years later, cause RCM [68].
In the initial stages of the disease, some patients are asymptomatic at the time of presentation. This is a particularly important challenge considering children with RCM are at a higher risk of sudden death [42]. Symptoms such as failure to thrive and fatigue may result in other patients seeking clinical attention. Other patients may present with palpitations, syncope or arrhythmias. As the disease progresses, symptoms of decreased cardiac output such as fatigue, dyspnea and exercise intolerance appear. Thromboembolic events can occur in patients with dilated atria when thrombi form in the atrial appendage [42,57,69].
Physical examination often reveals increased jugular venous distension, Kussmaul’s sign, pulsus paradoxus, peripheral edema, liver enlargement, ascites and pleural effusion. An audible third heart sound is often heard in RCM because of the abrupt cessation of ventricular filling.
Differentiating RCM from constrictive cardiomyopathy is important because, although both lead to diastolic dysfunction and abnormal ventricular filling, only patients with constrictive cardiomyopathy may recover completely after removal of the pericardium.
Chest radiographs reveal enlarged atria and variable degrees of pulmonary congestion without cardiomegaly. Echocardiography (Figure 17) shows a heart enlarged bilaterally, impaired systolic function and mitral inflow Doppler velocities indicative of severe diastolic dysfunction. The ratio of early diastolic filling to atrial filling is increased and the E deceleration time and isovolumic relaxation time are decreased. Reversed hepatic venous flow during inspiration indicates RCM. Recently, data from the PCMR revealed that decreased LV fractional shortening and increased LV end-diastolic posterior wall thickness are risk factors for poorer prognosis in those diagnosed with RCM. In addition, the extent of posterior wall hypertrophy in children with a mixed HCM and RCM phenotype was found to be a risk factor for both death and transplantation [42].
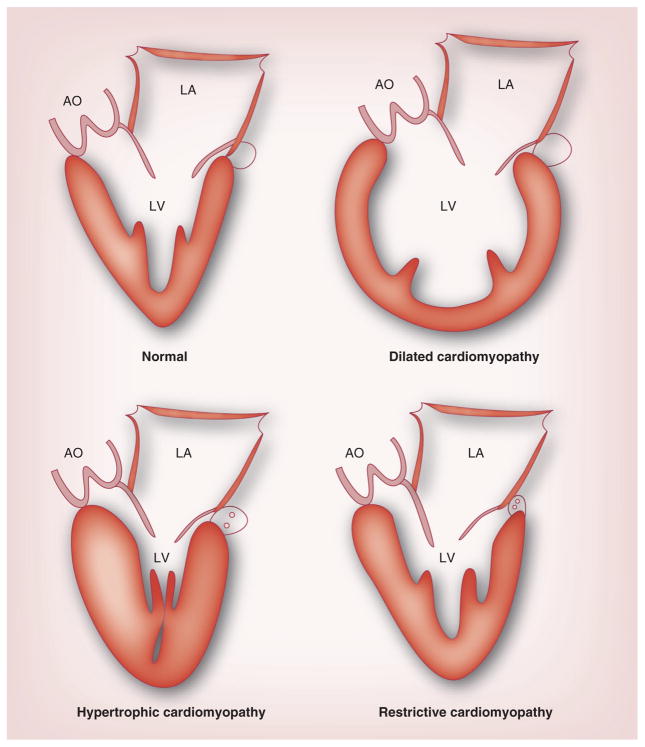
Figure 17. Characteristics of the normal heart and the three main types of cardiomyopathy.
AO: Aorta; LA: Left atrium; LV: Left ventricle.
Reproduced with permission from [225].
Computed tomography and cardiac MR can provide more information about pericardial anatomy and may help differentiate the various types of cardiomyopathy from other pathologies. A pericardium thicker than 4 mm suggests constrictive pericarditis and is detectable with these imaging modalities. In addition to providing additional thoracic information, differentiating RCM and constrictive pericarditis is one of the roles of these modern technologies.
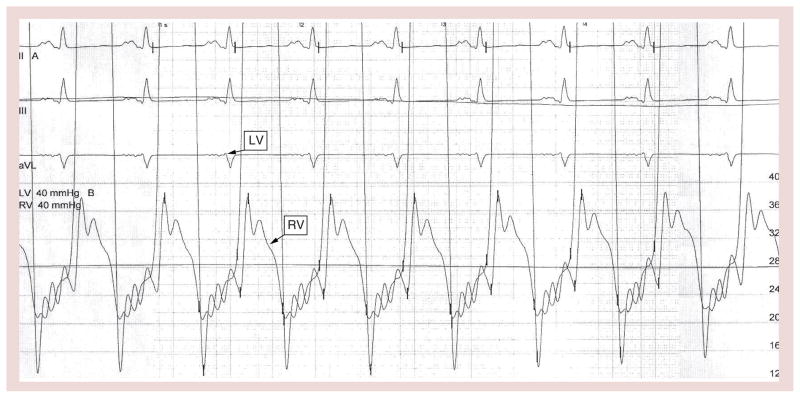
End-diastolic pressure is increased during cardiac catheterization and shows a characteristic square root sign (Figure 18). This ‘dip and plateau’ sign reflects the rapid filling during early diastole that is followed by a decrease in ventricular filling from the end of the first third of diastole onward [70,71]. Right ventricular end-diastolic pressure and LVEDP are elevated in both ventricles; however, the LVEDP may be 5–7 mm/Hg higher than the right ventricular end-diastolic pressure. The elevated diastolic pressure in the ventricles is preload and heart rate dependent. Therefore, if the end-diastolic pressure were the same in both ventricles, a fluid challenge would increase the LVEDP above the right ventricular end-diastolic pressure in patients with RCM.
Figure 18. Equalization of right ventricular and left ventricular end-diastolic pressures with a ‘dip and plateau’ pattern.
ECG was recorded at a chart speed of 50 mm/s.
LV: Left ventricular; RV: Right ventricular.
Plasma concentrations of brain natriuretic peptide are increased in many conditions where ventricular dysfunction stretches the ventricular walls [72]. Some studies of patients with RCM have reported increased brain natriuretic peptide concentrations and, in others, near-normal concentrations [73–75].
Despite optimal medical treatment, RCM carries a poor prognosis. Between 66 and 100% of patients die or undergo cardiac transplantation within a few years of diagnosis [42,76,77]. It is apparent that children with RCM undergo transplantation at a larger proportion than children with other types of cardiomyopathy. However, the decision to list RCM pediatric patients for heart transplantation is mostly based on local pediatric heart center listing philosophy. There are currently no widely accepted evidence-based criteria on decisions to list children with RCM for heart transplantation. Findings from the PCMR suggest that the current era’s aggressive approach to listing and early transplantation is associated with improved survival of these children when compared with historical data [42]. In one study of 18 RCM patients, five died suddenly without signs of heart failure [77]. However, these individuals did have angina and ECG evidence of ischemia. Autopsies of four hearts of children who died suddenly revealed acute myocardial infarcts, subendocardial ischemic necrosis and chronic ischemic scarring, despite normal-appearing coronary arteries [77]. Patients who present with ischemia, chest pain and syncope are at an increased risk of sudden death, and Holter monitor evaluation every 6 months would be prudent. Placement of implantable cardioverter–defibrillators may be considered in patients with ischemia and ventricular arrhythmias while awaiting transplantation [77]. When comparing pure RCM with a combined phenotype of RCM and HCM, the time from diagnosis to death or transplantations is less for those with a pure RCM phenotype [42]. Two studies of adults with a total of 113 patients have reported that 32–44% with RCM died from cardiovascular-related causes within 5 years after diagnosis [78,79]. Patients with large atria and tachyarrhythmias had evidence of embolic complications. Therefore, these findings suggest that prophylactic anticoagulant therapy should be considered in all RCM patients with enlarged atria, even before supraventricular tachycardia develops, and in one recent study, 43% of children with RCM had received some form of anticoagulation or antithrombotic therapy [42].
Treatment of RCM should target the cause [67]. For instance, in patients with amyloidosis, chemotherapy may be the proper treatment. In patients with risk factors for poor outcomes, such as pulmonary congestion, heart failure, low fractional shortening, diagnosis before the age of 2 years, thromboembolic events and increased pulmonary vascular resistance, and more aggressive treatment strategies including transplantation, have been advocated [42,54,57,80]. In most cases, however, no clear cause of RCM can be determined, thus requiring individual-specific treatment. Antiarrhythmics and anticoagulants are sometimes prescribed, but little evidence supports their use. Genotype testing has only just recently become available, in addition to the pediatric cardiomyopathy blood and tissue repository. A search for candidate gene mutations could potentially become a focus of future studies. An understanding of the genetic manifestations of RCM could help characterize cellular and molecular events leading to myocardial restriction in order to better identify targets for therapeutic interventions.
LV noncompaction in childhood
LV noncompaction, formerly known as spongy cardiomyopathy, is a recently classified congenital cardiomyopathy that primarily involves the apical portion of the LV [53]. Once thought to be rare, LV noncompaction probably comprises at least 9% of all childhood cardiomyopathies [81–83]. Noncompaction is characterized by deep intertrabecular recesses and the separation and thickening of the myocardium into two distinct compacted and noncompacted layers [84]. Although the exact mechanism is not understood, LV noncompaction may result from disrupted embryogenesis [85–87]. There are seven different phenotypes of LV noncompaction, each with varying clinical outcomes. These phenotypes include isolated LV noncompaction, isolated LV noncompaction with arrhythmias, a dilated form of LV noncompaction, which mimics DCM, a hypertrophic form of LV noncompaction, which mimics HCM, a mixed hypertrophic and dilated form of LV noncompaction, a restrictive form of LV noncompaction and LV noncompaction with congenital heart disease [88]. The mixed hypertrophic and dilated form of LV noncompaction appears to have the worst prognosis.
The diagnosis of LV noncompaction is commonly made by echocardiogram [89]. Although both ventricles can be affected by myocardial noncompaction, dysfunction is most often isolated to the LV. In one retrospective study of 29 children with LV noncompaction, 22 (76%) experienced the disease only in the LV [90]. Noncompaction has often been retrospectively identified in patients with a diagnosis of DCM [91]. This finding suggests that the incidence and prevalence of LV noncompaction may be underestimated among patients with heart failure.
The etiology of LV noncompaction is still an area of active study. LV noncompaction is thought to result from the arrest of normal endomyocardial embryogenesis during weeks 5 and 8 [92,93]. Although the cause of this arrest is unknown, LV noncompaction has been associated with other congenital heart defects. Zuckerman et al. found 13 out of 58 (22%) patients with LV noncompaction had another form of congenital heart defect, most commonly ventricular septal defects (seven out of 13), followed by secundum atrial septal defects (three out of 13) [94]. Other abnormalities associated with LV noncompaction include Ebstein’s anomaly, hypoplastic right ventricle and hypoplastic left heart syndrome [81].
Metabolic, genetic, chromosomal, neuromuscular and mitochondrial diseases are also associated with LV noncompaction [94,95]. Examples include Barth syndrome, centronuclear myopathy, chromosomal deletions and Roifman syndrome [83]. Several studies have also reported LV noncompaction in patients with dysmorphic features without definitive diagnoses.
