Abstract
Dopaminergic neurons of the substantia nigra compacta (SNC), ventral tegmental area (VTA) and retrorubral field (RRF) play a role in reward, motivation, learning, memory, and movement. These neurons are intermingled with GABAergic neurons. Recent evidence shows that the VTA contains glutamatergic neurons expressing vesicular glutamate transporter type 2 (VGluT2); some of them co-express tyrosine hydroxylase (TH). Here, we used a combination of radioactive in situ hybridization and immunohistochemistry to explore whether any of the vesicular glutamate transporters [vesicular glutamate transporter type 1 (VGluT1), VGluT2, or vesicular glutamate transporter type 3 (VGluT3)] were encoded by neurons in the SNC or RRF. We found expression of VGluT2 mRNA, but not of VGluT1 or VGluT3, in the SNC and RRF. These VGluT2 neurons rarely showed TH immunoreactivity. Within the SNC, the VGluT2 neurons were infrequently found at the rostral level, but were often seen at the medial and caudal levels intercalated in the mediolateral portion of the dorsal tier, at a ratio of one VGluT2 neuron per 4.4 TH neurons. At this level, VGluT2 neurons were also found in the adjacent substantia nigra reticulata and substantia nigra pars lateralis. Within the RRF, the VGluT2 neurons showed an increasing rostrocaudal gradient of distribution. The RRF proportion of VGluT2 neurons in relation to TH neurons was constant throughout the rostrocaudal levels, showing an average ratio of one VGluT2 neuron per 1.7 TH neurons. In summary, we provide evidence indicating that the SNC and RRF, which are traditionally considered to be dopaminergic areas, have neurons with the ability to participate in glutamate signaling.
Keywords: A9, A8, A10, dopamine, midbrain glutamatergic neurons, reward, VGluT2, VTA
Introduction
Midbrain dopaminergic neurons play a role in several brain functions, including reward processing, motivation, learning, memory, and movement. These neurons are characterized by their expression of tyrosine hydroxylase (TH), and are organized into three principal cell groups: A8 [in the retrorubral field (RRF)], A9 [in the substantia nigra compacta (SNC)], and A10 [in the ventral tegmental area (VTA)]. In addition to dopaminergic neurons, GABAergic neurons are present in the midbrain dopaminergic system, and recent studies have established the presence of glutamatergic neurons expressing vesicular glutamate transporter type 2 (VGluT2) mRNA in the VTA (Hur and Zaborszky, 2005; Kawano et al., 2006; Yamaguchi et al., 2007, 2011; Nair-Roberts et al., 2008). Although the majority of VGluT2 neurons within the VTA lack TH expression (VGluT2-only neurons) (Yamaguchi et al., 2007, 2011), there is a small subpopulation of VGluT2 neurons that co-express TH (VGluT2–TH neurons) (Kawano et al., 2006; Yamaguchi et al., 2011). The VGluT2-only and VGluT2–TH neurons both innervate the nucleus accumbens and the medial prefrontal cortex (Yamaguchi et al., 2011). A role for VGluT2–TH neurons in meso-accumbal glutamatergic signaling has been found in two independent optogenetic studies (Tecuapetla et al., 2010; Stuber et al., 2010). In these studies, selective optical stimulation of afferents from TH neurons expressing channelrhodopsin elicited excitatory postsynaptic currents (EPSCs) in all tested medium spiny neurons in the nucleus accumbens (Tecuapetla et al., 2010; Stuber et al., 2010). These responses appear to be mediated by VGluT2–TH neurons, as the glutamatergic signaling was absent in slice preparations of conditional knockout mice lacking VGluT2 within the population of TH neurons (Stuber et al., 2010).
The selective stimulation of channelrhodopsin-2 afferents from TH neurons of the SNC does not appear to elicit EPSCs in the dorsal striatum, suggesting a lack of VGluT2–TH nigrostriatal neurons (Stuber et al., 2010). Moreover, prior in situ hybridization studies, in which non-radioactive riboprobes were used for detection of transcripts, found a lack of expression of VGluT2 mRNA in both the SNC and the RRF (Nair-Roberts et al., 2008). Despite these recent findings, earlier electrophysiological and anatomical findings led to the proposal of the existence of a nigrostriatal glutamatergic pathway (Wilson et al., 1982; Hattori et al., 1991). In these studies, in vivo intracellular recordings showed EPSCs in dorsal striatal neurons after electrical stimulation of the SNC (Wilson et al., 1982), and ultrastructural analysis of retrograde labeled material provided evidence for symmetric TH synapses and putative excitatory asymmetric synapses lacking TH originating from the SNC (Hattori et al., 1991).
In this study, we applied a highly sensitive radioactive in situ hybridization procedure to determine whether neurons within the SNC or RRF express any of the known vesicular glutamate transporters [vesicular glutamate transporter type 1 (VGluT1), VGluT2, and vesicular glutamate transporter type 3 (VGluT3)]. We combined this method with TH detection (by immunoreactivity) to define the boundaries of the midbrain dopaminergic system, and to determine the distribution of neurons expressing vesicular glutamate transporter mRNA in relation to those containing TH. We found that VGluT2 mRNA is expressed in a subpopulation of neurons within the SNC and the RRF. In contrast to the VTA, which co-expresses VGluT2 mRNA and TH within a subgroup of neurons, the VGluT2 neurons in the SNC and RRF lack TH.
Materials and methods
Tissue preparation
Eight adult Sprague–Dawley male rats (body weight, 300–350 g; Charles River, Wilmington, MA, USA) were anesthetized with chloral hydrate (35 mg/100 g), and perfused transcardially with 4% (w/v) paraformaldehyde (PF) in 0.1 m phosphate buffer (PB) (pH 7.3). Brains were left in 4% PF for 2 h at 4 °C, rinsed with PB, and transferred sequentially to 12%, 14% and 18% sucrose solutions in PB. Coronal serial sections 12 μm (five rats) or 20 μm (three rats) in thickness were prepared. All animal procedures were approved by the NIH/NIDA Animal Care and Use Committee, and experiments were carried out in accordance with the guidelines laid down by the NIH regarding the care and use of animals for experimental procedures.
