Abstract
Introduction
Differentiated paediatric epithelial cells can be used to study the role of epithelial cells in asthma. Nasal epithelial cells are easier to obtain and may act as a surrogate for bronchial epithelium in asthma studies. We assessed the suitability of nasal epithelium from asthmatic children to be a surrogate for bronchial epithelium using air-liquid interface cultures.
Methods
Paired nasal and bronchial epithelial cells from asthmatic children (n = 9) were differentiated for 28 days under unstimulated and IL-13-stimulated conditions. Morphological and physiological markers were analysed using immunocytochemistry, transepithelial-electrical-resistance, Quantitative Real-time-PCR, ELISA and multiplex cytokine/chemokine analysis.
Results
Physiologically, nasal epithelial cells from asthmatic children exhibit similar cytokine responses to stimulation with IL-13 compared with paired bronchial epithelial cells. Morphologically however, nasal epithelial cells differed significantly from bronchial epithelial cells from asthmatic patients under unstimulated and IL-13-stimulated conditions. Nasal epithelial cells exhibited lower proliferation/differentiation rates and lower percentages of goblet and ciliated cells when unstimulated, while exhibiting a diminished and varied response to IL-13.
Conclusions
We conclude that morphologically, nasal epithelial cells would not be a suitable surrogate due to a significantly lower rate of proliferation and differentiation of goblet and ciliated cells. Physiologically, nasal epithelial cells respond similarly to exogenous stimulation with IL-13 in cytokine production and could be used as a physiological surrogate in the event that bronchial epithelial cells are not available.
Introduction
Airway epithelial cells not only provide a physical barrier to potentially harmful insults but they also play a significant role in the first line of immunological defence. There is increasing evidence that allergic diseases such as asthma are associated with epithelial disorders and, furthermore, that a primary abnormality of the airway epithelium may be central to disease causation [1], [2]. Chronic inflammation and airway remodelling are the main characteristics of asthma however they have been observed to occur in young children before asthma has become firmly established [3], [4]. In asthma, bronchial epithelial airway remodelling is characterised by goblet cell hyperplasia, reduced ciliated cell numbers and mucus hypersecretion [5], defective repair and proliferation [6], increased basal cell number [7] and impaired barrier function [8], [9].
Differentiated ALI cultures using the Transwell system have recently been shown to be an authentic model representing the airway epithelium ex vivo [10]. Differentiated ALI cultures from healthy subjects displayed a polarised pseudostratified multi-layered epithelium comprising basal, ciliated and goblet cells whereas cultures from asthmatic subjects displayed a dysfunctional epithelium consistent with the asthmatic airway in vivo [5]. The cultures closely mimic in vivo airway epithelial physiology in terms of cilia coverage and cilial beating, mucus production and formation of intact tight junctions [5], [11].
Samples from the lower airways in children can be obtained at the time of clinically indicated bronchoscopy but it is difficult to justify sampling the lower airways in otherwise healthy control children and in those with milder disease. The nasal epithelium therefore represents an attractive alternative although it is unclear how well the nasal epithelium represents the bronchial epithelium in paediatric asthma. According to the ‘united airway concept’ there is a close connection between the upper and lower airways [12]–[16]. This suggests that changes found in the nasal epithelium might mirror similar changes occurring in the lower airways. The link between allergic rhinitis and asthma has been underlined by epidemiological and clinical studies [12]–[16] which suggest that upper airway inflammation may reflect and provide an additional insight into lower airway involvement. Devalia et al have demonstrated that adult human nasal and bronchial epithelial cells cultured in vitro resemble the cells in vivo [17]. These studies involved using samples from adults and extrapolating data from adult studies cannot accurately represent the paediatric epithelium, especially since functional differences exist between adult and paediatric epithelium [18]. Several studies have shown similar morphology and response to cytokine stimulation between nasal and bronchial epithelium using submerged monolayer cultures, which only poorly represents the epithelium [19], [20]. Differentiated ALI cultures on the other hand are more authentic and can be analysed for markers of differentiation including MUC5AC (the major mucus-forming mucin) and SAM-pointed domain containing Ets-transcription factor (SPDEF) (a transcription factor in the goblet cell hyperplasia pathway) production as well as for goblet and ciliated cells and tight junction formation. As a result the differentiated ALI culture model is the most appropriate platform with which to conduct a comparison of paired nasal and bronchial differentiated ALI cultures.
In this study we aimed to compare the morphological and physiological profiles of nasal differentiated ALI cultures (PNECs) with bronchial differentiated ALI cultures (PBECs) under basal unstimulated and IL-13 stimulated conditions to determine the ability of PNECs to act as an in vitro surrogate for PBECs in asthma studies.
Methods
Subjects
Children less than 12 years of age (mean age 7.2 years [range: 1 to 12 years]) attending elective surgical procedures at the Royal Belfast Hospital for Sick Children were recruited. A doctor administered pro-forma was used to record the clinical history. Of the nine children with atopic asthma, defined as recurrent wheezing within the last year, 5 children had asthma plus allergic rhinitis and 4 children had asthma plus eczema but no allergic rhinitis (Table 1).
Table 1. Patient details including clinical status and clinical atopy.
| Clinical Status | Clinical Atopy (assessed by questionnaire) | Serum IgE Concentration (kU/L) | Age (y) | Gender (M/F) | Treatment |
| Asthmatic | Allergic Rhinitis, Allergic Conjunctivitis | 2258 | 6 | M | ICS/LABA, LTA |
| Asthmatic | Eczema | 154 | 7 | M | ICS/LABA |
| Asthmatic | Eczema | 446 | 4 | F | SABA, when required |
| Asthmatic | Eczema, Allergic Rhinitis | 5158 | 12 | M | ICS/LABA |
| Asthmatic | Allergic Rhinitis | 101 | 9 | M | ICS/LABA |
| Asthmatic | Eczema | 10 | 2 | M | ICS, SABA |
| Asthmatic | Eczema | 627 | 7 | M | ICS/LABA |
| Asthmatic | Eczema, Allergic Rhinitis | 100 | 11 | M | ICS |
| Asthmatic | Allergic Rhinitis | 40 | 1 | M | ICS |
Current treatment abbreviations are classed as follows: inhaled corticosteroid (ICS), long-acting beta agonist (LABA), short-acting beta agonist (SABA) and leukotriene antagonist (LTA).