Several genes and related proteins have been associated with LV noncompaction, including the G4.5 gene on chromosome Xq28 encoding tafazzins, the ZASP gene on chromosome 10q22 and the α-dystrobrevin gene on chromosomes 18q12 and 11p15 [95–98]. The inheritance patterns include X-linked and autosomal dominant.
An inheritance pattern of 18–50% was found among first- or second-degree relatives [99]. However, this rate was determined on a group including asymptomatic patients screened after the diagnosis was made in the index case [99]. In one study, 16 out of 22 LV noncompaction patients (73%) reported a family history of cardiomyopathy or sudden death [85].
Manifestations of LV noncompaction range from asymptomatic cardiac dysfunction, to arrhythmia, to thromboembolism, to severe decompensated heart failure [100]. In turn, patterns of arrhythmia can range from atrial fibrillation to sustained ventricular tachycardia. Abnormal ECG findings can be attributed to branch blocks and arrhythmias, although arrhythmias rarely develop in children and are more common in adults [90,93,101,102]. Early reports of LV noncompaction described symptomatic children presenting with heart failure accompanied by ventricular trabeculations, deep intertrabecular recesses and facial dysmorphism [84,92,103].
The prognosis of children with LV noncompaction has been found to be evidenced by chronic, progressive LV dysfunction. More recent studies have found that patients asymptomatic at the time of diagnosis initially experience a prolonged clinical course marked by gradual dysfunction of the LV. One study of 36 children found the course of disease to be marked by periods of deterioration and recovery with survival into adulthood [81]. Ichida et al. reported that patients with heart failure, arrhythmia or embolic events at initial presentation had an accelerated disease course [104]. In addition, the majority of both symptomatic and asymptomatic patients experienced ventricular dysfunction during more than 10 years of follow-up.
Symptoms associated with impaired LV systolic function are the most frequent clinical manifestations in children. Although the origin of systolic dysfunction is not fully understood, subendocardial hypoperfusion and microcirculatory dysfunction are believed to contribute to impaired ventricular function [92,102]. Subendocardial perfusion has been identified in patients with LV noncompaction undergoing cardiac MR, PET and scintigraphy with 201Tl [102,105]. An initial presentation that includes RCM has also been reported in children [104]. Prominent trabeculae can cause abnormal relaxation and restrictive filling of the ventricle, leading to diastolic dysfunction. Asynchrony between noncompacted and compacted segments of the myocardium leads to global dysfunction of the LV. Successful resynchronization therapy was been reported in one 3-year-old with LV noncompaction [106].
Clinical manifestations at the time of diagnosis can include dyspnea, New York Heart Association class III or IV heart failure, chest pain and chronic atrial fibrillation. Heart failure is one of the more common symptomatic manifestations, secondary to sustained or paroxysmal arrhythmias and thromboembolic episodes. Younger patients diagnosed incidentally or by family referral have less severe LV diastolic dysfunction and a lower prevalence of ECG abnormalities [107]. Consequently, these patients were more likely to be stable during the first few years after diagnosis. More than likely, this is a form of lead-time bias, as these differences may be attributed to identifying these younger individuals at an earlier stage of disease [107]; for example, Murphy et al. found that familial enlargement of the LV and asymptomatic LV noncompaction suggested prolonged silent gestation before the clinical onset of the disease [91].
Treatment of LV noncompaction should be based on echocardiographic findings. Patients presenting with systolic dysfunction can be treated with angiotensin-converting enzyme inhibitors, such as enalapril, or β-blockers, such as carvedilol. β-blocker therapy with propranolol or atenolol is appropriate treatment for patients with diastolic dysfunction [88].
Endocrinology & pediatric cardiomyopathy
Abnormal thyroid function can be associated with cardiomyopathy. Although cardiomyopathy is not rare in adults with thyroid dysfunction, it is fortunately much less common in children and adolescents in the absence of underlying heart disease. The PCMR specifically excluded children with cardiomyopathy who also had endocrine issues known to cause heart muscle disease, such as thyroid dysfunction [19].
Cardiomyopathy in hyperthyroidism is thought to be secondary to either the direct toxic effects of excess thyroid hormone, hyperdynamic stress caused by the thyroid hormone or both [108]. Other cardiovascular manifestations are more common than cardiomyopathy, including arrhythmias, such as sinus tachycardia, atrial fibrillation and atrial flutter, decreased systemic vascular resistance, increased resting heart rate, increased LV contractility, and increased blood volume leading to high cardiac output [109].
Thyrotoxicosis may cause reversible compensated functional cardiomyopathy [110] and the severity of thyrotoxicosis may be a determining factor in its development. In addition, cases of cardiomyopathy and cardiac failure in infants with neonatal thyrotoxicosis have been reported [111]. Cardiomyopathy can also occur in patients with severe hypothyroidism, especially if it is untreated for long periods, including central hypothyroidism in children with hypopituitarism, which is usually associated with growth hormone (GH) deficiency [112]. This cardiomyopathy may markedly improve with thyroxine treatment [113]. Both hypothyroidism and cardiomyopathy may occur with other rare multiorgan syndromes, such as Alström syndrome and Kearns–Sayre syndrome. Takotsubo cardiomyopathy, stress-induced cardiomyopathy, has been reported with both severe hypothyroidism and thyroid storm in adults [114].
GH therapy has had mixed results in adults with DCM. Multiple studies have reported increased LV wall thickness and mass with treatment, but not all have found improved cardiac function. However, few studies have tested GH treatment in children with cardiomyopathy. McElhinney et al. found improvement in LV functional indices after 6 months of GH treatment in children with DCM [115]. Treatment increased LV wall thickness in anthracycline-treated survivors of childhood cancer, but the effect was lost when therapy was discontinued. In addition, the therapy did not affect progression to LV dysfunction [116].
Other investigations of GH treatment have shown no effect. For example, treatment over 14 weeks with synthetic GH increased serum IGF1 concentrations, but did not affect the degree of apoptosis or improve cardiac structure and function in 38 children with DCM [117]. Another study found that GH therapy worsened HCM [118]. Recombinant IGF1 has also been used to treat cardiomyopathy, with limited benefit. Thus, the benefit of GH therapy on cardiomyopathy has yet to be determined.
Diabetes mellitus (DM) is an increasingly common disease with extensive micro- and macro-vascular complications. Diabetic cardiomyopathy refers to myocardial dysfunction occurring in the absence of coronary artery disease or other primary cardiac abnormalities, but nevertheless increases the risk of heart failure in a patient with diabetes [119]. The condition is especially common in patients with Type 2 DM, a diagnosis that should prompt early cardiac assessment, but it can also occur in patients with Type 1 DM.
Hyperglycemia is thought to be important in the development of cardiomyopathy by increasing glucose oxidation that damages cells [120,121]. Von Bibra et al. and Grandi et al. found that increasing insulin therapy to decrease glycated hemoglobin concentrations improved the rate of peak LV diameter lengthening [122,123]. Another study of Type 1 DM reported that the incidence of early precursors to diabetic cardiomyopathy, specifically changes in myocardial relaxation, may be increased in teenaged girls. Although this study found sex-related differences, BMI and glycated hemoglobin were not found to be predictors of cardiac dysfunction in patients with diabetes [124]. Other studies have shown no sex bias [125].
In addition to echocardiography, other modalities can help in the early diagnosis of diabetic cardiomyopathy by showing early diastolic dysfunction, even when traditional imaging indicates normal cardiac function; for example, N-terminal pro-brain natriuretic peptide concentrations are significantly higher in patients with diabetic cardiomyopathy than in healthy controls [125]. Currently, there are no specific treatment guidelines, given the scarcity of therapeutic studies on the early stages of diabetic cardiomyopathy, but it is evident that early treatment may prevent or delay more severe cardiac dysfunction [119]. Early treatment should focus on reducing the effect of causative factors, including hyperglycemia and subsequent metabolic disturbances [119]. New therapies under investigation focus on the enhanced fibrosis and cardiomyocyte metabolism abnormalities that lead to marked cardiac dysfunction [119].
Obesity-related cardiomyopathy is defined as myocardial disease caused by obesity in the absence of other comorbidities, such as DM, hypertension and coronary artery disease [126]. Obesity is an independent risk factor for heart failure in adults. Individuals with a BMI greater than 30 kg/m2 have a risk of heart failure that is twice that of lean individuals. Both the duration and severity of obesity increase the probability of heart failure. A 1-kg/m2 change in BMI increases the risk of heart failure by 5% in men and 7% in women, and for every year of severe obesity, the odds of heart failure increase by 1.5 [127].
In children and adolescents, obesity has been related to changes in heart structure, including increased LV mass and LV hypertrophy as the heart adapts to high total blood volume and low systemic arterial resistance. LV diastolic dysfunction has been reported in children 10–18 years old who had a BMI greater than 25 kg/m2 [128]. Most recently, early LV regional diastolic and systolic dysfunction was documented in severely obese adolescents. Such changes may be related to dysfunction in subendocardial myofibers [129]. Fatty acid storage, delivery and oxidation accelerate with obesity and insulin resistance, resulting in myocardial dysfunction [130]. Echocardiography can detect the early changes of obesity-related cardiomyopathy, mainly LV diastolic dysfunction. Other more sophisticated echocardiographic studies, computed tomography and cardiac MR can provide additional and valuable information, but are not currently used in asymptomatic patients.
Treatment is directed to weight control, starting at childhood. Strategies to improve nutrition and exercise are the primary interventions to control weight. Bariatric surgery markedly reduces weight and comorbidities in adolescents, and now has specific indications, which must first be met prior to being deemed a surgical candidate. Pharmacotherapeutic options for obesity are limited in adolescents [131].
Other endocrinopathies, such as Addison’s disease, type II autoimmune polyendocrine syndrome and pheochromocytoma, may infrequently lead to DCM. Thus, children with these conditions need to be made aware of their increased risk of cardiomyopathy with age.
Nephrology & pediatric cardiomyopathy
Uremic cardiomyopathy is a common complication in patients with chronic kidney disease (CKD). Such patients are more likely to have cardiovascular disease (CVD). In fact, CVD is a risk factor of progressive CKD as a result of a complex bidirectional relationship between the heart and the kidneys, a relationship exemplified by the cardiorenal and renocardiac syndromes [132]. Furthermore, CVD is the leading cause of morbidity and death in patients with CKD and, conversely, CKD affects survival in patients with CVD [133]. The mortality rate of patients with CKD receiving dialysis is approximately 20-times as high as that of the general population, largely as a result of the CVD. In young adults with a childhood diagnosis of CKD, the mortality rate from CVD is more than 100-times as great as that in similarly aged people in the general population [134], and children on maintenance dialysis have a cardiac death rate that is 1000-times as high as that of the general population [135]. LV hypertrophy, which is present in up to 85% of children with advanced CKD and on dialysis [136], can be detected in children during the earliest stages of CKD and tends to progress with age [137]. Moreover, LV hypertrophy is a major cause of cardiovascular morbidity and mortality in young adults treated with dialysis since childhood [138].