Combination of in situ hybridization and TH immunolabeling
Coronal free-floating sections (thickness, 12 μm) were processed as described previously (Yamaguchi et al., 2011). Sections were incubated for 10 min in PB containing 0.5% Triton X-100, rinsed twice for 5 min each with PB, treated with 0.2 m HCl for 10 min, rinsed twice for 5 min each with PB, and then acetylated in 0.25% acetic anhydride in 0.1 m triethanolamine (pH 8.0) for 10 min. Sections were rinsed twice for 5 min each with PB, and post-fixed with 4% PF for 10 min. Prior to hybridization and after a final rinse with PB, the free-floating sections were incubated in hybridization buffer [50% formamide; 10% dextran sulfate; 5 × Denhardt's solution; 0.62 m NaCl; 50 mm dithiothreitol; 10 mm EDTA; 20 mm PIPES (pH 6.8); 0.2% sodium dodecylsulfate; 250 μg/mL salmon sperm DNA; 250 μg/mL tRNA] for 2 h at 55 °C. Sections were hybridized for 16 h at 55 °C in hybridization buffer containing 35S-labeled and 33P-labeled single-stranded antisense or sense rat VGluT1 (nucleotides 53–2077; accession no. NM-053859.1), VGluT2 (nucleotides 317–2357; accession no. NM-053427) or human VGluT3 (nucleotides 1–1729; accession no. BC117229.1) probes at 107 c.p.m./mL. Plasmids that contained VGluT1 and VGluT2 were generously provided by R. H. Edwards (University of California, San Francisco, CA, USA). Sections were treated with 4 μg/mL RNase A at 37 °C for 1 h, and then washed with 1 × SSC and 50% formamide at 55 °C for 1 h, and with 0.1 × SSC at 68 °C for 1 h. After the last SSC wash, sections were rinsed with PB and incubated for 1 h in PB supplemented with 4% bovine serum albumin and 0.3% Triton X-100. This was followed by overnight incubation at 4 °C with an anti-TH mouse monoclonal antibody (1 : 500, MAB 318; Millipore, Billerica, MA, USA) whose specificity has been documented (Tagliaferro and Morales, 2008). After being rinsed three times for 10 min each in PB, sections were processed with an ABC kit (Vector Laboratories, Burlingame, CA, USA). The material was incubated for 1 h at room temperature in a 1 : 200 dilution of the biotinylated secondary antibody, rinsed with PB, and incubated with avidin-biotinylated horseradish peroxidase for 1 h. Sections were rinsed, and the peroxidase reaction was then developed with 0.05% 3,3′-diaminobenzidine-4HCl and 0.03% hydrogen peroxide. Free-floating sections were mounted on coated slides. Slides were dipped in Ilford K.5 nuclear tract emulsion (Polysciences, Warrington, PA, USA; 1 : 1 dilution in double-distilled water), and exposed in the dark at 4 °C for 4 weeks prior to development.
Data analysis of neuronal subpopulations
Sections were viewed, analyzed and photographed with bright-field or epiluminescence microscopy by use of a Nikon BX51 microscope fitted with a ×4 (aperture, 0.16) and ×20 (aperture, 0.75) objective lens. Subdivisions of the midbrain dopaminergic system were traced according to Phillipson (1979), Halliday and Törk (1986), German and Manaye (1993), and Paxinos and Watson (2007). Boundaries between the VTA and the SNC were based on detection of corticotropin-releasing factor-binding protein (CRF-BP) in sections adjacent to those used for VGluT2 mRNA detection, as the selective expression of this protein in VTA dopaminergic neurons demarcates the boundaries between the SNC and the VTA (Wang and Morales, 2008). Single-labeled and double-labeled neurons were observed within each traced region at high power (×20 objective lens) and marked electronically. TH/VGluT2-double-labeled material was analyzed with epiluminescence to increase the contrast of silver grains (neither dark-field nor bright-field optics allow clear visualization of silver grains when they are colocalized with high concentrations of immunoproduct). A neuron was considered to express VGluT2 mRNA when its soma contained concentric aggregates of silver particles above background level. A neuron was considered to express TH when its soma was clearly labeled as brown. A TH-immunolabeled neuron was included in the calculation of total population of TH neurons when the stained neuron was at least 5 μm in diameter. The neurons expressing VGluT2 mRNA, TH or both markers were counted separately. To determine the coexistence of VGluT2 mRNA and TH immunolabel: (i) silver grains corresponding to VGluT2 expression were focused under an epiluminescence microscope; (ii) the path of epiluminescence light was blocked without changing the focus; and (iii) bright-field light was used to determine whether a brown neuron (expressing TH) in focus contained the aggregates of silver grains seen under epiluminescence. Labeled neurons were counted three times, each time by a different observer. Data collected for the SNC, the substantia nigra reticulata (SNR) and the substantia nigra lateralis (SNL) were from a total of 49 sections (from five rats). Data collected for the retrorubral RRF were from 11 sections (from three rats). For initial statistical analysis of the VGluT2 rostrocaudal distribution within the SNC, SNL and RRF, we conducted a Kruskal–Wallis analysis in which the VGluT2 neuron population was compared at three bregma levels. For the SNC and SNL, we compared the VGluT2 neuron populations at the rostral (–5.16 to –5.40 mm), medial (–5.52 to –6.12 mm) and caudal (–6.24 to –6.60 mm) levels. For the RRF, the three analyzed levels were: –6.36 mm for the rostral level, –6.48 mm for the medial level, and –6.60 mm for the caudal level. For the SNR, a non-parametric Mann–Whitney U-test was used, because here we were able to compare the VGluT2 neuron populations at only two bregma levels (–5.52 to –6.12 mm, and –6.24 to –6.60 mm). The initial analysis was followed by a Dunn's post hoc test to determine the bregma in which the VGluT2 neurons were more abundant. Finally, we conducted a chi-square (χ2) test to compare the ratio of TH neurons to VGluT2 neurons counted at each bregma level. Statistical significance was achieved at P < 0.05. Statistical tests were performed with graphpad prism 5. The percentage of neurons expressing VGluT2 mRNA or TH within the total population of counted neurons was expressed as mean ± standard error of the mean. The background was evaluated from slides hybridized with sense probes. Pictures were adjusted to match contrast and brightness with adobe photoshop (Adobe Systems Incorporated, Seattle, WA, USA).