Ethics Statement
Written informed parental consent was obtained. This study was approved by the Office of the Research Ethics Committees of Northern Ireland (ORECNI).
Isolation of PBECs and PNECs
Primary bronchial epithelial cells were obtained from asthmatic children as previously described [21]. Nasal brushings were performed by rotating an endocervical brush in each nostril. Asthmatic PBECs and asthmatic PNECs were cultured as previously described [5]. All brush washings were analysed for viruses using a multi-viral PCR analysis and only uncontaminated cultures were used [22].
Differentiated ALI culture
ALI cultures (n = 9) were grown as previously described [5], [11]. Briefly, cells from subjects used in this study were grown at ALI at passage 2 for 28 days. At confluence, ALI was created by removing the apical medium and restricting the culture feeding to the basolateral compartment. The culture medium was changed on alternate days during which the cells differentiated over 28 days to ensure full differentiation as assessed by the presence of beating cilia and mucus on the apical surface of the cultures. We sampled 12 patients in total however 3 of the cultures either did not proliferate due to lack of cells from the initial sample or they did not form a 100% confluent monolayer prior to differentiation and so were rejected from the study.
Stimulation of PBECs and PNECs with IL-13
Following the establishment of ALI, cells were fed basolaterally on alternate days with ALI medium [5] supplemented with recombinant human IL-13 (PeProTech EC Ltd, UK) at 20 ng/ml throughout the duration of the cultures (28 days) starting at day 0 ALI [11], [23]–[30].
Transepithelial Electrical Resistance measurements (TEER)
We used TEER as a measure of ‘tight junction’ formation in epithelial cultures [31]. TEER was measured weekly using an EVOM meter (World Precision Instruments, FL, USA) [5], [11].
Immunocytochemistry (ICC) for goblet and ciliated cell markers
Cytospin slides were made for the detection of goblet and ciliated cells from PBEC and PNEC cultures [5], [11]. Negative controls were subjected to routine conditions with the omission of the primary antibody. The results are expressed as the percentage differential goblet or ciliated cell count corrected for cell number from 3 slides per stain per insert per patient.
RNA extraction and Quantitative Real-time PCR for MUC5AC and SPDEF mRNA
RNA extraction and cDNA synthesis were carried out using the RNeasy Mini kit (Qiagen, Crawley, UK) and First Strand cDNA Synthesis Kit (AMV) (Roche, UK) according to the manufacturer's protocol. Quantitative Real time PCR was carried out as previously described [11].
Measurement of MUC5AC secreted apically using ELISA
Production of MUC5AC secreted mucin in the apical washes from PBECs and PNECs was measured as previously described [5], [11] using an adapted in-house MUC5AC ELISA [32]. We used mouse monoclonal antibody to MUC5AC [45M1] clone (Abcam, UK) as the primary antibody and Goat anti-mouse IgG HRP secondary antibody (Jackson Laboratories, USA) for detection of primary antibody binding. Results are expressed as the optical density corrected for MUC5AC positive control.
Cytokine Analysis
Apical washings and basolateral supernatants from unstimulated and IL-13-stimulated PBECs and PNECs were analyzed using a 27-plex bead array assay (Bio-Plex Pro Human Cytokine 27-plex) (BioRad, UK) as per manufacturers' instructions. Results are only shown for analytes that were within the limits of detection (IL-1rα, IL-6, IL-7, IL-8, IL-12p70, granulocyte colony–stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), regulated upon activation, normal T cell expressed and secreted (RANTES) and vascular endothelial growth factor (VEGF).
Statistical Analysis
Comparisons between PBECs and PNECs were made using paired t-tests and repeated measures ANOVA, with logarithmic transformation where appropriate. Cytokine concentrations were corrected for total cell number (pg/cell) and then the fold change in cytokine secretion following IL-13 stimulation was compared with unstimulated cytokine secretion (IL-13-stimulated/unstimulated). These results were then plotted on a log axis due to the varying ranges of all analytes involved. Individual cytokine profiles are included as supplemental data (Figures S1, S2, S3, S40, S5, S6) along with additional detail on all of the mentioned methods in the online data supplement (Methods S1).
Results
It is unlikely given the number of passages the cells go through along with the length of time from sampling to completion of culture (7–10 weeks) that there would be any carry-over effect of inhaled corticosteriods in the following results.
TEER
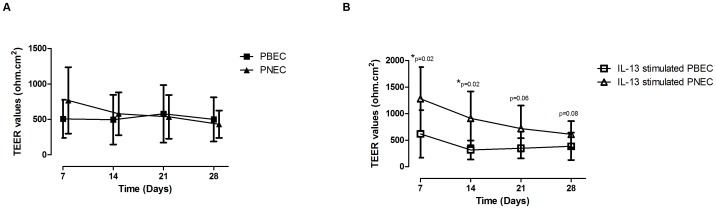
We detected no overall differences in TEER values over time under unstimulated conditions between PBECs and PNECs [Figure 1A]. In contrast, following IL-13 stimulation, there was a significant difference in TEER values on days 7 (p = 0.02) & 14 (p = 0.02) between PBECs and PNECs with the difference in resistance becoming similar by days 21 & 28 [Figure 1B].
Figure 1. Measurement of Transepithelial Electrical Resistance (TEER).
A - TEER of unstimulated PNECs and PBECs cultured for 28 d. Values are expressed as mean ± SD. No significant difference was observed in the TEER values between the groups. B - TEER of PNECs and PBECs cultured with 20 ng/ml IL-13 for 28 d. Values are expressed as mean ± SD. On days 7 and 14 there was a significant difference in TEER values between PNECs and PBECs (p<0.02 for each time point) however by the end of the culture period the TEER values were similar between groups.