Both LV hypertrophy and elevated LV mass index have important prognostic value in patients with advanced CKD because they often precede various adverse outcomes, such as heart failure, cardiac ischemia, arrhythmias and cardiac death [139]. In older adults with advanced CKD, arrhythmogenic heart disease related to coronary atherosclerosis is the major underlying cause of sudden cardiac death. However, in children and adolescents with advanced CKD, lethal arrhythmias are the leading cause of cardiac death [140]. These arrhythmias may be facilitated by structural myocardial changes related to LV hypertrophy. Such structural changes include fibrosis and cellular hypertrophy, in a manner analogous to the pathogenic mechanisms of HCM in children and adolescents with intact renal function [141]. In addition, several myocardial changes underlie LV hypertrophy in experimental models of renal insufficiency and occur in patients with CKD. These changes include cardiomyocyte hypertrophy, loss of cardiomyocytes (cardiomyocyte ‘drop out’), as evidenced by a reduced number of myocytes per unit of myocardial volume [142], thickening of intramyocardial arterioles and, eventually, a marked capillary deficit, causing a mismatch between cardiomyocyte hypertrophy and capillary density [143].
Although LV hypertrophy in CKD was thought to be a response to increased LV preload or afterload (LV wall stress), other load-independent factors have proved to be important in the initiation and progression of LV hypertrophy in CKD [136,144]. Some of the causes identified as nonclassical risk factors for cardiovascular mortality and LV hypertrophy in CKD patients include disturbances of mineral metabolism, including hyperphosphatemia, secondary hyperparathyroidism and vitamin D deficiency [145]. More recently, FGF-23, a member of the FGF family of signaling proteins, has also been identified as another mechanistic link in the development of LV hypertrophy in CKD [146,147].
Vitamin D
Vitamin D obtained either through dietary intake or by skin exposure to UVB radiation is eventually converted by D-25-hydroxylase in the liver to 25-hydroxyvitamin D (25OHD; calcidiol) and then to the active form, 1,25-dihydroxyvitamin D (1,25[OH]2D3; calcitriol), by the kidneys’ proximal tubule 1α-hydroxylase [148]. Various cell types, such as vascular smooth muscle cells, osteoblasts and endothelial cells, also express 1α-hydroxylase to form 1,25(OH)2D3 locally, which eventually binds to its cognate intracellular receptor, the vitamin D receptor (VDR), which is responsible for the ultimate effects of activated vitamin D on specific target genes [149]. The VDR is expressed in several organs, including cardiomyocytes [150]. Thus, the heart is believed to be a vitamin D target organ. Low levels of 25OHD and high prevalence rates of vitamin D insufficiency or deficiency are often found in children at all stages of CKD [151].
Vitamin D insufficiency and deficiency, as defined by variable cut-off serum concentrations of 25OHD, are associated with a higher relative risk for cardiovascular mortality in CKD [152]. They are also associated with cardiomyopathy and cardiac death in children with intact renal function [153], as well as in the general population [154]. By contrast, high 25OHD concentrations are associated with improved survival [155] and several studies suggest that higher vitamin D intake or supplements reduce the risk of CVD [156,157].
In renal insufficiency, the decline in renal 1α-hydroxylase activity and the increased catabolic rate of 1,25(OH)2D3 result in reduced circulating concentrations of 1,25(OH)2D3 [158] that have also been associated with cardiovascular mortality [152]. The cardiovascular mortality of patients on dialysis and those with progressive CKD predialysis can be reduced by treatment with a VDR agonist, such as calcitriol or its less calcemic analog, paricalcitol (19-nor-1,25[OH]2D2) [159,160]. Therapy with calcitriol is also associated with attenuation of LV hypertrophy in patients on dialysis [161], and the analog paricalcitol suppresses cardiac hypertrophy in animals with chronic renal insufficiency [162]. However, a recent randomized trial did not confirm this finding in adults with CKD and LV hypertrophy [163].
Although the best known function of 1,25(OH)2D3 is to maintain calcium and phosphorus homeostasis, and to promote bone mineralization, recent evidence indicates that the vitamin D endocrine system is also critical in regulating blood pressure, volume homeostasis and cardiac function, largely by modulating the renin–angiotensin system (RAS), a cascade that profoundly affects the cardiovascular system [164–167]. Activated 1,25(OH)2D3 negatively regulates RAS. In null mutant mice lacking the VDR [164] or 1α-hydroxylase [168], cardiac hypertrophy develops and intracardiac renin concentrations increase. The ratio of heart:weight to body:weight also increases, with marked cardiomyocyte hypertrophy. Captopril improved cardiac hypertrophy in VDR-null mice and lowered markedly elevated cardiac renin concentrations to nearly normal in the 1α-hydroxylase-mutant mice. The cardiac hypertrophic phenotype was rescued by replacing 1,25(OH)2D3 [168].
Paricalcitol and 1,25(OH)2D3 can suppress renin gene transcription by binding to its cyclic AMP response element [169,170], further explaining the ability of VDR activators to attenuate RAS-mediated prohypertrophic processes [171]. In addition to renin, all other local RAS components have been found in the kidney and in the heart [172]. Renin inhibition by the vitamin D analog, paricalcitol, has provided a novel mechanism to suppress the intrarenal RAS, attenuate renal dysfunction and improve histological damage in experimental uremia [173]. The local cardiac RAS is critical to the development of cardiac hypertrophy and, because all the components required for angiotensin II production are present in the heart [174], blocking RAS with an angiotensin-converting-enzyme inhibitor expectedly ameliorates the remodeling of the heart and arrests the development of LV hypertrophy and heart failure in experimental models of uremia [143]. Paricalcitol also ameliorates LV hypertrophy in uremic rats by upregulating the VDR, as well as by reducing myocardial oxidative stress [162].
All RAS components have been systematically studied and are found in the myocardium of animals with intact renal function. The above observations also suggest that VDR activator therapy improves cardiac hypertrophy and overall cardiovascular mortality in CKD by upregulating VDR expression and downregulating renin and other RAS components in the myocardium. However, studies documenting specific upregulation of RAS components in the myocardium of uremic animals have not been conducted.
Furthermore, the effects of therapy with a VDR activator, such as calcitriol or paricalcitol, on the myocardial expression of RAS components in animal models of experimental uremia and with cardiac hypertrophy remain largely unknown, and are the focus of ongoing investigations.
FGF-23
FGF-23, a bone-derived phosphaturic hormone that also reduces the activity of the renal 1α-hydroxylase and renal synthesis of 1,25(OH)2D3, is critical in maintaining mineral homeostasis in early-stage CKD [175]. Its concentrations are universally elevated in adults and young patients with advanced CKD [176,177]. In adults with CKD, elevated serum FGF-23 concentrations are strong predictors of premature death [178] and are independently associated with LV hypertrophy in adults on hemodialysis and on those not yet on dialysis [146,147,176]. However, until recently, these associations were recognized only in adults whose exposure to the metabolic derangements and comorbidities of CKD associated with cardiovascular death is longer than it is in children.
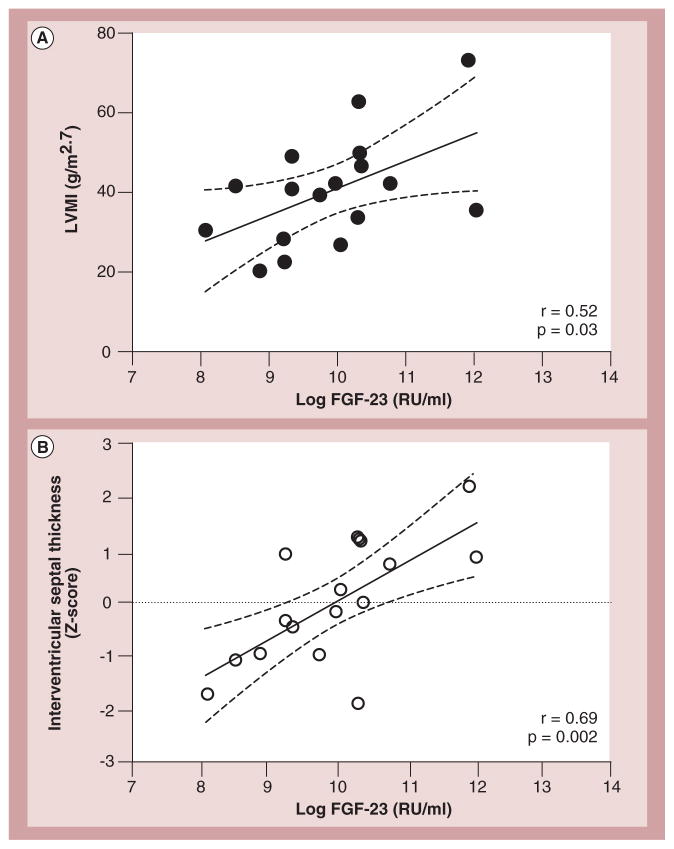
In a study of children with CKD, we found that elevated plasma FGF-23 concentrations correlated significantly with LV mass index (p < 0.03) and were independently associated with interventricular septal thickness Z-scores (p < 0.001) in patients on maintenance hemodialysis [179]. Each one SD increase in log-transformed FGF-23 concentration was associated with a 17% increase in LV mass index, corresponding to an increase of 6.78 g/m2.7 (Figure 19). The mean (SD) LV mass index was 43 (13) g/m2.7, and 55% of 26 patients had LV hypertrophy, as indicated by height- and age-adjusted values. A recent cross-sectional study of 20 children with heart failure had similar findings. Children with heart failure were found to have elevated FGF-23 concentrations. Additionally, high FGF-23 concentrations were correlated with greater LV end-diastolic diameter [180].
Figure 19. Correlations between plasma FGF-23 levels and echocardiographic measurement of cardiac hypertrophy.
(A) LVMI and (B) Z-score of interventricular septal thickness correlated positively with log-transformed FGF-23 levels measured with C-terminal assay. Dashed lines represent 95% CIs.
LVMI: Left ventricular mass index; RU: Relative units.
Reproduced with permission from [179].
Although LV hypertrophy has been reported in up to 85% of children receiving dialysis [137,181], the substantially lower prevalence of LV hypertrophy in our study (55%) probably reflects the stricter definition of LV hypertrophy created by adjusting the LV mass index for height and age [182], rather than to chronological age [183,184]. The prevalence rate we found was similar to that reported recently in a large cohort of children receiving chronic peritoneal dialysis and employing similar height-adjusted criteria [185]. We also found that the only variables significantly associated with LV mass index were hypertension and elevated FGF-23 concentrations. In addition, only FGF-23 concentrations were correlated significantly with interventricular septal thickness, another marker of myocardial hypertrophy [179]. Whether the markedly elevated FGF-23 concentrations in patients with CKD could be cardiotoxic and eventually result in LV hypertrophy independently of other factors remains debatable.
FGF receptor-1, one of the four FGF receptors that are members of the tyrosine kinase superfamily, is expressed in cardiomyocytes [186,187]. However, under physiologic conditions, FGF-23 has a low affinity for FGF receptor-1 and also requires prior activation by the obligatory co-receptor Klotho, which is conspicuously absent in normal cardiomyocytes. Nonetheless, the elevated FGF-23 concentrations seen in CKD and dialysis patients could conceivably bind nonselectively to these receptors in the absence of Klotho, subsequently inducing myocardial hypertrophy.