Results
Detection of VGluT2 mRNA, but not VGluT1 or VGluT3 mRNA, within the SNC and RRF
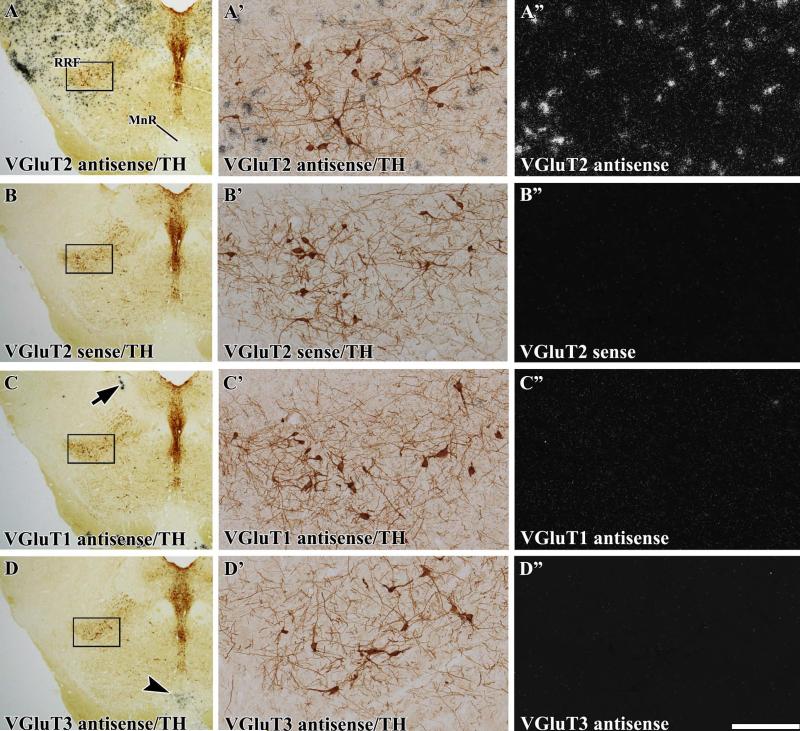
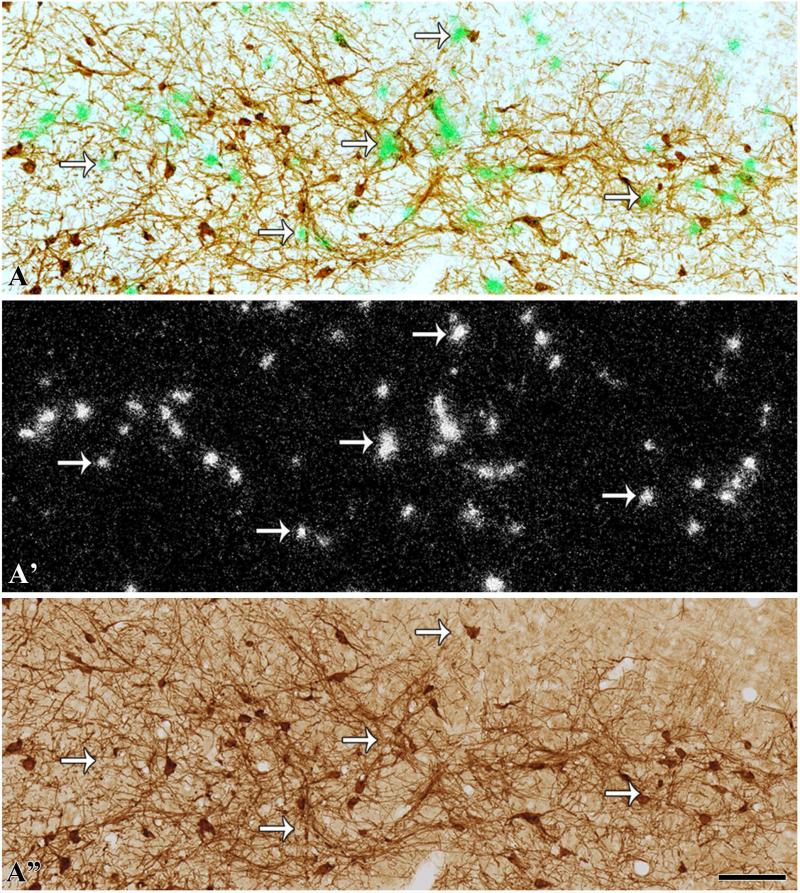
By using a VGluT2 radioactive antisense riboprobe, we detected neurons expressing VGluT2 mRNA intermingled with TH cells in the SNC and RRF (Fig. 1A–A″). The specificity of VGluT2 mRNA detection was demonstrated by a lack of signal when sections were hybridized with the VGluT2 radioactive sense riboprobe (Fig. 1B″). We did not detect transcripts encoding either VGluT1 or VGluT3 in the SNC or RRF (Fig. 1C″ and D″).
Fig. 1.
Expression of VGluT2 mRNA, but lack of expression of either VGluT1 or VGluT3 mRNAs, in the RRF (radioactive in situ hybridization). Coronal sections were incubated with anti-TH antibodies and hybridized with either VGluT2 antisense (A–A″), VGluT2 sense (B–B″), VGluT1 antisense (C– C″) or VGluT3 antisense (D–D″) radioactive riboprobes. (A–D) Coronal sections at low magnification under bright-field microscopy showing TH immunoreactivity (dark brown label) in the RRF and the dorsal raphe. The delimited areas in A–D are shown at higher magnification under bright-field microscopy for visualization of TH immunoreactivity (A′–D′) or under epiluminescence microscopy for visualization of cells expressing VGluT2 mRNA (silver white grains in A″). Note numerous cells expressing VGluT2 mRNA in the RRF (A″), but a lack of signal in the section hybridized with sense radioactive VGluT2 riboprobe (B″). (C–C″) High expression of VGluT1 mRNA is seen in the mesencephalic trigeminal nucleus (arrow), but a lack of VGluT1 mRNA in the RRF (C″). (D–D″) Expression of VGluT3 mRNA is seen in the median raphe nucleus (arrowhead in D), but there is a lack of VGluT3 mRNA in the RRF (D″). MnR, median raphe nucleus. The scale bar shown in D″ is 1125 μm for A–D and 175 μm for A′–D″ (–7.08 mm from bregma).