Total Cell Count
On day 28 we found a significantly higher total cell number between PBECs [mean 5.7×105 cells/ml (SD 1.2)] and PNECs [mean 3.4×105 cells/ml (SD 0.8), (p = 0.002)] under unstimulated conditions [Table 2]. However there was no significant difference in the total cell number under IL-13 stimulated conditions [PBECs [mean 5.3×105 cells/ml (SD 0.9); PNECs: mean 4.2×105 cells/ml (SD 1.1)] [Table 2]. All subsequent results have been adjusted for total cell number.
Table 2. Total cell count on day 28 of ALI culture.
| Sample | Mean number of cells (×105 cells/ml) | Standard Deviation | P value | Treatment |
| PBEC(A) | 5.7 | 1.2 | 0.002 | Unstimulated |
| PNEC(A) | 3.4 | 0.8 | Unstimulated | |
| PBEC(A) | 5.3 | 0.9 | >0.05 | 20 ng/ml IL-13 |
| PNEC(A) | 4.2 | 1.1 | 20 ng/ml IL-13 |
Under unstimulated conditions there were significant differences between PBECs and PNECs (p = 0.002). However under IL-13 stimulated conditions there were no significant differences between the same groups.
Goblet Cell Quantification
IL-13 stimulation resulted in a similar increase in the percentage of goblet cells in both PBECs [43.3% (SD 18.1), p = 0.0036] and PNECs [19.4% (SD 4.0), p = 0.0001] compared with unstimulated controls [PBEC: 27.1% (SD 18.4); PNEC: 8.4% (SD 2.4)] [Figure 2]. In addition there was a significant difference in goblet cell percentage between PNEC unstimulated and PBEC unstimulated [8.4% (SD 2.4) versus 27.1% (SD 18.4) respectively, p = 0.033] and PNEC IL-13 stimulated and PBEC IL-13 stimulated [19.4% (SD 4.0) versus 43.3% SD (18.1) respectively, p = 0.009].
Figure 2. Quantification of Goblet Cell Number.
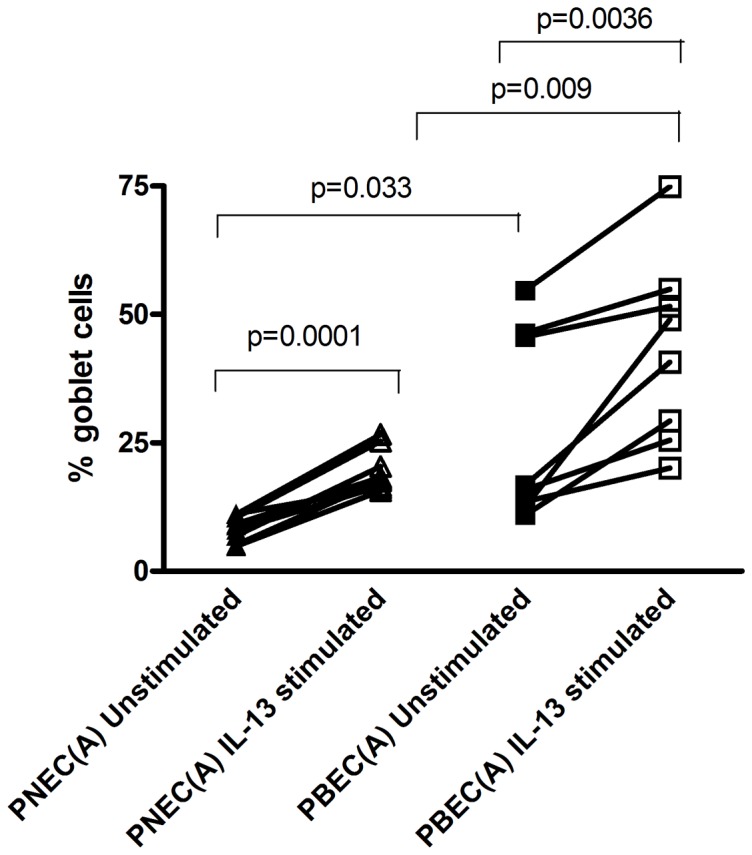
Number of goblet cells from PNECs and PBECs treated with 20/ml IL-13 expressed as the percentage differential goblet cell count corrected for cell number on d 28 of ALI culture. Comparisons of average values between groups were performed using paired t-tests. There was a significant difference seen between unstimulated and IL-13 stimulated PNECs (p = 0.0001) and in IL-13 stimulated PBECs when compared to unstimulated PBECs (p = 0.0036). Additionally, there was a significant difference between PNEC unstimulated and PBEC unstimulated (p = 0.033) and when cells where stimulated with IL-13, the percentage of goblet cells was significantly higher in stimulated PBECs compared to stimulated PNECs (p = 0.009).
Real Time PCR
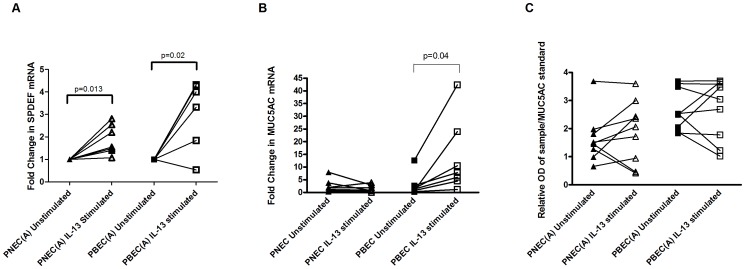
SPDEF mRNA was higher in IL-13 stimulated PNECs (p = 0.013) and IL-13 stimulated PBECs (p = 0.02) compared with unstimulated PNECs and PBECs demonstrating a similar response to exogenous stimulation between PBECs and PNECs [Figure 3A]. However under IL-13 stimulation there was a similar level of MUC5AC mRNA between stimulated and unstimulated PNECs whereas IL-13 stimulated PBECs had higher levels of MUC5AC mRNA than unstimulated PBECs (p = 0.04) [Figure 3B].