A recent series of elegant experiments found FGF-23-mediated development of LV hypertrophy in wild-type mice receiving intramyocardial injections of FGF-23. This finding established that FGF-23 exerts a direct hypertrophic effect on the heart rather than changes in the circulating concentrations of other substances it regulates, which could potentially be linked to the development of LV hypertrophy [188]. Furthermore, Klotho-deficient mice with elevated FGF-23 concentrations had LV hypertrophy, showing that Klotho is not essential for the development of cardiac hypertrophy. In addition, in uremic animals, the administration of a pan-FGF receptor inhibitor markedly attenuated LV hypertrophy, thereby indicating that the hypertrophic process is mediated by the FGF receptor [188].
In an experimental model of chronic renal insufficiency, FGF receptor-1 was upregulated in myocardial tissue of uremic rats with cardiac hypertrophy [189], a model characterized by universally elevated FGF-23 concentrations, indicating that, indeed, the FGF-23 prohypertrophic effect seems to be mediated by at least one of the four recognized FGF receptors and is independent of Klotho.
Whether other FGF receptors involved in cardiac hypertrophy, such as FGF receptor-4 or -3, are capable of binding FGF-23 with high affinity under nonuremic conditions and without the presence of Klotho remains unknown. Because hypertension is a predictor of LV hypertrophy in patients on dialysis [185] and because blood pressure is also significantly associated with the LV mass index [179], we cannot categorically support the notion that FGF-23 constitutes the sole and independent cause of elevated LV mass index and LV hypertrophy in these patients. However, the attenuating effects of the pan-FGF receptor blocker on LV hypertrophy in the above study of uremic animals with elevated FGF-23 concentrations did not alter the severity of hypertension, providing evidence for an important and blood pressure-independent role of FGF-23 in the development of LV hypertrophy [188]. Other cardiovascular complications of CKD, such as coronary calcification, have also been associated with elevated FGF-23 concentrations in both children [190] and adults with CKD [191].
Vitamin D and FGF-23 may have opposing effects on the cardiovascular system, whereas active vitamin D or its analogs improve LV hypertrophy in CKD patients and attenuate cardiac hypertrophy in experimental models associated with uremia. FGF-23 is associated with LV hypertrophy and seems to promote cardiac hypertrophy. In addition, vitamin D administration may further elevate circulating FGF-23 [192]. The protective effects of vitamin D on the renal and cardiovascular system appear to be mediated, at least in part, through the suppression of renal and cardiac renin expression [167,173]. It is plausible that VDR activators may be able to overcome the off-target adverse effects of FGF-23 on LV hypertrophy by modulating the intracardiac RAS, as well as by exerting RAS-independent antihypertrophic effects of direct VDR activation [193].
As the most frequent cardiac complication in CKD, LV hypertrophy is the result of multiple abnormalities, including load-independent factors. Emerging evidence indicates that VDR activators negatively regulate RAS, providing reno- and cardio-protective effects in experimental uremia models, and in human CKD. In addition to its effects on vitamin D metabolism, elevated concentrations of FGF-23 are associated with increased cardiovascular morbidity and mortality, and appear to contribute directly to the initiation and progression of LV hypertrophy in CKD. Nonetheless, additional studies are needed to identify the precise molecular mechanisms underlying the effectiveness of VDR activators in preventing and reversing LV hypertrophy in experimental models and in patients with CKD patients, despite elevated FGF-23 concentrations.
Based on the above considerations, measurements of circulating 25OHD (calcidiol) and FGF-23 levels is recommended in patients with all stages of CKD. Calcidiol levels should be maintained >30 ng/ml with early supplemental vitamin D therapy and, with advancing CKD, the use of VDR activators is usually required. Attempts to reduce elevated FGF-23 levels by restricting chronic dietary phosphate overload is desirable, but remains a clinical challenge. The development of agents capable of selectively blocking the cardiotoxic effects of FGF-23 while preserving its phosphaturic properties is much needed and promises to more effectively impact the high prevalence of LVH in patients with CKD.
Nutritional status of children with cardiomyopathy
Medications or heart transplant may be the most direct way to improve cardiac function in children with cardiomyopathy; ancillary therapies to improve nutritional status can also improve quality of life and overall health. Chronic cardiomyopathy in children leads to an imbalance of energy in which the added metabolic demands of the disease outpace the child’s ability to consume adequate calories. Several other factors, often synergistic, contribute to growth failure in children with cardiomyopathy. These factors include gastrointestinal malabsorption, low and suboptimal dietary intake of both macro- and micro-nutrients, and psychosocial problems.
The delivery of adequate nutrition begins with early detection for those children at risk of malnutrition. Both undernutrition (a BMI <10% for age and sex) and overnutrition (BMI >95% for age and sex) are associated with poorer outcomes [194].
Macronutrients
Children with cardiomyopathy and heart failure should receive adequate calories to compensate for their heart failure and provide for normal growth. Children in heart failure often do not grow according to expected standards for age and sex, and poor growth may either contribute to poor cardiac function or be a result of it [195–199]. Optimal caloric intake is estimated to be 110–125% of the estimated energy requirement for age and sex [195–200]. Caloric requirements are increased because of the additional metabolic demands associated with routine activities of daily living in the context of heart failure. Intestinal malabsorption of critical nutrients often results in gut edema in these children. Furthermore, children with cardiomyopathy can be anorexic, possibly as a result of a proinflammatory condition (cachexia) or because of delayed gastric emptying secondary to increased edema. Providing optimal dietary macronutrition makes good clinical sense, but its importance in developing or preventing LV hypertrophy or heart failure in children with cardiomyopathy is less clear.
Micronutrients
Children with cardiomyopathy and heart failure may require greater-than-standard intakes of certain micronutrients to optimize cardiac function [201,202]. Serum levels of micronutrients may not necessarily reflect adequate tissue stores. The micronutrient, mineral and other nutritional deficiencies discussed below can be associated with heart failure, and treating these deficiencies may augment cardiac function as an ancillary intervention. Children with cardiomyopathy and heart failure may require greater-than-standard intakes of certain micronutrients to optimize cardiac function, although much of the current literature is derived from adult studies [201,202].
Antioxidants
Free radicals are products of oxygen metabolism, and their rate of production is usually equal to their metabolism under typical conditions in the human body. Production of free radicals can increase when the body is under stress [203]. An excessive increase in reactive oxygen species is linked to atherosclerosis, CVD, hypertension, ischemia/reperfusion injury, DM and neurodegenerative and immunoinflammatory diseases [204]. Reactive oxygen species are important in modulating vascular function, either by direct oxidative damage or by activating cellular signaling pathways that lead to abnormal contractile, inflammatory, proliferative or remodeling properties of the blood vessels [205]. Increased reactive oxygen species can lower necessary antioxidant defenses. It is recommended that heart failure patients should increase their antioxidant intake by consuming more fruits, vegetables and grains [203]. Since antioxidants can benefit patients with heart failure, they presumably have similar effects in patients with cardiomyopathy [201].
Vitamins A and E are antioxidants that can decrease oxidative stress. Short-term interventions with diets rich in fruits and vegetables, which contain antioxidants such as vitamins A and E, can improve coronary risk factors, and, therefore, probably reduce CVD [204]. Carotenoids may be cardioprotective and this effect may extend to patients with cardiomyopathy [206]. No specific dosing has been recommended to prevent heart disease [206].
Vitamin E levels are below normal among patients with heart failure [207]. Recent studies have reported cardioprotective benefits of vitamins and trace elements, including vitamin E, but the results have been inconsistent [208]. Large clinical studies have not found a benefit of vitamin E in either the primary or secondary prevention of CVD [209]. The American Heart Association does not support the use of vitamin E supplements to prevent CVD [204]. Other nutrients that may provide cardiovascular protection include folate, fiber and potassium.
Selenium and zinc are important antioxidants that maintain cellular function [203,210]. Zinc may protect against cardiomyopathy and coronary artery disease [210], while a deficiency of zinc is associated with cardiomyocyte apoptosis [211]. Zinc and selenium deficiencies have been associated with reversible cardiomyopathy when treated with supplementation in patients with intestinal malabsorption secondary to gastric bypass surgery [211–214]. Although some studies show that heart failure is associated with lower selenium and zinc concentrations [214], these concentrations were the same in patients with idiopathic DCM and with ischemic cardiomyopathy [214]. The long-term use of the ketogenic diet in children with refractory seizure disorders is a risk factor of selenium-associated cardiomyopathy. Selenium supplements and the cessation of a ketogenic diet can reverse this cardiomyopathy [215].
Amino acids & derivatives
Taurine is a semi-essential amino acid that helps to regulate the flow of calcium through cells [216,217]. It is found in high concentrations in the heart and muscle, and is important in ion movement, calcium handling, osmoregulation and cytoprotection. Taurine deficiency has been associated with cardiomyopathy [218], cardiomyocyte atrophy and mitochondrial and myofiber damage, as well as cardiac dysfunction. Studies on the effects of supplementation in children are limited.
L-carnitine is an amino acid derivative that helps in transporting long-chain fatty acids from the cytoplasm to the sites of β-oxidation within the mitochondrion [203,219]. L-carni-tine deficiency can be caused by an autosomal recessive disorder and is associated with cardiomyopathy, arrhythmias and fatigability in both children and adults [220]. In case studies, DCM associated with primary carnitine deficiency improved rapidly with oral carnitine supplementation [219,221]. L-carnitine is a potentially important nutrient for children with cardiomyopathy, although its value remains to be determined.
Vitamin D & calcium
Vitamin D and calcium are important in bone homeostasis and the cardiovascular system. Hypocalcemia is a rare and reversible cause of DCM [222,223], especially in areas of the world where nutritional rickets is common [224]. Vitamin D deficiency is associated with several cardiovascular-related diseases, including hypertension, coronary artery disease, cardiomyopathy and diabetes, and is a strong overall predictor of all-cause death [153]. In one study, vitamin D supplementation was also associated with improved survival [153]. Vitamin D deficiency is prevalent in children, with many children consuming less than 50% of the recommended daily intake of calcium and vitamin D [203]. Nutritional intakes of these nutrients should be monitored and optimized in children with cardiomyopathy.
The value of optimal nutrition and nutritional supplementation in children with cardiomyopathy is not well studied. Specific nutrients, such as L-carnitine, can improve the prognosis of children who are deficient in a particular nutrient, thereby optimizing heart function with little to no risk to the child. However, more studies are needed to fully understand the impact of nutrition on cardiac function in children with cardiomyopathy.