Neurons expressing VGluT2 mRNA in the different aspects of the substantia nigra
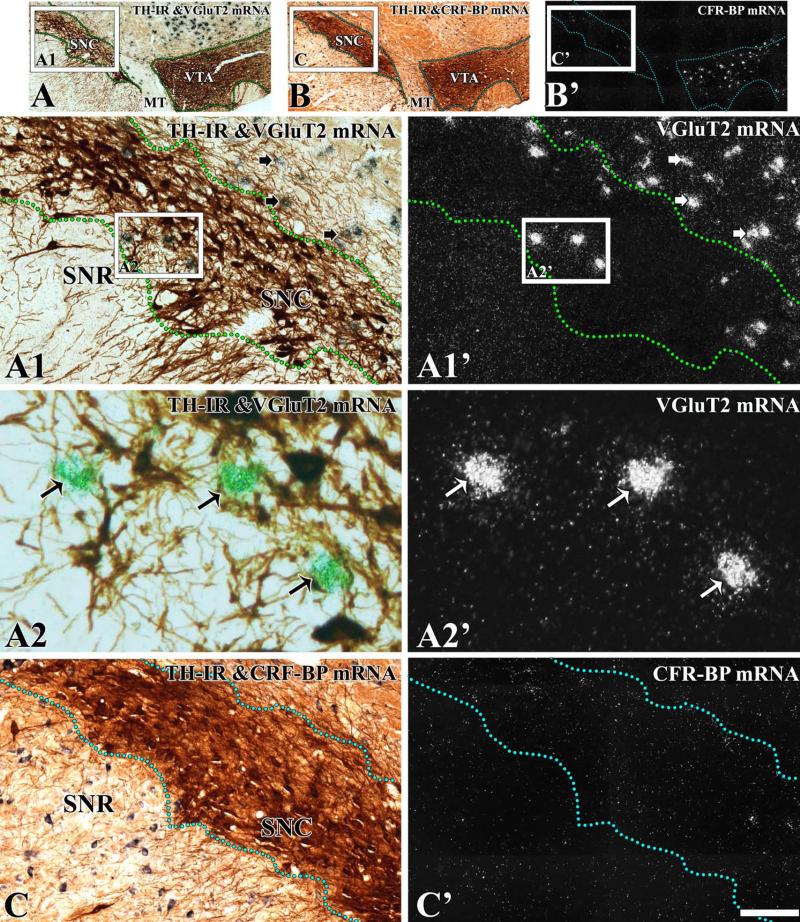
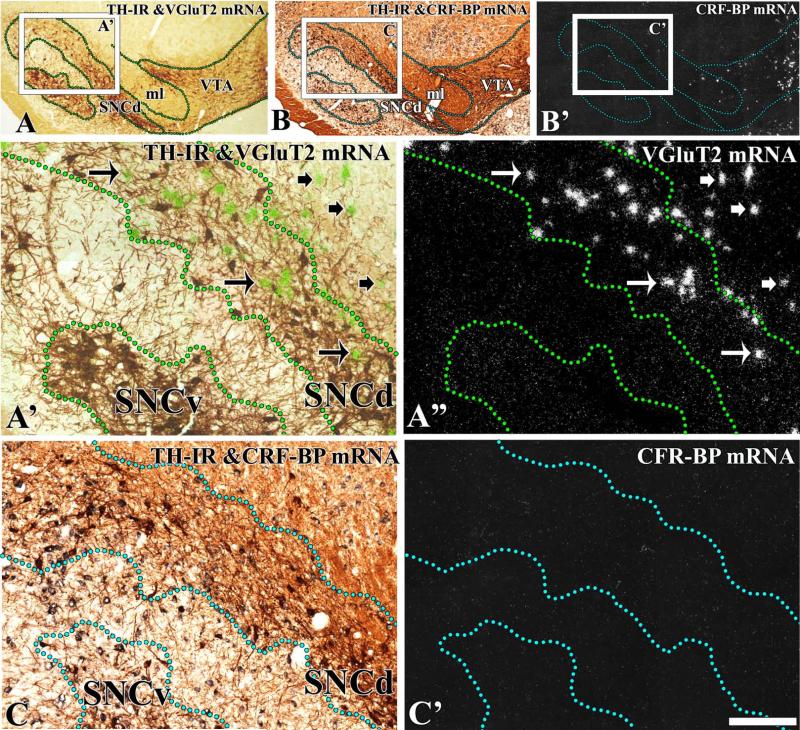
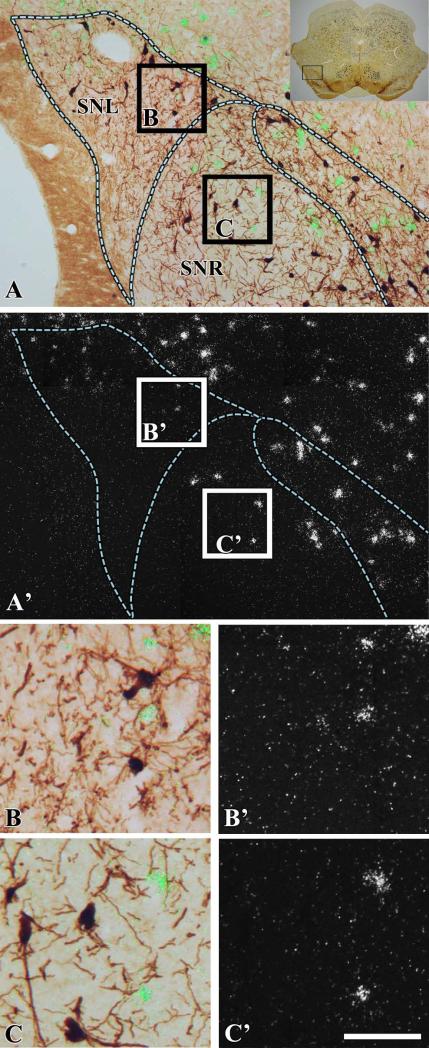
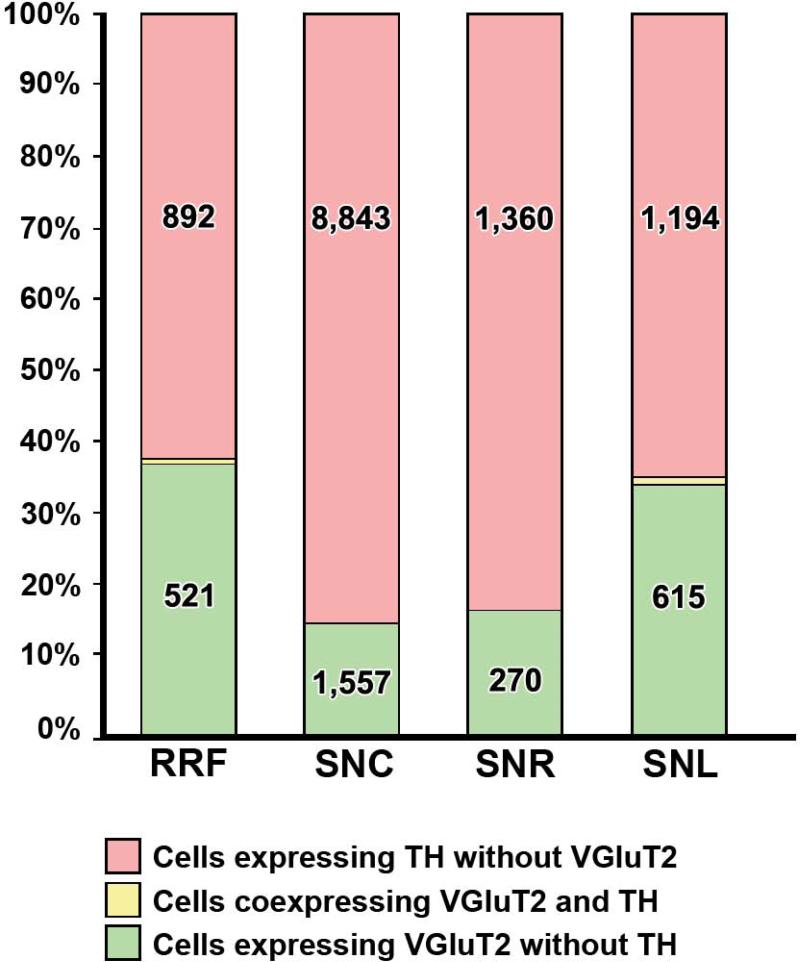
To define the boundaries of the midbrain dopaminergic system and to determine the distribution of neurons expressing VGluT2 mRNA in relation to TH neurons, brain sections were processed for the detection of VGluT2 mRNA by radioactive in situ hybridization and TH by immunohistochemistry (Figs 2 and 3). Boundaries between the VTA and the SNC were based on detection of CRF-BP in sections adjacent to those used for VGluT2 mRNA detection (Figs 2 and 3), as the selective expression of this protein in VTA dopaminergic neurons demarcates the boundaries between the SNC and the VTA (Wang and Morales, 2008). Analysis of co-expression of VGluT2 mRNA and TH showed that only 0.09% of the TH neurons co-expressed VGluT2 mRNA in the SNC (seven VGluT2–TH neurons in a total of 7780 TH neurons and 1547 VGluT2 neurons; Table 1). VGluT2 neurons were confined to the lateral and dorsal zones of the SNC (Figs 2 and 3). A pairwise multiple comparison Dunn's test among three rostrocaudal levels of the SNC showed that VGluT2 neurons were more concentrated in the medial level (–5.16 to –5.40 mm) than in the rostral (–5.16 to –5.40 mm) or the caudal (–6.24 to –6.60 mm) levels (Table 2). At the rostral level, we found 7.8 TH neurons for each VGluT2 neuron (Table 1). In contrast, we detected 4.4 TH neurons for each VGluT2 neuron at the medial level (Table 1; χ2 = 57.1, P < 0.01) and 4.7 TH neurons for each VGluT2 neuron at the caudal level (Table 1; χ2 = 63.27, P < 0.01). VGluT2 neurons were also found in the dorsal portion of the SNR adjacent to the SNC (Fig. 4B and B′), and in the