Figure 3. Gene expression of SPDEF & MUC5AC mRNA and ELISA for MUC5AC mucin secretion.
A - Gene expression of SPDEF mRNA using comparative quantitation real time PCR in IL-13 stimulated PNECs and PBECs expressed as fold change compared to unstimulated cells. In both cell types, stimulation with IL-13 caused a significant increase in SPDEF mRNA levels (PNECs: p = 0.013; PBECs: p = 0.02). B - Gene expression of MUC5AC mRNA using comparative quantitation real time PCR in IL-13 stimulated PNECs and PBECs expressed as fold change compared to unstimulated cells. There was no significant increase in MUC5AC mRNA levels in PNECs, however, stimulation with IL-13 caused a significant increase in MUC5AC mRNA levels in PBECs (p = 0.04). C - Relative optical density (OD λ = 450 nm) of MUC5AC secreted apically using ELISA corrected for MUC5AC positive control. There was no significant difference between unstimulated and IL-13 stimulated PNECs or PBECs.
Measurement of MUC5AC secreted apically using ELISA
Under IL-13 stimulation similar quantities of apically secreted mucin were measured between PNECs and PBECs [Figure 3C].
Ciliated Cell Quantification
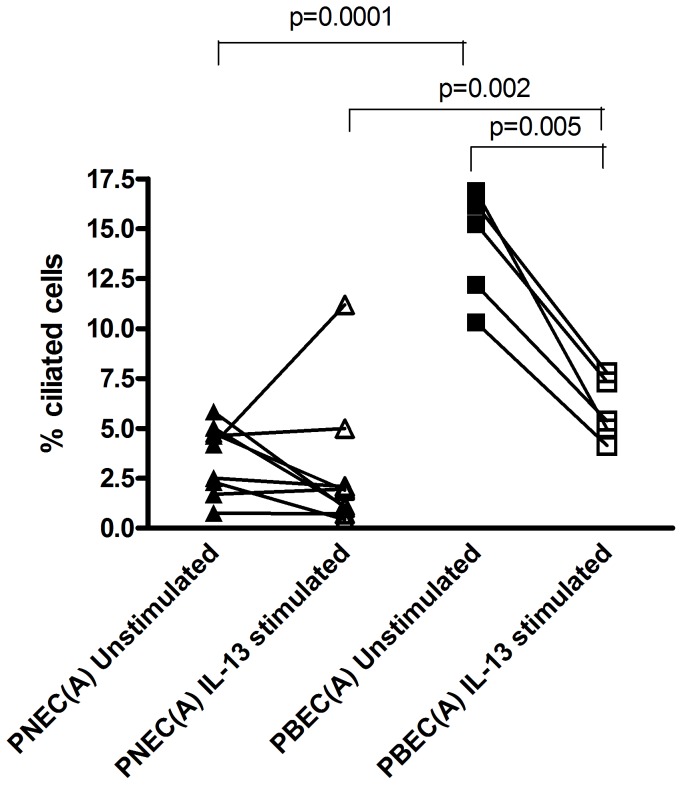
IL-13 stimulated PBECs had lower ciliated cell numbers than unstimulated PBECs [5.9% (SD 1.6) versus 14.8% (SD 2.5) respectively, p = 0.005] which was not observed between PNEC IL-13 stimulated and PNEC unstimulated [2.8% (SD 3.4) versus 3.5% (SD 1.7) respectively] [Figure 4]. There was a significant difference between PBEC unstimulated and PNEC unstimulated [14.8% (SD 2.5) versus 3.5% (SD 1.7) respectively, p = 0.0001] [Figure 4]. In addition there was a significant difference between PBEC IL-13 stimulated and PNEC IL-13 stimulated [5.9% (SD 1.6) versus 2.8% (SD 3.4) respectively, p = 0.002] [Figure 4].
Figure 4. Quantification of Ciliated Cells.
Number of ciliated cells from PNECs and PBECs treated with 20/ml IL-13 expressed as the percentage differential ciliated cell count corrected for cell number on d 28 of ALI culture. Comparisons of average values between groups were performed using paired t-tests. In unstimulated conditions, PBEC cultures contained a significantly higher percentage of ciliated cells than PNECs (p = 0.0001). However, when stimulated with IL-13, the percentage of ciliated cells significantly decreased in PBECs (p = 0.005), but IL-13 had no effect on ciliated cell numbers in PNECs. Additionally, IL-13 stimulated PBECs had significantly higher numbers of ciliated cells compared with IL-13 stimulated PNECs (p = 0.002).
Cytokine production from PBECs and PNECs
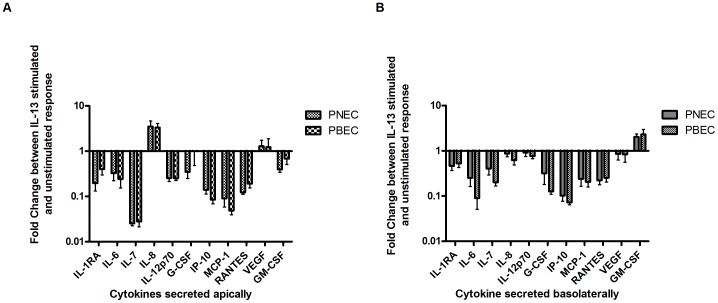
Analytes that were within the limits of detection were IL-1rα, IL-6, IL-7, IL-8, IL-12p70, granulocyte colony–stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), regulated upon activation, normal T cell expressed and secreted (RANTES) and vascular endothelial growth factor (VEGF). The fold change of apically secreted cytokines after IL-13 stimulation [Figure 5A] showed there to be no significant difference between PBECs and PNECs. Similarly, there was no significant difference in basolaterally secreted cytokines between PBECs and PNECs stimulated with IL-13 [Figure 5B] demonstrating an overall similar response to IL-13 stimulation between PNECs and PBECs. Individual cytokine secretion graphs are included in the supplementary data section of this paper.