Conclusion & future perspective
Cardiomyopathy is a rare, yet serious disorder of the heart that stands as a frequent cause of heart failure and the most common cause of heart transplantation in children older than 1 year of age. Although there is a spectrum of disease types, the majority of children are diagnosed with dilated cardiomyopathy or HCM. Both of these functional types are associated with abnormal cardiac structure and function, and poor prognosis. The PCMR remains one of the largest and most important cohorts in the study of pediatric cardiomyopathy. Since the 1990s, it has helped refine the estimate of pediatric cardiomyopathy incidence and outcomes, identify risk factors associated with pediatric cardiomyopathy and provide a description of the care being provided to children with cardiomyopathy. As follow-up of this aging cohort continues and is linked with data gathered from stored blood and tissue specimens, our understanding of this chronic disease will continue to improve. Advances in genomic characterization, such as genome-wide analysis, should provide additional insights into the etiology and prognosis in children with cardiomyopathy.
Executive summary.
Dilated cardiomyopathy in childhood
One of several phenotypic classifications of cardiomyopathy, dilated cardiomyopathy (DCM), is usually progressive and is a leading indication for cardiac transplantation in adults and children.
Approximately 40% of children undergo cardiac transplantation or die within 5 years of being diagnosed with DCM.
Children with DCM differ from adults in the causes, pharmacodynamics, expected duration of treatment and therapeutic goals in terms of life expectancy and quality of life.
Hypertrophic cardiomyopathy in childhood
Hypertrophic cardiomyopathy (HCM) is a heterogeneous group of disorders characterized by left ventricular (LV) hypertrophy associated with nondilated ventricular chambers.
HCM is associated with symptomatic heart failure, pain, arrhythmia and sudden cardiac death.
Treatments for patients with HCM are diverse and range from no pharmacotherapy at all to transplantation.
Restrictive cardiomyopathy in childhood
Restrictive cardiomyopathy (RCM) is the least common of the cardiomyopathies, has the poorest prognosis and, when not idiopathic in nature, is associated with amyloidosis, inborn errors of metabolism, sarcoidosis, scleroderma and other diseases.
In the initial stages of the disease, some patients are asymptomatic at the time of presentation. This is a particularly important challenge considering children with RCM are at a higher risk of sudden death.
Treatment of RCM should target the cause. In patients with risk factors for poor outcomes, such as pulmonary congestion, heart failure, low LV fractional shortening, diagnosis before the age of 2 years, thromboembolic events and increased pulmonary vascular resistance, more aggressive treatment strategies, including cardiac transplantation, have been advocated.
LV noncompaction in childhood
LV noncompaction is a recently classified congenital cardiomyopathy that primarily involves the apical portion of the LV.
LV noncompaction is characterized by deep intertrabecular recesses and the separation and thickening of the myocardium into two distinct compacted and noncompacted layers.
Manifestations of LV noncompaction range from asymptomatic cardiac dysfunction, to arrhythmias, to thromboembolism, to severe decompensated heart failure.
Patients presenting with systolic dysfunction can be treated with angiotensin-converting enzyme inhibitors, such as enalapril, or β-blockers, such as carvedilol. β-blocker therapy with propranolol or atenolol is appropriate treatment for patients with diastolic dysfunction.
Endocrinology & pediatric cardiomyopathy
Abnormal thyroid function can be associated with cardiomyopathy. Although cardiomyopathy is not rare in adults with thyroid dysfunction, it is fortunately much less common in children and adolescents in the absence of underlying heart disease.
Diabetes mellitus is an increasingly common disease with extensive micro- and macro-vascular complications.
In addition to echocardiography, other modalities, such as tissue Doppler imaging and N-terminal pro-brain natriuretic peptide concentrations, can help in the early diagnosis of diabetic cardiomyopathy.
Currently, there are no specific treatment guidelines, given the scarcity of therapeutic studies on the early stages of diabetic cardiomyopathy, but it is evident that early treatment may prevent or delay more severe cardiac dysfunction.
Nephrology & pediatric cardiomyopathy
Uremic cardiomyopathy is a common complication in patients with chronic kidney disease (CKD).
Vitamin D insufficiency and deficiency, as defined by variable cut-off serum concentrations of 25-hydroxyvitamin D, are associated with a higher relative risk for cardiovascular mortality in CKD.
Elevated concentrations of FGF-23 are associated with increased cardiovascular morbidity and mortality, and appear to contribute directly to the initiation and progression of LV hypertrophy in CKD.
Nutritional status of children with cardiomyopathy
Ancillary therapies to improve nutritional status can also improve quality of life and overall health.
Children with cardiomyopathy and heart failure should receive adequate calories to compensate for their heart failure and provide for normal growth, but may require greater-than-standard intakes of certain micronutrients to optimize cardiac function.
Reactive oxygen species are important in modulating vascular function. Vitamins A and E are antioxidants that can decrease oxidative stress.
Conclusion
Cardiomyopathy is a rare, yet serious constellation of disorders of the heart that stands as a frequent cause of heart failure and is the most common cause of heart transplantation in children older than 1 year of age.
Advances in genomic characterization, such as genome-wide analysis, should provide additional insights into the etiology and prognosis in children with cardiomyopathy.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Wilkinson JD, Sleeper LA, Alvarez JA, Bublik N, Lipshultz SE for the Pediatric Cardiomyopathy Study Group. The pediatric cardiomyopathy registry: 1995–2007. Prog Pediatr Cardiol. 2008;25(1):31–36. doi: 10.1016/j.ppedcard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2■.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. Found that the incidence of cardiomyopathy was significantly higher in children younger than 1 year old. The estimated incidence of pediatric cardiomyopathy was determined to be 1.13 cases per 100,000 children. Incidence varied according to sex, region and racial origin. [DOI] [PubMed] [Google Scholar]
- 3.Bublik N, Alvarez JA, Lipshultz SE. Pediatric cardiomyopathy as a chronic disease: a perspective on comprehensive care programs. Prog Pediatr Cardiol. 2008;25(1):103–111. doi: 10.1016/j.ppedcard.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez JA, Wilkinson JD, Lipshultz SE for the Pediatric Cardiomyopathy Registry Study Group. Outcome predictors for pediatric dilated cardiomyopathy: a systematic review. Prog Pediatr Cardiol. 2007;23(1):25–32. doi: 10.1016/j.ppedcard.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5■■.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. Found that the annual incidence of dilated cardiomyopathy in children younger than 18 years of age was 0.57 cases per 100,000. It also found that annual incidence was higher in boys than girls. The majority of children had idiopathic disease, with the most common causes being myocarditis and neuromuscular disease. [DOI] [PubMed] [Google Scholar]
- 6.Towbin JA, Sleeper LA, Lowe AM, et al. Etiology, incidence, and outcome of dilated cardiomyopathy in children: the pediatric cardiomyopathy registry experience. JAMA. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV multicenter study. Am Heart J. 2005;150(3):439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipshultz SE. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med. 1998;339(16):1153–1155. doi: 10.1056/NEJM199810153391609. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 10■■.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. Examined the course of left ventricular structure and function. Found that after 11.8 years, abnormalities of cardiac structure and function were persistent and progressive in children diagnosed with acute lymphoblastic leukemia treated with any amount of doxorubicin, but were particularly worse in children who received more than 300 mg/m2. [DOI] [PubMed] [Google Scholar]
- 11.Singh TP, Sleeper LA, Lipshultz SE, et al. Association of left ventricular dilation at listing for heart transplant with post-listing and early post-transplant mortality in children with dilated cardiomyopathy. Circ Heart Fail. 2009;2(6):591–598. doi: 10.1161/CIRCHEARTFAILURE.108.839001. [DOI] [PubMed] [Google Scholar]
- 12.Kantor PF, Orav EJ, Wilkinson JD, et al. Progressive left ventricular changes predict the likelihood of survival in pediatric dilated cardiomyopathy: findings from the pediatric cardiomyopathy registry. Presented at. ACC Scientific Sessions; Chicago, IL, USA. 24–27 March 2012. [Google Scholar]
- 13.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 14.Rusconi PG, Ludwig DA, Ratnasamy C, et al. Serial measurements of serum NT-proBNP as markers of left ventricular systolic function and remodeling in children with heart failure. Am Heart J. 2010;160(4):776–783. doi: 10.1016/j.ahj.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmon WG, Sleeper LA, Cuniberti L, et al. Treating children with idiopathic dilated cardiomyopathy (from the Pediatric Cardiomyopathy Registry) Am J Cardiol. 2009;104(2):281–286. doi: 10.1016/j.amjcard.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmon WG, Sleeper LA, Cuniberti L, et al. Practice patterns in treating children with dilated cardiomyopathy: findings from the North American Pediatric Cardiomyopathy Registry. Am J Cardiol. 2009;104(2):281–286. doi: 10.1016/j.amjcard.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez JA, Orav EJ, Wilkinson JD, et al. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the Pediatric Cardiomyopathy Registry. Circulation. 2011;124(7):814–823. doi: 10.1161/CIRCULATIONAHA.110.973826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connuck DM, Sleeper LA, Colan SD, et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: A comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2008;155(6):998–1005. doi: 10.1016/j.ahj.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson JD, Landy DC, Colan SD, et al. The Pediatric Cardiomyopathy Registry and heart failure: key results from the first 15 years. Heart Fail Clin. 2010;6(4):401–413. doi: 10.1016/j.hfc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs229. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipshultz SE, Wilkinson JD. Beta-adrenergic adaptation in idiopathic dilated cardiomyopathy presenting in pediatric patients contrasted with adult presentations. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs402. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Pahl E, Sleeper LA, Canter CE, et al. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: a report from the Pediatric Cardiomyopathy Registry. J Am Coll Cardiol. 2012;59(6):607–615. doi: 10.1016/j.jacc.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen RL, Canter CE, Naftel DC, et al. The impact of heart failure severity at time of listing for cardiac transplantation on survival in pediatric cardiomyopathy. J Heart Lung Transplant. 2011;30(7):755–760. doi: 10.1016/j.healun.2011.01.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsirka AE, Trinkaus K, Chen SC, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of cardiac transplantation. J Am Coll Cardiol. 2004;44(2):391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 25.Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3(6):689–697. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 26.Pietra BA, Kantor PF, Bartlett HL, et al. Early predictors of survival to and after heart transplantation in children with dilated cardiomyopathy. Circulation. 2012;126(9):1079–1086. doi: 10.1161/CIRCULATIONAHA.110.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):2761–2796. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 28.Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26(7):1699–1708. doi: 10.1016/0735-1097(95)00390-8. [DOI] [PubMed] [Google Scholar]