neighboring SNL (Fig. 4C and C′). Within the SNR, the VGluT2 neurons were intermingled with TH neurons, with higher concentrations at the caudal level (one VGluT2 neuron for every 3.2 TH neurons; Table 2) than at the medial level (one VGluT2 neuron for every five TH neurons) (Table 2). Co-expression of VGluT2 mRNA and TH was not detected in the SNR (1084 TH neurons and 262 VGluT2 neurons were counted). VGluT2 cells neurons also found throughout the SNL, with a significant rostrocaudal increase (Table 3). The VGluT2 neurons within the SNL were more abundant in the medial and caudal levels than in the rostral level (Table 3). The SNL VGluT2 neurons rarely co-expressed TH (15 VGluT2–TH neurons in a total of 1139 TH neurons and in a total of 610 VGluT2 neurons).
Fig. 2.
Expression of VGluT2 mRNA in the SNC at the middle level (–5.28 mm from bregma). Coronal sections were incubated with anti-TH antibodies and hybridized with antisense riboprobes for detection of VGluT2 (A–A2′) or CRF-BP (B–C′). (A) Section at low magnification showing TH immunoreactivity (TH-IR) (dark brown label) under bright-field microscopy. (A1′–A2′) The delimited area in A is shown at higher magnification; TH neurons are seen as brown cells (A1 and A2) and VGluT2 neurons as aggregates of white (A1′ and A2′) or green (A2) grains. (A1–A2′) Long arrows indicate examples of VGluT2 neurons intermingled with TH neurons. Short arrows indicate examples of VGluT2 neurons intermingled with TH dendrites. (B and B′) At low magnification, note expression of CRF-BP mRNA in the VTA, but not in the SNC. Delimited areas in B and B′ are shown at higher magnification in C and C′. Note the lack of CRF-BP in the SNC and SNR (C′). MT. The scale bar shown in C″ is 625 μm for A–B′, 150 μm for A1 and A1′, and C and C′, and 50 μm for A2 and A2′.
Fig. 3.