Figure 5. Cytokine Analysis of Apical Washings and Basolateral Supernatants using Bioplex.
A – Apical secretion of cytokines in PBEC and PNEC cultures. Results were corrected for cell numbers and a fold change ratio was calculated (IL-13 stimulated/unstimulated). In order to graphically represent all detectable cytokines the results were plotted on a logarithmic axis. There were no significant differences in any of the detected cytokines secreted between PNECs and PBECs. B - Basolateral secretion of cytokines in PBEC and PNEC cultures. Results were corrected for cell number and a fold change ratio was then calculated (IL-13 stimulated/unstimulated). In order to graphically represent all detectable cytokines the results were plotted on a logarithmic axis. There were no significant differences in any of the cytokines secreted between PNECs and PBECs.
Discussion
Nasal epithelial cells provide an attractive alternative to bronchial epithelial cells for use in asthma research due to their accessibility without the need for anaesthesia and intubation however it is unclear whether differentiated nasal ALI cultures represent the differentiated bronchial ALI cultures morphologically and physiologically. We have compared paired nasal and bronchial epithelial cells differentiated at ALI from asthmatic children in order to determine whether nasal epithelial cells can act as a surrogate for bronchial epithelial cells. We have found that PNECs exhibit different morphological features such as lower proliferation rates and differentiation of goblet and ciliated cells compared with PBECs however their physiological response of secreted cytokines to exogenous stimulation with IL-13, a key cytokine in the pathogenesis of asthma is strikingly similar when corrected for cell number.
In considering morphology, unstimulated PNECs do not exhibit the constitutive goblet cell hyperplasia seen in unstimulated PBECs [5]. This is to be expected as asthma is a lower airways disease. Mouse models of allergic rhinitis have reported that a goblet cell hyperplasia may only exist following stimulation with ovalbumin [33], [34] or TGF-β [35]. IL-13 has been shown to increase goblet cell hyperplasia in non-asthmatic and asthmatic paediatric bronchial epithelium [11] and in this study we have shown that PNECs also respond with a goblet cell increase to IL-13 stimulation along with a significant increase in SAM-pointed domain-containing Ets-like factor (SPDEF) mRNA which is implicated in the goblet cell hyperplasia pathway [36]. Miyahara and colleagues found that although IL-13 is a major contributor to the late nasal response it did not induce a goblet cell hyperplasia [37] however our data is at odds with this finding. The IL-13 mediated reduction of ciliated cells in PBECs is not observed with IL-13 stimulation in PNECs however.
In a previous study by McDougall et al. which compared bronchial and nasal epithelial monolayers from adults and children they found the cells to look morphologically similar [19]. However, differentiated nasal ALI cultures display notable morphological differences when compared with the differentiated bronchial ALI cultures which could not have been detected in submerged monolayer cultures. We found that PNECs did not proliferate or differentiate at the same rate as PBECs with a significant lower total cell number at the end of the culture period in addition to the differences seen in goblet and ciliated cells under basal and stimulated conditions. PBECs appeared to exhibit consistent distinct morphological changes in response to IL-13 stimulation whereas PNECs responded in a variable manner. We did however observe some similarities between PBECs and PNECs: TEER measurements, which is at odds with the TEER data generated by Lopez-Souza and colleagues [38] and MUC5AC secretion under unstimulated conditions. Taken together, morphologically there does not appear to be enough consistency between PBECs and PNECs which would suggest that for studies focussing on the morphology of the asthmatic bronchial epithelium, PNECs would not serve as an appropriate surrogate.
In considering physiological response we examined the effects of an exogenous cytokine, namely IL-13, on cytokine/mediator release from PBECs and PNECs and found a similar response profile when corrected for cell number. McDougall et al. found that constitutive and cytokine-stimulated release of particular cytokines (IL-6, IL-8, MMP-9 and RANTES) was significantly higher in nasal monolayer than bronchial monolayer cultures but that on further analysis nasal and bronchial epithelial cells responded in the same way showing significant correlation [19]. In a follow-up study from the same group, looking specifically at mediator release between nasal and bronchial epithelial monolayer cultures from children, Pringle and colleagues found that RANTES, MMP-9, TIMP-1, MCP-1 and VEGF were similar between nasal and bronchial monolayer cultures whereas IL-6, IL-8 and G-CSF were higher [20]. The difference in observations of MMP-9 and RANTES between the studies is proposed to be due to the first study having a mixed population of adult and paediatric epithelial cells. We demonstrated a similar finding to McDougall et al. and Pringle et al. in those cytokines from our panel that were detectable. Both PNECs and PBECs responded to IL-13 stimulation (corrected for cell numbers) in a similar manner, albeit with PNECs generally expressing higher levels of cytokine. This would suggest that physiologically for the cytokines measured, PNECs could be used as a surrogate for PBECs. Further studies would hope to confirm this observation however it would suggest that the similarities seen in monolayer mediator release are maintained throughout the differentiation process.
In a more recent study using well-differentiated paediatric asthmatic and non-asthmatic paired bronchial and nasal epithelial cells, Lopez-Guisa and colleagues also suggested that there was correlation in mediator release between PBEC and PNEC cultures [39]. This group showed TGF-β2 and VEGF to be significantly increased in asthmatic bronchial and nasal epithelium compared to non-asthmatic under unstimulated conditions. TGF-β2 and periostin were significantly up-regulated in nasal and bronchial asthmatic epithelium under unstimulated and following IL-4/IL-13 stimulation [39]. Unfortunately our study did not have a sample of non-asthmatic children to perform a similar comparison. However, our study broadly agrees with Lopez-Guisa et al. in terms of the similarities observed in mediator release between nasal and bronchial asthmatic epithelium.