- 29.Maskatia SA. Hypertrophic cardiomyopathy: infants, children, and adolescents. Congenit Heart Dis. 2012;7(1):84–92. doi: 10.1111/j.1747-0803.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- 30.Decker JA, Rossano JW, Smith EO, et al. Risk factors and mode of death in isolated hypertrophic cardiomyopathy in children. J Am Coll Cardiol. 2009;54(3):250–254. doi: 10.1016/j.jacc.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 31.Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115(6):773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 32.Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–715. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 33.Botvinick EH, Dae MW, Krishnan R, Ewing S. Hypertrophic cardiomyopathy in the young: another form of ischemic cardiomyopathy? J Am Coll Cardiol. 1993;22(3):805–807. doi: 10.1016/0735-1097(93)90194-6. [DOI] [PubMed] [Google Scholar]
- 34.Garson A., Jr Sudden death in the young. Hosp Pract (Off Ed) 1991;26(6):51–60. doi: 10.1080/21548331.1991.11704188. [DOI] [PubMed] [Google Scholar]
- 35.Gow RM. Sudden cardiac death in the young. Can J Cardiol. 1996;12(11):1157–1160. [PubMed] [Google Scholar]
- 36.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334(16):1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 37.Driscoll DJ, Edwards WD. Sudden unexpected death in children and adolescents. J Am Coll Cardiol. 1985;5(Suppl 6):B118–B121. doi: 10.1016/s0735-1097(85)80540-4. [DOI] [PubMed] [Google Scholar]
- 38.Lipshultz SE, Orav EJ, WIkinson JD, et al. A risk stratification analysis of predictors of death or transplant in children with hypertrophic cardiomyopathy. Circulation. 2008;118:S964, Abstract 4956. [Google Scholar]
- 39.Cox GF, Sleeper LA, Lowe AM, et al. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118(4):1519–1531. doi: 10.1542/peds.2006-0163. [DOI] [PubMed] [Google Scholar]
- 40.Ho CY. New paradigms in hypertrophic cardiomyopathy: insights from genetics. Prog Pediatr Cardiol. 2011;31(2):93–98. doi: 10.1016/j.ppedcard.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41■■.Wilkinson JD, Lowe AM, Salbert BA, et al. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: a study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2012;164(3):442–448. doi: 10.1016/j.ahj.2012.04.018. Compared children with Noonan syndrome and hypertrophic cardiomyopathy with children with idiopathic or familial hypertrophic cardiomyopathy. Found that children who had Noonan syndrome with hypertrophic syndrome had worse risk profiles. The authors suggested that an aggressive therapeutic approach, potentially including listing for cardiac transplantation, may be warrented. [DOI] [PubMed] [Google Scholar]
- 42■■.Webber SA, Lipshultz SE, Sleeper LA, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation. 2012;126(10):1237–1244. doi: 10.1161/CIRCULATIONAHA.112.104638. Analyzed outcomes of children from the Pediatric Cardiomyopathy Registry with restrictive cardiomyopathy, with a focus on the impact of phenotype. Survival was found to be independent of phenotype, although children with the mixed restrictive cardiomyopathy and hypertrophic cardiomyopathy had significantly better transplant-free survival, whereas children with pure restrictive cardiomyopathy had worse transplant-free survival. [DOI] [PubMed] [Google Scholar]
- 43.Maron BJ, Douglas PS, Graham TP, Nishimura RA, Thompson PD. Task Force 1: preparticipation screening and diagnosis of cardiovascular disease in athletes. J Am Coll Cardiol. 2005;45(8):1322–1326. doi: 10.1016/j.jacc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Ostman-Smith I, Wettrell G, Riesenfeld T. A cohort study of childhood hypertrophic cardiomyopathy: improved survival following high-dose beta-adrenoceptor antagonist treatment. J Am Coll Cardiol. 1999;34(6):1813–1822. doi: 10.1016/s0735-1097(99)00421-0. [DOI] [PubMed] [Google Scholar]
- 45.Ostman-Smith I. Hypertrophic cardiomyopathy in childhood and adolescence – strategies to prevent sudden death. Fund Am Clin Pharmacol. 2010;24(5):637–652. doi: 10.1111/j.1472-8206.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 46.Theodoro DA, Danielson GK, Feldt RH, Anderson BJ. Hypertrophic obstructive cardiomyopathy in pediatric patients: results of surgical treatment. J Thorac Cardiovasc Surg. 1996;112(6):1589–1597. doi: 10.1016/S0022-5223(96)70018-1. discussion 1597–1589. [DOI] [PubMed] [Google Scholar]
- 47.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 48.Lenarczyk R, Kowalski O, Kukulski T, Kowalczyk J, Kalarus Z. Resynchronization or dyssynchronization – successful treatment with biventricular stimulation of a child with obstructive hypertrophic cardiomyopathy without dyssynchrony. J Cardiovasc Electrophysiol. 2007;18(5):542–544. doi: 10.1111/j.1540-8167.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 49.Spirito P, Autore C, Rapezzi C, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119(13):1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 50.Dadlani GH, Harmon WG, Perez-Colon E, Sokoloski MC, Wilmot I, Lipshultz SE. Diagnosis and screening of hypertrophic cardiomyopathy in children. Prog Pediatr Cardiol. 2011;31(1):21–27. [Google Scholar]
- 51.Rodday AM, Triedman JK, Alexander ME, et al. Electrocardiogram screening for disorders that cause sudden cardiac death in asymptomatic children: a meta-analysis. Pediatrics. 2012;129(4):e999–e1010. doi: 10.1542/peds.2011-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maron BJ, Zipes DP. 36th Bethesda Conference: eligibility recommendataions for competitive atheletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45(8):1313–1375. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 54.Chen SC, Balfour IC, Jureidini S. Clinical spectrum of restrictive cardiomyopathy in children. J Heart Lung Transplant. 2001;20(1):90–92. doi: 10.1016/s1053-2498(00)00162-5. [DOI] [PubMed] [Google Scholar]
- 55.Keren A, Popp RL. Assignment of patients into the classification of cardiomyopathies. Circulation. 1992;86(5):1622–1633. doi: 10.1161/01.cir.86.5.1622. [DOI] [PubMed] [Google Scholar]
- 56.Russo LM, Webber SA. Idiopathic restrictive cardiomyopathy in children. Heart. 2005;91(9):1199–1202. doi: 10.1136/hrt.2004.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denfield SW, Rosenthal G, Gajarski RJ, et al. Restrictive cardiomyopathies in childhood Etiologies and natural history. Tex Heart Inst J. 1997;24(1):38–44. [PMC free article] [PubMed] [Google Scholar]
- 58.Mogensen J, Kubo T, Duque M, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest. 2003;111(2):209–216. doi: 10.1172/JCI16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angelini A, Calzolari V, Thiene G, et al. Morphologic spectrum of primary restrictive cardiomyopathy. Am J Cardiol. 1997;80(8):1046–1050. doi: 10.1016/s0002-9149(97)00601-2. [DOI] [PubMed] [Google Scholar]
- 60.Feld S, Caspi A. Familial cardiomyopathy with variable hypertrophic and restrictive features and common HLA haplotype. Isr J Med Sci. 1992;28(5):277–280. [PubMed] [Google Scholar]
- 61.Arbustini E, Morbini P, Grasso M, et al. Restrictive cardiomyopathy, atrioventricular block and mild to subclinical myopathy in patients with desmin-immunoreactive material deposits. J Am Coll Cardiol. 1998;31(3):645–653. doi: 10.1016/s0735-1097(98)00026-6. [DOI] [PubMed] [Google Scholar]
- 62.Bergman JE, Veenstra-Knol HE, Van Essen AJ, et al. Two related Dutch families with a clinically variable presentation of cardioskeletal myopathy caused by a novel S13F mutation in the desmin gene. Eur J Med Genet. 2007;50(5):355–366. doi: 10.1016/j.ejmg.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Kaminska A, Strelkov SV, Goudeau B, et al. Small deletions disturb desmin architecture leading to breakdown of muscle cells and development of skeletal or cardioskeletal myopathy. Hum Genet. 2004;114(3):306–313. doi: 10.1007/s00439-003-1057-7. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Tapscoft T, Gonzalez O, et al. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100(5):461–464. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- 65.Silva CP, Bacal F, Benvenuti LA, Bocchi EA. Desmin-related restrictive cardiomyopathy. Arg Bras Cardiol. 2007;89(6):165–168. [PubMed] [Google Scholar]
- 66.Lubitz SA, Goldbarg SH, Mehta D. Sudden cardiac death in infiltrative cardiomyopathies: sarcoidosis, scleroderma, amyloidosis, hemachromatosis. Prog Cardiovasc Dis. 2008;51(1):58–73. doi: 10.1016/j.pcad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336(4):267–276. doi: 10.1056/NEJM199701233360407. [DOI] [PubMed] [Google Scholar]
- 68.Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev. 2012;20(4):184–188. doi: 10.1097/CRD.0b013e3182431c23. [DOI] [PubMed] [Google Scholar]
- 69.Bakalli A, Georgievska-Ismail L, Kocinaj D, et al. Left ventricular and left atrial thrombi in sinus rhythm patients with dilated ischemic cardiomyopathy. Med Arh. 2012;66(3):155–158. doi: 10.5455/medarh.2012.66.155-158. [DOI] [PubMed] [Google Scholar]
- 70.Hirota Y, Kohriyama T, Hayashi T, et al. Idiopathic restrictive cardiomyopathy: differences of left ventricular relaxation and diastolic wave forms from constrictive pericarditis. Am J Cardiol. 1983;52(3):421–423. doi: 10.1016/0002-9149(83)90156-x. [DOI] [PubMed] [Google Scholar]
- 71.Meaney E, Shabetai R, Bhargava V, et al. Cardiac amyloidosis, contrictive pericarditis and restrictive cardiomyopathy. Am J Cardiol. 1976;38(5):547–556. doi: 10.1016/s0002-9149(76)80001-x. [DOI] [PubMed] [Google Scholar]
- 72.Kantor PF, Rusconi P, Lipshultz S, Mital S, Wilkinson JD, Burch M. Current applications and future needs for biomarkers in pediatric cardiomyopathy and heart failure: summary from the second international conference on pediatric cardiomyopathy. Prog Pediatr Cardiol. 2011;32(1):11–14. doi: 10.1016/j.ppedcard.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leya FS, Arab D, Joyal D, et al. The efficacy of brain natriuretic peptide levels in differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Coll Cardiol. 2005;45(11):1900–1902. doi: 10.1016/j.jacc.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 74.Babuin L, Alegria JR, Oh JK, Nishimura RA, Jaffe AS. Brain natriuretic peptide levels in constrictive pericarditis and restrictive cardiomyopathy. J Am Coll Cardiol. 2006;47(7):1489–1491. doi: 10.1016/j.jacc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Sengupta PP, Krishnamoorthy VK, Abhayaratna WP, et al. Comparison of usefulness of tissue Doppler imaging versus brain natriuretic peptide for differentiation of constrictive pericardial disease from restrictive cardiomyopathy. Am J Cardiol. 2008;102(3):357–362. doi: 10.1016/j.amjcard.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 76.Kaski JP, Syrris P, Burch M, et al. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart. 2008;94(11):1478–1484. doi: 10.1136/hrt.2007.134684. [DOI] [PubMed] [Google Scholar]
- 77.Rivenes SM, Kearney DL, Smith EO, Towbin JA, Denfield SW. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000;102(8):876–882. doi: 10.1161/01.cir.102.8.876. [DOI] [PubMed] [Google Scholar]
- 78.Kubo T, Gimeno JR, Bahl A, et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol. 2007;49(25):2419–2426. doi: 10.1016/j.jacc.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 79.Ammash NM, Seward JB, Bailey KR, Edwards WD, Tajik AJ. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101(21):2490–2496. doi: 10.1161/01.cir.101.21.2490. [DOI] [PubMed] [Google Scholar]
- 80.Cetta F, O’Leary PW, Seward JB, Driscoll DJ. Idiopathic restrictive cardiomyopathy in childhood: diagnostic features and clinical course. Mayo Clin Proc. 1995;70(7):634–640. doi: 10.4065/70.7.634. [DOI] [PubMed] [Google Scholar]