Expression of VGluT2 mRNA in the SNC at the caudal level (–6.0 mm from bregma). Coronal sections were incubated with anti-TH antibodies and hybridized with antisense riboprobes for detection of VGluT2 (A–A″’) or CRF-BP (B–C′). (A) Section at low magnification showing TH immunoreactivity (TH-IR) under bright-field microscopy. The delimited area in A is shown at higher magnification under bright-field/epiluminescence microscopy (A′) and under epiluminescence microscopy (A″). Outlines demarcate the dorsal (SNCd) or the ventral (SNCv) portions of the SNC. The TH neurons cells are seen as brown cells, and the VGluT2 neurons as green grain aggregates (arrows in A′) under bright-field/epiluminescence microscopy. These VGluT2 neurons are seen as silver grain aggregates (arrows in A″) under epiluminescence microscopy. Long arrows indicate examples of VGluT2 neurons intermingled with TH neurons. Short arrows indicate examples of VGluT2 neurons intermingled with TH dendrites. VGluT2 neurons are seen in the SNCd, but not in the SNCv. (B and B′) At low magnification, note expression of CRF-BP mRNA in the VTA, but not in the SNC. Delimited areas in B and B′ are shown at higher magnification in C and C′. There is a lack of CRF-BP in the SNCd and SNCv (C′). ml. The scale bar shown in C′ is 625 μm for A–B′ and 150 μm for A′–C′.
Table 1.
Frequency of neurons expressing VGluT2 mRNA or TH in the SNC
| Bregma (mm) | Percentage of neurons expressing VGluT2 in the total counted neurons, mean ± SEM | Percentage of neurons expressing TH in the total counted neurons, mean ± SEM | TH neuron/VGluT2 neuron ratio |
|---|---|---|---|
| –5.16 to –5.40 (100%) (n = 2196) | 11.6 ± 1.7* (n = 249) | 88.3 ± 1.7 (n = 1950) | 7.8 |
| –5.52 to –6.12 (100%) (n = 4748) | 18.3 ± 1.1 (n = 879) | 81.7 ± 1.1 (n = 3870) | 4.4** |
| –6.24 to –6.60 (100%) (n = 2376) | 13.6 ± 2.8* (n = 419) | 86.3 ± 2.8 (n = 1960) | 4.7** |
| Total | 15.4 ± 1.0 (n = 1547) | 84.5 ± 1.0 (n = 7780) | 5.0 |
SEM, standard error of the mean. VGluT2 neurons and TH neurons were counted at three different levels of the SNC from five rats (11 sections at bregma –5.16 to –5.40 mm, 22 sections at bregma –5.52 to –6.12 mm, and 16 sections at bregma –6.24 to –6.60 mm).
P < 0.05 for comparison with the neuronal population counted at bregma –5.52 to –6.12 mm (Dunn's post hoc test).
P < 0.01 for comparison with the TH/VGluT2 neuron ratio found at bregma –5.16 to –5.40 mm (chi-square test).
n = counted neurons. Seven neurons were found to coexpress VGluT2 mRNA and TH in a total of 7780 counted TH neurons.
Table 2.
Frequency of neurons expressing VGluT2 mRNA or TH in the SNR
| Bregma (mm) | Percentage of neurons expressing VGluT2 in the total counted neurons, mean ± SEM | Percentage of neurons expressing TH in the total counted neurons, mean ± SEM | TH neuron/VGluT2 neuron ratio |
|---|---|---|---|
| –5.52 to –6.12 (100%) (n = 810) | 16.4 ± 2.2 (n = 133) | 83.6 ± 2.2 (n = 677) | 5.0 |
| –6.24 to –6.60 (100%) (n = 536) | 23.6 ± 3.0* (n = 129) | 76.4 ± 3.0 (n = 407) | 3.2** |
| Total | 19.0 ± 1.8 (n = 262) | 81.0 ± 1.8 (n = 1084) | 4.1 |
SEM, standard error of the mean. VGluT2 neurons and TH neurons were counted at two different levels of the SNR from five rats (22 sections at bregma –5.52 to –6.12 mm, and 16 sections at bregma –6.24 to –6.60 mm).
P < 0.05 for comparison with the neuronal population counted at bregma –5.52 to –6.12 mm (Mann–Whitney U-test).
P <0.01 for comparison with the TH/VGluT2 neuron ratio found at bregma –5.52 to –6.12 mm (chi-square test).
n = counted neurons. We did not find coexpression of VGluT2 mRNA and TH in a total of 1084 counted TH neurons.
Fig. 4.
Expression of VGluT2 mRNA in the SNR and SNL (–6.24 mm from bregma). Coronal sections were incubated with anti-TH antibodies and hybridized with VGluT2 antisense radioactive riboprobes. (A) Section at low magnification showing TH immunoreactivity under bright-field microscopy. The delimited area in the inset is shown at higher magnification under bright-field/epiluminescence microscopy (A) and under epiluminescence microscopy (A′). Delimited areas in A are shown at higher magnification under bright-field/epiluminescence microscopy (B and C) and under epiluminescence microscopy (B′ and C′). Note that several VGluT2 neurons are found in the dorsal portion of the SNL (B and B′) and in the dorsal portion of the SNR (C and C′). The scale bar shown in C′ is 4375 μm for the inset, 175 μm for A and A′, and 50 μm for B–C′.
Table 3.
Frequency of neurons expressing VGluT2 mRNA or TH in the SNL
| Bregma (mm) | Percentage of neurons expressing VGluT2 in the total counted neurons, mean ± SEM | Percentage of neurons expressing TH in the total counted neurons, mean ± SEM | TH neuron/VGluT2 neuron ratio |
|---|---|---|---|
| –5.16 to –5.40 (100%) (n = 264) | 11.6 ± 3.5 (n = 39) | 87.0 ± 3.5 (n = 229) | 5.9 |
| –5.52 to –6.12 (100%) (n = 1162) | 34.0 ± 3.8* (n = 420) | 65.5 ± 3.8 (n = 749) | 1.8** |
| –6.24 to –6.60 (100%) (n = 308) | 45.2 ± 4.5* (n = 151) | 53.3 ± 4.5 (n = 161) | 1.0** |
| Total | 30.5 ± 2.7 (n = 610) | 68.5 ± 2.7 (n = 1139) | 1.9 |
SEM, standard error of the mean. VGluT2 neurons and TH neurons were counted at three different levels of the SNL from five rats (11 sections at bregma –5.16 to –5.40 mm, 22 sections at bregma –5.52 to –6.12 mm, and 10 sections at bregma –6.24 to –6.60 mm).
P < 0.01 for comparison with the neuronal population counted at bregma –5.16 to –5.40 mm (Dunn's post hoc test).