IL-13 resulted in the reduction of the majority of cytokines, with the exceptions of IL-8 and VEGF, when secreted apically and basolaterally and GM-CSF when secreted basolaterally. Surprisingly, a number of cytokines that we expected to increase actually decreased including IL-1ra, GM-CSF and MCP-1 [40]–[43]. While there are various studies that have noted increases in these cytokines under stimulation with IL-13 we would add that many used different culture systems, stimulation concentrations and treatment regimens compared to our study, which may go some way to explaining the unexpected decrease in these mediators. We believe this is a true reflection of the epithelium as in both PBECs and PNECs we saw strikingly similar responses to our chronic stimulation.
Our data confirm that characteristics and responses in submerged monolayer cultures differ from differentiated ALI cultures. We believe that our differentiated ALI model authentically represents the true state of the epithelium in vivo [10]. A recent study by Ogilvie and colleagues, who compared paired CF nasal and bronchial epithelial brushings using microarray, found that differences in the global gene expression profiles of CF and non-CF nasal and bronchial epithelial cells existed. They recommended not using nasal epithelial cells as a surrogate for pre-screening prior to lung-directed therapies [44]. Whether nasal epithelium should be used in place of bronchial epithelium will inevitably be determined by the focus and design of future studies and with a growing literature on the many comparisons now taking place, we will be much better informed of the benefits or drawbacks of using nasal as a surrogate for bronchial epithelial cells.
Our study was limited in a number of ways. Firstly, we were unable to sample a large enough number of non-asthmatic controls to perform a comparison between health and disease. Secondly, in terms of stimulation only one cytokine (IL-13) was used due to the quantity of cells available. It would be interesting to know if other stimuli in PNECs reflect the response in PBECs. Additional studies are required to further explore the use of nasal epithelial cells as a surrogate.
In conclusion, we have demonstrated that our models of differentiated PNECs and PBECs display notable morphological differences which would question the use of PNECs as a reliable and reproducible morphological surrogate, especially in asthma where characteristic traits such as constitutive goblet cell hyperplasia are absent in PNECs from asthmatics. However, we have also demonstrated that physiologically, both PNECs and PBECs respond in the same way to stimulation with IL-13 suggesting that PNECs could be used as a physiological surrogate for PBECs in asthmatic studies in the event that bronchial epithelial cells are not available.
Supporting Information
Cytokine analysis of IL-1ra, IL-6, IL-7 & IL-8 secreted apically. A - Analysis of IL-1ra secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-1ra compared with unstimulated PNECs (p = 0.02) and PBECs (p = 0.001). Additionally unstimulated PNECs secreted higher levels of IL-1ra compared with unstimulated PBECs (p = 0.04). B - Analysis of IL-6 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-6 compared with unstimulated PNECs (p = 0.02) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted higher levels of IL-6 compared with unstimulated PBECs (p = 0.04). C - Analysis of IL-7 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-7 compared with unstimulated PNECs (p = 0.0003) and PBECs (p = 0.0004). Additionally unstimulated PNECs secreted higher levels of IL-7 compared with unstimulated PBECs (p = 0.04). D - Analysis of IL-8 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted higher levels of IL-8 compared with unstimulated PNECs and PBECs however they did not reach statistical significance. Additionally IL-13 stimulated PNECs secreted higher levels of IL-8 compared with IL-13 stimulated PBECs (p = 0.03).
(TIF)
Cytokine analysis of IL-12p70, G-CSF, IP-10 & MCP-1 secreted apically. A - Analysis of IL-12p70 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-12p70 compared with unstimulated PNECs (p = 0.007) and PBECs (p = 0.0009). Additionally unstimulated PNECs secreted higher levels of IL-12p70 compared with unstimulated PBECs although this was not statistically significant (p = 0.08). B - Analysis of G-CSF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly less G-CSF compared with unstimulated PNECs (p = 0.03). There was no significant difference between IL-13 stimulated and unstimulated PBECs. Additionally unstimulated PNECs secreted higher levels of G-CSF compared with unstimulated PBECs although this was not statistically significant. C - Analysis of IP-10 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of IP-10 compared with unstimulated PNECs (p = 0.006) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted significantly higher levels of IP-10 compared with unstimulated PBECs (p = 0.047) and IL-13 stimulated PNECs secreted higher levels of IP-10 compared with IL-13 stimulated PBECs (p = 0.04). D - Analysis of MCP-1 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of MCP-1 compared with unstimulated PNECs (p = 0.005) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted significantly higher levels of IP-10 compared with unstimulated PBECs (p = 0.001).
(TIF)
Cytokine analysis of RANTES, VEGF & GM-CSF secreted apically. A - Analysis of RANTES secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of RANTES compared with unstimulated PNECs (p = 0.003) and PBECs (p = 0.049). Additionally unstimulated PNECs secreted significantly higher levels of RANTES compared with unstimulated PBECs (p = 0.047). B - Analysis of VEGF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. C - Analysis of GM-CSF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted lower levels of GM-CSF compared with unstimulated PNECs (p = 0.009). There was no significant change in GM-CSF between IL-13 stimulated and unstimulated PBECs. Additionally unstimulated PNECs secreted significantly higher levels of GM-CSF compared with unstimulated PBECs (p = 0.01).
(TIF)
Cytokine analysis of IL-1ra, IL-6, IL-7 & IL-8 secreted basolaterally. A - Analysis of IL-1ra secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly less IL-1ra compared with unstimulated PNECs (p = 0.04). There was no significant difference between IL-13 stimulated and unstimulated PBECs. Additionally there was no difference in IL-1ra secretion between PNECs and PBECs. B - Analysis of IL-6 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted less IL-6 compared with unstimulated PNECs however, this did not reach statistical significance. There was a significant difference in IL-6 secretion between IL-13 stimulated and unstimulated PBECs (p = 0.04). Additionally the IL-13 stimulated PNECs secreted higher levels of IL-1ra compared with IL-13 stimulated PBECs (p = 0.04). C - Analysis of IL-7 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted less IL-7 compared with unstimulated PNECs (p = 0.06) and PBECs (p = 0.05) but these did not reach statistical significance. Additionally there was no significant difference in IL-7 secretion between PBECs and PNECs. D - Analysis of IL-8 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference in IL-8 secretion between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs.