- 81.Pignatelli RH, Mcmahon CJ, Dreyer WJ, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108(21):2672–2678. doi: 10.1161/01.CIR.0000100664.10777.B8. [DOI] [PubMed] [Google Scholar]
- 82.Tsai SF, Ebenroth ES, Hurwitz RA, Cordes TM, Schamberger MS, Batra AS. Is left ventricular noncompaction in children truly an isolated lesion? Pediatr Cardiol. 2009;30(5):597–602. doi: 10.1007/s00246-008-9382-1. [DOI] [PubMed] [Google Scholar]
- 83.Wald R, Veldtman G, Golding F, Kirsh J, McCrindle B, Benson L. Determinants of outcome in isolated ventricular noncompaction in childhood. Am J Cardiol. 2004;94(12):1581–1584. doi: 10.1016/j.amjcard.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 84.Jenni R, Rojas J, Oechslin E. Isolated noncompaction of the myocardium. N Engl J Med. 1999;340(12):966–967. doi: 10.1056/NEJM199903253401215. [DOI] [PubMed] [Google Scholar]
- 85.Ergul Y, Nisli K, Demirel A, et al. Left ventricular non-compaction in children and adolescents: clinical features, treatment and follow-up. Cardiol J. 2011;18(2):176–184. [PubMed] [Google Scholar]
- 86.Dusek J, Ostadal B, Duskova M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol. 1975;99(6):312–317. [PubMed] [Google Scholar]
- 87.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258(4):319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 88.Towbin JA. Left ventricular noncompaction: a new form of heart failure. Heart Fail Clin. 2010;6(4):453–469. doi: 10.1016/j.hfc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Baca Lopez F, Alonso Bravo RM, Rodriguez Huerta DA. Echocardiographic features of non-compaction cardiomyopathy: missed and misdiagnosed disease. Arq Bras Cardiol. 2009;93(2):e33–e35. doi: 10.1590/s0066-782x2009000800024. [DOI] [PubMed] [Google Scholar]
- 90.Ozgur S, Senocak F, Orun UA, et al. Ventricular non-compaction in children: clinical characteristics and course. Interact Cardiovasc Thorac Surg. 2011;12(3):370–373. doi: 10.1510/icvts.2010.246694. [DOI] [PubMed] [Google Scholar]
- 91.Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187–192. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 92.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 93.Rosa LV, Salemi VM, Alexandre LM, Mady C. Noncompaction cardiomyopathy: a current view. Arq Bras Cardiol. 2011;97(1):e13–e19. doi: 10.1590/s0066-782x2011000900021. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman WA, Richmond ME, Singh RK, Carroll SJ, Starc TJ, Addonizio LJ. Left-ventricular noncompaction in a pediatric population: predictors of survival. Pediatr Cardiol. 2011;32(4):406–412. doi: 10.1007/s00246-010-9868-5. [DOI] [PubMed] [Google Scholar]
- 95.Richards A, Mao CY, Dobson NR. Left ventricular noncompaction: a rare cause of hydrops fetalis. Pediatr Cardiol. 2009;30(7):985–988. doi: 10.1007/s00246-009-9465-7. [DOI] [PubMed] [Google Scholar]
- 96.Ichida F, Tsubata S, Bowles KR, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103(9):1256–1263. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 97.Sasse-Klaassen S, Probst S, Gerull B, et al. Novel gene locus for autosomal dominant left ventricular noncompaction maps to chromosome 11p15. Circulation. 2004;109(22):2720–2723. doi: 10.1161/01.CIR.0000131865.21260.56. [DOI] [PubMed] [Google Scholar]
- 98.Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42(11):2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 99.Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109(24):2965–2971. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- 100.Jenni R, Oechslin EN, Van Der Loo B. Isolated ventricular non-compaction of the myocardium in adults. Heart. 2007;93(1):11–15. doi: 10.1136/hrt.2005.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 102.Ichida F. Left ventricular noncompaction. Circ J. 2009;73(1):19–26. doi: 10.1253/circj.cj-08-0995. [DOI] [PubMed] [Google Scholar]
- 103.Yasukawa K, Terai M, Honda A, Kohno Y. Isolated noncompaction of ventricular myocardium associated with fatal ventricular fibrillation. Pediatr Cardiol. 2001;22(6):512–514. doi: 10.1007/s002460010286. [DOI] [PubMed] [Google Scholar]
- 104.Ichida F, Hamamichi Y, Miyawaki T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol. 1999;34(1):233–240. doi: 10.1016/s0735-1097(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 105.Hamamichi Y, Ichida F, Hashimoto I, et al. Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2001;17(4):305–314. doi: 10.1023/a:1011658926555. [DOI] [PubMed] [Google Scholar]
- 106.Saito K, Ibuki K, Yoshimura N, et al. Successful cardiac resynchronization therapy in a 3-year-old girl with isolated left ventricular non-compaction and narrow QRS complex: a case report. Circ J. 2009;73(11):2173–2177. doi: 10.1253/circj.cj-08-0806. [DOI] [PubMed] [Google Scholar]
- 107.Lofiego C, Biagini E, Pasquale F, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart. 2007;93(1):65–71. doi: 10.1136/hrt.2006.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 109.Cavallo A, Joseph CJ, Casta A. Cardiac complications in juvenile hyperthyroidism. Am J Dis Child. 1984;138(5):479–482. doi: 10.1001/archpedi.1984.02140430055014. [DOI] [PubMed] [Google Scholar]
- 110.Cavallo A, Casta A, Fawcett HD, Nusynowitz ML, Wolf WJ. Is there a thyrotoxic cardiomyopathy in children? J Pediatr. 1985;107(4):531–536. doi: 10.1016/s0022-3476(85)80010-x. [DOI] [PubMed] [Google Scholar]
- 111.Gilbert-Barness E. Review: metabolic cardiomyopathy and conduction system defects in children. Ann Clin Lab Sci. 2004;34(1):15–34. [PubMed] [Google Scholar]
- 112.Jain V, Kannan L, Kumar P. Congenital hypopituitarism presenting as dilated cardiomyopathy in a child. J Pediatr Endocrinol Metab. 2011;24(9–10):767–769. doi: 10.1515/jpem.2011.366. [DOI] [PubMed] [Google Scholar]
- 113.Khochtali I, Hamza N, Harzallah O, et al. Reversible dilated cardiomyopathy caused by hypothyroidism. Int Arch Med. 2011;4:20. doi: 10.1186/1755-7682-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Micallef T, Gruppetta M, Cassar A, Fava S. Takotsubo cardiomyopathy and severe hypothyroidism. J Cardiovasc Med (Hagerstown) 2011;12(11):824–827. doi: 10.2459/JCM.0b013e3283403454. [DOI] [PubMed] [Google Scholar]
- 115.McElhinney DB, Colan SD, Moran AM, et al. Recombinant human growth hormone treatment for dilated cardiomyopathy in children. Pediatrics. 2004;114(4):e452–e458. doi: 10.1542/peds.2004-0072. [DOI] [PubMed] [Google Scholar]
- 116■.Lipshultz SE, Vlach SA, Lipsitz SR, Sallan SE, Schwartz ML, Colan SD. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115(6):1613–1622. doi: 10.1542/peds.2004-1004. Analyzed serial cardiac findings of anthracycline-treated childhood cancer survivors with several years of growth hormone therapy. Left ventricular contractility was decreased in patients who received growth hormone therapy before, during and after treatment. Although growth hormone therapy increased the wall thickness of the left ventricle, this effect was lost shortly after growth hormone therapy ended. [DOI] [PubMed] [Google Scholar]
- 117.Ibe W, Saraste A, Lindemann S, et al. Cardiomyocyte apoptosis is related to left ventricular dysfunction and remodelling in dilated cardiomyopathy, but is not affected by growth hormone treatment. Eur J Heart Fail. 2007;9(2):160–167. doi: 10.1016/j.ejheart.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi D, Cook AL, Williams DA. Progressively worsening hypertrophic cardiomyopathy in a child with newly diagnosed Costello syndrome while receiving growth hormone therapy. Cardiol Young. 2010;20(4):459–461. doi: 10.1017/S1047951110000260. [DOI] [PubMed] [Google Scholar]
- 119.Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17(3):325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 120.Maisch B, Alter P, Pankuweit S. Diabetic cardiomyopathy – fact or fiction? Herz. 2011;36(2):102–115. doi: 10.1007/s00059-011-3429-4. [DOI] [PubMed] [Google Scholar]
- 121.Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta Diabetol. 2011;48(3):173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 122.Von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. 2010;53(6):1033–1045. doi: 10.1007/s00125-010-1682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grandi AM, Piantanida E, Franzetti I, et al. Effect of glycemic control on left ventricular diastolic function in Type 1 diabetes mellitus. Am J Cardiol. 2006;97(1):71–76. doi: 10.1016/j.amjcard.2005.07.110. [DOI] [PubMed] [Google Scholar]
- 124.Suys BE, Katier N, Rooman RP, et al. Female children and adolescents with Type 1 diabetes have more pronounced early echocardiographic signs of diabetic cardiomyopathy. Diabetes Care. 2004;27(8):1947–1953. doi: 10.2337/diacare.27.8.1947. [DOI] [PubMed] [Google Scholar]
- 125.Salem M, El Behery S, Adly A, Khalil D, El Hadidi E. Early predictors of myocardial disease in children and adolescents with Type 1 diabetes mellitus. Pediatr Diabetes. 2009;10(8):513–521. doi: 10.1111/j.1399-5448.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 126.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4(8):436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 127.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 128.Mehta SK, Holliday C, Hayduk L, Wiersma L, Richards N, Younoszai A. Comparison of myocardial function in children with body mass indexes >/=25 versus those <25 kg/m2. Am J Cardiol. 2004;93(12):1567–1569. doi: 10.1016/j.amjcard.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 129.Obert P, Gueugnon C, Nottin S, et al. Two-dimensional strain and twist by vector velocity imaging in adolescents with severe obesity. Obesity (Silver Spring) 2012;20(12):2397–2405. doi: 10.1038/oby.2012.111. [DOI] [PubMed] [Google Scholar]
- 130.Unger RH. Lipotoxic disease. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 131.Qureshi MY, Wilkinson JD, Lipshultz SE. The relationship of childhood obesity with cardiomyopathy and heart failure. In: Lipshultz SE, Messiah SE, Miller TL, editors. Pediatric Metabolic Syndrome Comprehensive Clinical Review and Related Health Issues. Springer-Verlag; London, UK: 2012. pp. 199–215. [Google Scholar]
- 132.Schrier RW. Cardiorenal versus renocardiac syndrome: is there a difference? Nat Clin Pract Nephrol. 2007;3(12):637. doi: 10.1038/ncpneph0673. [DOI] [PubMed] [Google Scholar]
- 133.Anavekar NS, Mcmurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 134.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 135.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141(2):191–197. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 136.Shroff R, Weaver DJ, Jr, Mitsnefes MM. Cardiovascular complications in children with chronic kidney disease. Nat Rev Nephrol. 2011;7(11):642–649. doi: 10.1038/nrneph.2011.116. [DOI] [PubMed] [Google Scholar]
- 137.Mitsnefes MM, Barletta GM, Dresner IG, et al. Severe cardiac hypertrophy and long-term dialysis: the Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2006;21(8):1167–1170. doi: 10.1007/s00467-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 138.Gruppen MP, Groothoff JW, Prins M, et al. Cardiac disease in young adult patients with end-stage renal disease since childhood: a Dutch cohort study. Kidney Int. 2003;63(3):1058–1065. doi: 10.1046/j.1523-1755.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- 139.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 140.Chavers BM, Li S, Collins AJ, Herzog CA. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 2002;62(2):648–653. doi: 10.1046/j.1523-1755.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- 141.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 142.Amann K, Tyralla K, Gross ML, et al. Cardiomyocyte loss in experimental renal failure: prevention by ramipril. Kidney Int. 2003;63(5):1708–1713. doi: 10.1046/j.1523-1755.2003.00927.x. [DOI] [PubMed] [Google Scholar]
- 143.Tyralla K, Adamczak M, Benz K, et al. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS ONE. 2011;6(1):e15287. doi: 10.1371/journal.pone.0015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. 2009;75(8):771–773. doi: 10.1038/ki.2009.35. [DOI] [PubMed] [Google Scholar]