P < 0.01 for comparison with the TH/VGluT2 neuron ratio found at bregma –5.16 to –5.40 mm (chi-square test).
n = counted neurons. Fifteen neurons were found to coexpress VGluT2 mRNA and TH in a total of 1139 counted TH neurons.
Neurons expressing VGluT2 mRNA in the RRF
VGluT2 neurons were intermingled with TH neurons at all rostrocaudal levels of the RRF (Fig. 5A and A′; Table 4). Within the RRF, the VGluT2 neurons showed a rostrocaudal increase in concentration. The number of VGluT2 neurons in relation to TH neurons was constant at all levels of the RRF, with an average ratio of one VGluT2 neuron per 1.7 TH neurons (Table 4). We found that only 0.2% of the TH neurons co-expressed VGluT2 mRNA in the RRF (two VGluT2–TH neurons in a total of 892 TH neurons and in a total of 521 VGluT2 neurons).
Fig. 5.
Expression of VGluT2 mRNA in the RRF (–6.48 mm from bregma) (radioactive in situ hybridization). Coronal sections were incubated with anti-TH antibodies and hybridized with VGluT2 antisense radioactive riboprobes. (A) TH neurons are seen as brown cells and VGluT2 neurons as green grain aggregates (arrows) under bright-field/epiluminescence microscopy. (A′) These VGluT2 neurons are seen as silver grain aggregates under epiluminescence microscopy (arrows). Note numerous VGluT2 neurons intermingled with TH immunoreactivity (brown cells) throughout the RRF. (A″) TH cells after silver grains were removed. The scale bar shown in B″ is 750 μm for A and A′, and 100 μm for B and B″.
Table 4.
Frequency of neurons expressing VGluT2 mRNA or TH in the RRF
| Bregma (mm) | Percentage of neurons expressing VGluT2 in the total counted neurons, mean ± SEM | Percentage of neurons expressing TH in the total counted neurons, mean ± SEM | TH neuron/VGluT2 neuron ratio |
|---|---|---|---|
| –6.36 (100%) (n = 259) | 30.2 ± 3.2 (n = 79) | 69.4 ± 2.9 (n = 181) | 2.3 |
| –6.48 (100%) (n = 376) | 37.0 ± 1.1* (n = 139) | 63.0 ± 1.1 (n = 237) | 1.7** |
| –6.60 (100%) (n = 776) | 38.3 ± 4.0* (n = 303) | 61.5 ± 4.0 (n = 474) | 1.6*** |
| Total | 36.0 ± 2.4 (n = 521) | 63.8 ± 2.4 (n = 892) | 1.7 |
SEM, standard error of the mean. VGluT2 neurons and TH neurons were counted at three different levels of the RRF (three sections from three rats at bregma –6.36 mm, two sections from two rats at bregma –6.48 mm, and six sections from three rats at bregma –6.60 mm).
No significant difference for comparison with the neuronal population counted at –6.36 mm bregma (Dunn's post hoc test).
No significant difference or
P < 0.05 for comparison with the TH/VGluT2 neuron ratio found at bregma –6.36 mm (chi-square test).
n = counted neurons. Two neurons were found to coexpress VGluT2 mRNA and TH in a total of 892 counted TH neurons.
In summary, we detected neurons expressing transcripts encoding VGluT2, but not VGluT1 or VGluT3, in all aspects of the substantia nigra and at all levels of the RRF (Fig. 6). The subpopulation of VGluT2-TH neurons previously found in the VTA appears to be absent in the SNC and RRF. Within the SNC, the VGluT2 neurons were confined to the laterodorsal portion of this structure; they were rarely observed in the rostral SNC, and had their highest concentration in the caudal SNC. In contrast, the RRF VGluT2 neurons were found in all rostrocaudal levels.
Fig. 6.
VGluT2 mRNA-expressing neurons; distribution and degree of colocalization with TH. Differential proportions of VGluT2 neurons (green bars), VGluT2–TH neurons (yellow bars) and TH neurons (pink bars) in the RRF, SNC, SNR, and SNL. Note the presence of VGluT2 neurons in all of these regions. The total number of neurons counted is indicated in each bar.
Discussion
The substantia nigra and the RRF have been identified as dopaminergic structures; however, the presence of non-dopaminergic neurons within these structures has been also documented, and some of these non-dopaminergic neurons utilize GABA as a signaling neurotransmitter (Nair-Roberts et al., 2008). We now provide evidence that neurons expressing VGluT2 are present in the SNC and RRF. These findings indicate that these structures also have glutamatergic neurons, underscoring the neuronal heterogeneity and functional complexity of these structures that are known mainly as dopaminergic structures.
Here, we used detection of transcripts encoding vesicular glutamate transporters as a reliable method to identify cell bodies of glutamatergic neurons. We found that glutamatergic neurons expressing transcripts encoding VGluT2, but not VGluT1 or VGluT3, are intermingled with TH neurons in the SNC and RRF. Our findings of VGluT2 neurons in the SNC and RRF (by radioactive riboprobes) are in contrast to findings of the lack of expression of VGluT2 mRNA in these two brain structures, in which non-radioactive riboprobes were applied (Nair-Roberts et al., 2008). These findings may be explained by differences in sensitivity between radioactive and non-radioactive in situ hybridization for the detection of VGluT2 transcripts within the midbrain dopaminergic system.
We found that, in contrast to the VTA, in which a subset of TH neurons express VGluT2, the TH neurons of the RRF (A8 neurons) and SNC (A9 neurons) lack VGluT2. These findings do not support the view that all dopaminergic neurons are glutamatergic neurons, as initially suggested by Kaneko et al. (1990) based on the detection of glutaminase in all catecholaminergic neurons. Later studies have shown that glutaminase is present in all types of cell (Márquez et al., 2009), so it cannot be considered to be a selective marker for glutamatergic neurons.
Glutamatergic neurons within the A9 region
The lack of VGluT2 mRNA in A9 cells indicates that glutamatergic neurons and dopaminergic neurons are two distinct subpopulations of neurons in the SNC, and does not support the idea that a single nigrostriatal dopaminergic neuron forms two chemically distinct synaptic classes, a dopaminergic class and an excitatory class, as originally suggested by Hattori et al. (1991).