(TIF)
Cytokine analysis of IL-12p70, G-CSF, IP-10 & MCP-1 secreted basolaterally. A - Analysis of IL-12p70 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs. However unstimulated PNECs secreted higher levels of IL-12p70 compared with unstimulated PBECs (p = 0.009). B - Analysis of G-CSF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs although there was a large decrease in concentration. However IL-13 stimulated PNECs secreted higher levels of IL-12p70 compared with IL-13 stimulated PBECs (p = 0.048). C - Analysis of IP-10 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs. However unstimulated PNECs secreted higher levels of IP-10 compared with unstimulated PBECs (p = 0.009). D - Analysis of MCP-1 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was lower MCP-1 secretion between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs however this did not reach statistical significance. Unstimulated PNECs secreted significantly higher levels of MCP-1 compared with unstimulated PBECs (p = 0.003) as did IL-13 stimulated PNECs compared with IL-13 stimulated PBECs which was close to reaching significance (p = 0.05).
(TIF)
Cytokine analysis of RANTES, VEGF & GM-CSF secreted basolaterally. A - Analysis of RANTES secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. B - Analysis of VEGF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. C - Analysis of GM-CSF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly more GM-CSF compared with unstimulated PNECs (p = 0.039). Additionally there was no significant difference in GM-CSF secretion between IL-13 stimulated and unstimulated PBECs or between PBECs and PNECs.
(TIF)
Full descriptions of each method used in the study are provided in the supplementary methods section.
(DOC)
Acknowledgments
The authors would like to thank the parents and children involved for facilitating this study along with Prof. Madeleine Ennis and Dr. Bettina Schock for their critical comments on this manuscript.
Funding Statement
This study was funded by Northern Ireland Chest Heart and Stroke Association and Surendran Thavagnanam was employed on an Irish Thoracic Soceity/Boehringer Ingleheim Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cookson W (2004) The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 4 12: 978–988. [DOI] [PubMed] [Google Scholar]
- 2. Holgate ST (2011) The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 242 1: 205–219. [DOI] [PubMed] [Google Scholar]
- 3. Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, et al. (2007) Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 176 9: 858–864. [DOI] [PubMed] [Google Scholar]
- 4. Thavagnanam S, Williamson G, Ennis M, Heaney LG, Shields MD (2010) Does airway allergic inflammation pre-exist before late onset wheeze in children? Pediatr Allergy Immunol 21 7: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 5. Parker J, Sarlang S, Thavagnanam S, Williamson G, O'Donoghue D, et al. (2010) A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates un-stimulated morphological differences between asthmatic and non-asthmatic cells. Ped Res 67: 17–22. [DOI] [PubMed] [Google Scholar]
- 6. Puddicombe SM, Torres-Lozano C, Richter A, Bucchieri F, Lordan JL, et al. (2003) Increased expression of p21(waf) cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol 28 1: 61–68. [DOI] [PubMed] [Google Scholar]
- 7. Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, et al. (2009) Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 180 2: 122–133. [DOI] [PubMed] [Google Scholar]
- 8. Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, et al. (2011) Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol 45 5: 1090–1100. [DOI] [PubMed] [Google Scholar]
- 9. deBoer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, et al. (2008) Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol 86 3: 105–112. [DOI] [PubMed] [Google Scholar]
- 10. Pezzulo AA, Starner TD, Scheetz TE, Traver GL, Tilley AE, et al. (2011) The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol 300: L25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG, et al. (2011) Effects of Interleukin-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Ped Res 69: 95–100. [DOI] [PubMed] [Google Scholar]
- 12. Gaga M, Vignola AM, Chanez P (2001) Upper and lower airways: similarities and differences. Eur Respir Monogr 6: 1–15. [Google Scholar]
- 13. Hellings PW, Hens G (2009) Rhinosinusitis and the lower airways. Immunol Allergy Clin North Am 29: 733–740. [DOI] [PubMed] [Google Scholar]
- 14. Jeffery PK, Haahtela T (2006) Allergic rhinitis and asthma: inflammation in a one-airway condition. BMC Pulm Med 6: Suppl 1: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, et al. (2010) Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 126: 466–476. [DOI] [PubMed] [Google Scholar]
- 16. Bousquet J, Vignola AM, Demoly P (2003) Links between rhinitis and asthma. Allergy 58: 691–706. [DOI] [PubMed] [Google Scholar]
- 17. Devalia JL, Davies RJ (1991) Human nasal and bronchial epithelial cells in culture: an overview of their characteristics and function. Allergy Proc 12: 71–79. [DOI] [PubMed] [Google Scholar]
- 18. Legget JJ, Doherty GM, Skibinski G (2004) Susceptibility of bronchial epithelial cells from asthmatics to oxidant injury. Eur Respir J 24: 100. [Google Scholar]
- 19. McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, et al. (2008) Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol 39: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pringle EJ, Richardson HB, Miller D, Cornish DS, Devereux GS, et al. (2012) Nasal and bronchial airway epithelial cell mediator release in children. Pediatr Pulmonol 47 12: 1215–1225. [DOI] [PubMed] [Google Scholar]
- 21. Doherty GM, Christie SN, Skibinski G, Puddicombe SM, Warke TJ, et al. (2003) Non-bronchoscopic sampling and culture of bronchial epithelial cells in children. Clin Exp Allergy 33: 1221–1225. [DOI] [PubMed] [Google Scholar]
- 22. Coyle PV, Ong GM, O'Neill HJ, McCaughey C, DeOrnellas D, et al. (2004) A touchdown nucleic acid amplification protocol as an alternative to culture backup for immunofluorescence in the routine diagnosis of acute viral respiratory tract infections. BMC Microbiol 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laoukili J, Perret E, Willems T, Minty A, Parthoens E, et al. (2001) IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 108: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skowron M, Perret E, Marano F, Caput D, Tournier F (2003) Interleukin-13 alters mucociliary differentiation of human nasal epithelial cells. Chest (Suppl) 123: 3. [DOI] [PubMed] [Google Scholar]