- 145.Pilz S, Tomaschitz A, Marz W, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75(5):575–584. doi: 10.1111/j.1365-2265.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 146.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337(2):116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 147.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 149.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 150.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2):558–564. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr. 2009;154(6):906–911. e901. doi: 10.1016/j.jpeds.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 152.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 153.Maiya S, Sullivan I, Allgrove J, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. 2008;94(5):581–584. doi: 10.1136/hrt.2007.119792. [DOI] [PubMed] [Google Scholar]
- 154.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58(3):374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 156.Sun Q, Shi L, Rimm EB, et al. Vitamin D intake and risk of cardiovascular disease in US men and women. Am J Clin Nutr. 2011;94(2):534–542. doi: 10.3945/ajcn.110.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152(5):315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 158.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 159.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 160.Kovesdy CP. Survival benefits with vitamin D receptor activation: new insights since 2003. Clin J Am Soc Nephrol. 2010;5(9):1704–1709. doi: 10.2215/CJN.02590310. [DOI] [PubMed] [Google Scholar]
- 161.Kim HW, Park CW, Shin YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102(1):c21–c29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 162.Mizobuchi M, Nakamura H, Tokumoto M, et al. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol. 2010;121(1–2):188–192. doi: 10.1016/j.jsbmb.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 164.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90(1–5):387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 166.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin–angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):e125–e132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 167.Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA. 2007;104(43):16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin–angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 169.Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 170.Fryer RM, Rakestraw PA, Nakane M, et al. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol. 2007;106(4):76–81. doi: 10.1159/000104875. [DOI] [PubMed] [Google Scholar]
- 171.Li YC. Vitamin D: roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens. 2012;21(1):72–79. doi: 10.1097/MNH.0b013e32834de4ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II Role of an intracardiac renin–angiotensin system. Annu Rev Physiol. 1992;54:227–241. doi: 10.1146/annurev.ph.54.030192.001303. [DOI] [PubMed] [Google Scholar]
- 173.Freundlich M, Quiroz Y, Zhang Z, et al. Suppression of renin–angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74(11):1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 174.Wollert KC, Drexler H. The renin–angiotensin system and experimental heart failure. Cardiovasc Res. 1999;43(4):838–849. doi: 10.1016/s0008-6363(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 175.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78(10):975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 176.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wesseling-Perry K, Pereira RC, Wang H, et al. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94(2):511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol. 2012;27(11):2129–2136. doi: 10.1007/s00467-012-2224-7. [DOI] [PubMed] [Google Scholar]
- 180.Isakova T, Houston J, Santacruz L, et al. Associations between fibroblast growth factor 23 and cardiac characteristics in pediatric heart failure. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2515-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, Strife CF. Severe left ventricular hypertrophy in pediatric dialysis: prevalence and predictors. Pediatr Nephrol. 2000;14(10–11):898–902. doi: 10.1007/s004670000303. [DOI] [PubMed] [Google Scholar]
- 182.Borzych D, Bakkaloglu SA, Zaritsky J, et al. Defining left ventricular hypertrophy in children on peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6(8):1934–1943. doi: 10.2215/CJN.11411210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol. 1995;76(10):699–701. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 184.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22(6):709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 185.Bakkaloglu SA, Borzych D, Soo Ha I, et al. Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol. 2011;6(8):1926–1933. doi: 10.2215/CJN.05990710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45(7):1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 187.Liu L, Pasumarthi KB, Padua RR, et al. Adult cardiomyocytes express functional high-affinity receptors for basic fibroblast growth factor. Am J Physiol. 1995;268(5 Pt 2):H1927–H1938. doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- 188.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Freundlich M, Li YC, Weisinger JR, Quiroz Y, Bravo J, Rodriguez-Iturbe B. Paricalcitol downregulates myocardial renin–angiotensin and fibroblast growth factor expression and attenuates cardiac hypertrophy in uremic rats. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt177. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Srivaths PR, Goldstein SL, Silverstein DM, Krishnamurthy R, Brewer ED. Elevated FGF-23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26(6):945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 191.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23(7):2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 192.Razzaque MS. The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5(11):611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen S, Gardner DG. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J Steroid Biochem Mol Biol. 2012;136:150–155. doi: 10.1016/j.jsbmb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 194.Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9(1):29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 195.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 196.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 197.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its panel on folate, other B vitamins, and chlorine. The National Academies Collection: Reports Funded by National Institutes of Health. National Academies Press; DC, USA: 1998. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. [PubMed] [Google Scholar]
- 198.Electrolytes P.o.D.R.I.f., Water, and S.C.o.t.S.E.o.D.R. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press; Washington, DC, USA: 2005. [Google Scholar]
- 199.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 200.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine. DRI Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; DC, USA: 1997. [Google Scholar]
- 201.Broqvist M, Arnqvist H, Dahlstrom U, Larsson J, Nylander E, Permert J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure – effects of long-term dietary supplementation. Eur Heart J. 1994;15(12):1641–1650. doi: 10.1093/oxfordjournals.eurheartj.a060447. [DOI] [PubMed] [Google Scholar]
- 202.Sole MJ, Jeejeebhoy KN. Conditioned nutritional requirements: therapeutic relevance to heart failure. Herz. 2002;27(2):174–178. doi: 10.1007/s00059-002-2360-0. [DOI] [PubMed] [Google Scholar]
- 203.Miller TL, Neri D, Extein J, Somarriba G, Strickman-Stein N. Nutrition in pediatric cardiomyopathy. Prog Pediatr Cardiol. 2007;24(1):59–71. doi: 10.1016/j.ppedcard.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Szasz T, Thakali K, Fink GD, Watts SW. A comparison of arteries and veins in oxidative stress: producers, destroyers, function, and disease. Exp Biol Med (Maywood) 2007;232(1):27–37. [PubMed] [Google Scholar]
- 205.Riccioni G, D’orazio N, Franceschelli S, Speranza L. Marine carotenoids and cardiovascular risk markers. Mar Drugs. 2011;9(7):1166–1175. doi: 10.3390/md9071166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83(6):1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 207.Miwa K, Kishimoto C, Nakamura H, et al. Serum thioredoxin and alpha-tocopherol concentrations in patients with major risk factors. Circ J. 2005;69(3):291–294. doi: 10.1253/circj.69.291. [DOI] [PubMed] [Google Scholar]
- 208.Turan B, Vassort G. Vitamin E in oxidant stress-related cardiovascular pathologies: focus on experimental studies. Curr Pharm Des. 2011;17(21):2155–2169. doi: 10.2174/138161211796957454. [DOI] [PubMed] [Google Scholar]
- 209.Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17(3):e56–e65. doi: 10.1097/MJT.0b013e31819cdc9a. [DOI] [PubMed] [Google Scholar]
- 210.Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010;26(11–12):1050–1057. doi: 10.1016/j.nut.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 211.Strassburger M, Bloch W, Sulyok S, et al. Heterozygous deficiency of manganese superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium. in vivo Free Radic Biol Med. 2005;38(11):1458–1470. doi: 10.1016/j.freeradbiomed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 212.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114(1):217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 213.Frustaci A, Sabbioni E, Fortaner S, et al. Selenium- and zinc-deficient cardiomyopathy in human intestinal malabsorption: preliminary results of selenium/zinc infusion. Eur J Heart Fail. 2012;14(2):202–210. doi: 10.1093/eurjhf/hfr167. [DOI] [PubMed] [Google Scholar]
- 214.Kosar F, Sahin I, Taskapan C, et al. Trace element status (Se, Zn, Cu) in heart failure. Anadolu Kardiyol Derg. 2006;6(3):216–220. [PubMed] [Google Scholar]
- 215.Sirikonda NS, Patten WD, Phillips JR, Mullett CJ. Ketogenic diet: rapid onset of selenium deficiency-induced cardiac decompensation. Pediatr Cardiol. 2012;33(5):834–838. doi: 10.1007/s00246-012-0219-6. [DOI] [PubMed] [Google Scholar]
- 216.Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J. 1992;56(1):95–99. doi: 10.1253/jcj.56.95. [DOI] [PubMed] [Google Scholar]
- 217.Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17 (Suppl 1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ito T, Kimura Y, Uozumi Y, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol. 2008;44(5):927–937. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 219.Gesuete V, Ragni L, Picchio FM. The ‘big heart’ of carnitine. G Ital Cardiol (Rome) 2010;11(9):703–705. [PubMed] [Google Scholar]
- 220.Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7(1):68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baragou S, Pio M, Di Bernardo S, et al. A cause of dilated cardiomyopathy in child: primary carnitine deficiency. Ann Cardiol Angeiol (Paris) 2011 doi: 10.1016/j.ancard.2011.12.006. 10.1016/j. ancard.2011.12.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 222.Benzarouel D, Hasni K, Ashab H, El Hattaoui M. A reversible cause of cardiomyopathy: hypocalcemia. Ann Cardiol Angeiol (Paris) 2012 doi: 10.1016/j.ancard.2012.04.003. 10.1016/j. ancard.2012.04.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 223.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and Supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109(3):359–363. doi: 10.1016/j.amjcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 224.Verma S, Khadwal A, Chopra K, Rohit M, Singhi S. Hypocalcemia nutritional rickets: a curable cause of dilated cardiomyopathy. J Trop Pediatr. 2011;57(2):126–128. doi: 10.1093/tropej/fmq044. [DOI] [PubMed] [Google Scholar]
- 225.Cotran RM, Kumar V, Robbins SL. In: Robbin’s Pathologic Basis of Disease. 5. Michael B, Cohen MD, editors. Elsevier; PA, USA: 1994. [Google Scholar]