In vivo intracellular recordings have shown that electrical stimulation of the SNC evokes excitatory postsynaptic potentials in striatal neurons (Wilson et al., 1982). In addition, the anterograde transport of 3H-labeled proteins from the SNC to the striatum and TH immunolabeling clearly demonstrate two types of synapse in the striatum: symmetric TH synapses (on spine necks), and putative excitatory asymmetric synapses lacking TH (on dendritic spine heads) (Hattori et al., 1991). The existence of nigrostriatal symmetric TH synapses and nigrostriatal asymmetric synapses lacking TH is in agreement with our results showing that the subpopulation of SNC glutamatergic neurons is distinct from the dopaminergic subpopulation. A role for VGluT2 neurons within a nigrostriatal glutamatergic pathway may be considered on the basis of the presence of VGluT2 neurons in the SNC together with the reported glutamatergic signaling by nigrostriatal afferents (Wilson et al., 1982), and the detection of nigrostriatal non-dopaminergic asymmetric synapses (Hattori et al., 1991). However, further studies are needed to determine whether or not the SNC VGluT2 neurons innervate the striatum or other brain structures.
Glutamatergic neurons within the A8 region
We found numerous cells expressing VGluT2 mRNA intermingled with TH neurons at all levels of the RRF, with the highest concentration at the caudal level. It has long been known that RRF dopaminergic neurons project to the dorsal striatum, olfactory tubercle, amygdala, nucleus of the stria terminalis, and piriform and enthorhinal cortex (Swanson, 1982; Deutch et al., 1988). In addition, the RRF innervates the parvicellular reticular formation (von Krosigk et al., 1992), and it has been proposed that, throughout this pathway, the RRF regulates orofacial motor functions (Arts et al., 1998). Recent tracing studies have demonstrated that all RRF cells projecting into the parvicellular reticular formation lack TH and receive GABAergic inputs from the central amygdala (Tsumori et al., 2010). These observations led to the suggestion of a central amygdala–RRF–parvicellular reticular formation pathway that controls orofacial movements related to emotional behavior, in which GABAergic neurons from the central amygdala inhibit non-dopaminergic neurons of the RRF, which, in turn, regulate premotor interneurons in the parvicellular reticular formation (Tsumori et al., 2010). As GABAergic (Nair-Roberts et al., 2008) and glutamatergic neurons are present in the RRF, it remains uncertain whether the non-dopaminergic neurons within the central amygdala–RRF–parvicellular reticular formation pathway are GABAergic or glutamatergic.
Glutamatergic neurons within the A10 region
Whereas most of the VGluT2 neurons lack TH markers in the lateral aspects of the A10 region, there is a small subpopulation that co-express TH in the midline aspects of this structure (Kawano et al., 2006; Yamaguchi et al., 2011). On the basis of in vitro analysis of nucleus accumbens preparations enriched in vesicles, it has been suggested that VGluT2 facilitates dopamine accumulation in synaptic vesicles, and that dopamine and glutamate are co-released from the same pool of vesicles (Hnasko et al., 2010). However, findings from high-resolution imaging of intact brain tissue indicate that VGluT2 and TH are not co-expressed in the same terminals in the nucleus of adult rats (Berube-Carriere et al., 2009; Moss et al., 2011). Although selective optical stimulation of afferents from TH neurons expressing channelrhodopsin elicits EPSCs in neurons within the nucleus accumbens (Tecuapetla et al., 2010; Stuber et al., 2010), it is unclear whether these afferents also co-release dopamine.
Thus, in addition to the VTA, the SNC and the RRF, which are classically recognized as dopaminergic structures, contain neurons with the ability to use glutamate for signaling. Future studies are necessary to determine the targets of SNC and RRF glutamatergic neurons, and to determine whether or not the SNC VGluT2 neurons account for a previously reported fast non-dopaminergic excitatory nigrostrial signaling.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse.
Abbreviations
- CRF-BP
corticotropin-releasing factor-binding protein
- EPSC
excitatory postsynaptic current
- PB
phosphate buffer
- PF
paraformaldehyde
- RRF
retrorubral field
- SNC
substantia nigra compacta
- SNL
substantia nigra lateralis
- SNR
substantia nigra reticulata
- TH
tyrosine hydroxylase
- VGlut1
vesicular glutamate transporter type 1
- VGlut2
vesicular glutamate transporter type 2
- VGlut3
vesicular glutamate transporter type 3
- VTA
ventral tegmental area
References
- Arts MP, Bemelmans FF, Cools AR. Role of the retrorubral nucleus in striatally elicited orofacial dyskinesia in cats: effects of muscimol and bicuculline. Psychopharmacology. 1998;140:150. doi: 10.1007/s002130050752. [DOI] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine–glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J. Comp. Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Baldino F,, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann. N. Y. Acad. Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J. Comp. Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Törk I. Comparative anatomy of the ventromedial mesencephalic tegmentum in the rat, cat, monkey and human. J. Comp. Neurol. 1986;252:423–445. doi: 10.1002/cne.902520402. [DOI] [PubMed] [Google Scholar]
- Hattori T, Takada M, Moriizumi T, Van der Kooy D. Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J. Comp. Neurol. 1991;309:391–401. doi: 10.1002/cne.903090308. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J. Comp. Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Márquez J, Tosina M, de la Rosa V, Segura JA, Alonso FJ, Mates JM, Campos-Sandoval JA. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem. Int. 2009;55:64–70. doi: 10.1016/j.neuint.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur. J. Neurosci. 2011;33:1205–1211. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Phillipson OT. A Golgi study of the ventral tegmental area of Tsai and interfascicular nucleus in the rat. J. Comp. Neurol. 1979;187:99–115. doi: 10.1002/cne.901870107. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. The cytoarchitecture of the interfascicular nucleus and ventral tegmental area of Tsai in the rat. J. Comp. Neurol. 1979;187:85–98. doi: 10.1002/cne.901870106. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J. Comp. Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J. Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumori T, Qin Y, Yokota S, Niu JG, Yasui Y. Central amygdaloid axon terminals are in contact with retrorubral field neurons that project to the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res. 2010;1306:18–28. doi: 10.1016/j.brainres.2009.09.118. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience. 1992;50:531–549. doi: 10.1016/0306-4522(92)90445-8. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J. Comp. Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp. Brain Res. 1982;45:157–167. doi: 10.1007/BF00235775. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]