- 25. Kim CH, Song KS, Koo JS, Kim HU, Cho JY, et al. (2002) IL-13 suppresses MUC5AC gene expression and mucin secretion in nasal epithelial cells. Acta Otolaryngol 122: 638–643. [DOI] [PubMed] [Google Scholar]
- 26. Atherton HC, Jones G, Danahay H (2003) IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 285: L730–739. [DOI] [PubMed] [Google Scholar]
- 27. Gomperts BN, Kim LJ, Flaherty SA, Hackett BP (2007) IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol 37: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A (2002) Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 27: 536–541. [DOI] [PubMed] [Google Scholar]
- 29. Malavia NK, Mih JD, Raub CB, Dinh BT, George SC (2008) IL-13 induces a bronchial epithelial phenotype that is profibrotic. Respir Res 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, et al. (2007) IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 36: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denker BM, Nigam SK (1998) Molecular structure and assembly of the tight junction. Am J Physiol 274: F1–9. [DOI] [PubMed] [Google Scholar]
- 32. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, et al. (1999) Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A 96: 3081–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, et al. (2007) Prolonged allergen challenge in murine nasal allergic rhinitis: nasal airway remodeling and adaptation of nasal airway responsiveness. Laryngoscope 117: 881–885. [DOI] [PubMed] [Google Scholar]
- 34. Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, et al. (2008) Gamma-tocopherol prevents airway esosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy 38: 501–511. [DOI] [PubMed] [Google Scholar]
- 35. Ouyang Y, Miyata M, Hatsushika K, Ohnuma Y, Katoh R, et al. (2010) TGF-beta signaling may play a role in the development of goblet cell hyperplasia in a mouse model of allergic rhinitis. Allergol Int 59: 313–319. [DOI] [PubMed] [Google Scholar]
- 36. Haitchi HM, Davies DE, Holgate ST, Howarth PH, Whitsett JA, et al. (2011) The SPDEF network regulates mucus cell differentiation in asthma [abstract]. Am J Respir Crit Care Med 183: A2771. [Google Scholar]
- 37. Miyahara S, Miyahara N, Matsubara S, Takeda K, Koya T, et al. (2006) IL-13 is essential to the late-phase response in allergic rhinitis. J Allergy Clin Immunol 118: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 38. Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, et al. (2009) In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal epithelial cells in human subjects. J Allergy Clin Immunol 123 6: 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lopez-Guisa JM, Powers C, File D, Cochrane E, Jiminez N, et al. (2012) Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol 129 4: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, et al. (2011) Interleukins, from 1–37, and interferon-γ: Receptors, functions and roles in diseases. J Allergy Clin Immunol 127: 701–721.e70. [DOI] [PubMed] [Google Scholar]
- 41. Levine SJ, Wu T, Shelhamer JH (1997) Extracellular release of the type-1 intracellular receptor antagonist from human airway epithelial cells. J Immunol 158: 5949–5957. [PubMed] [Google Scholar]
- 42. Lordan JL, Bucchieri F, Richter A, Konstantinidis A, Holloway JW, et al. (2002) Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J Immunol 169 1: 407–414. [DOI] [PubMed] [Google Scholar]
- 43. Ip WK, Wong CK, Lam CWK (2006) Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not cJun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Allergy 145: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogilvie V, Passmore M, Hyndman L, Jones L, Stevenson B, et al. (2011) Differential global gene expression in cystic fibrosis nasal and bronchial epithelium. Genomics 98 5: 327–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytokine analysis of IL-1ra, IL-6, IL-7 & IL-8 secreted apically. A - Analysis of IL-1ra secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-1ra compared with unstimulated PNECs (p = 0.02) and PBECs (p = 0.001). Additionally unstimulated PNECs secreted higher levels of IL-1ra compared with unstimulated PBECs (p = 0.04). B - Analysis of IL-6 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-6 compared with unstimulated PNECs (p = 0.02) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted higher levels of IL-6 compared with unstimulated PBECs (p = 0.04). C - Analysis of IL-7 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-7 compared with unstimulated PNECs (p = 0.0003) and PBECs (p = 0.0004). Additionally unstimulated PNECs secreted higher levels of IL-7 compared with unstimulated PBECs (p = 0.04). D - Analysis of IL-8 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted higher levels of IL-8 compared with unstimulated PNECs and PBECs however they did not reach statistical significance. Additionally IL-13 stimulated PNECs secreted higher levels of IL-8 compared with IL-13 stimulated PBECs (p = 0.03).
(TIF)
Cytokine analysis of IL-12p70, G-CSF, IP-10 & MCP-1 secreted apically. A - Analysis of IL-12p70 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted significantly less IL-12p70 compared with unstimulated PNECs (p = 0.007) and PBECs (p = 0.0009). Additionally unstimulated PNECs secreted higher levels of IL-12p70 compared with unstimulated PBECs although this was not statistically significant (p = 0.08). B - Analysis of G-CSF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly less G-CSF compared with unstimulated PNECs (p = 0.03). There was no significant difference between IL-13 stimulated and unstimulated PBECs. Additionally unstimulated PNECs secreted higher levels of G-CSF compared with unstimulated PBECs although this was not statistically significant. C - Analysis of IP-10 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of IP-10 compared with unstimulated PNECs (p = 0.006) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted significantly higher levels of IP-10 compared with unstimulated PBECs (p = 0.047) and IL-13 stimulated PNECs secreted higher levels of IP-10 compared with IL-13 stimulated PBECs (p = 0.04). D - Analysis of MCP-1 secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of MCP-1 compared with unstimulated PNECs (p = 0.005) and PBECs (p = 0.01). Additionally unstimulated PNECs secreted significantly higher levels of IP-10 compared with unstimulated PBECs (p = 0.001).
(TIF)
Cytokine analysis of RANTES, VEGF & GM-CSF secreted apically. A - Analysis of RANTES secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted lower levels of RANTES compared with unstimulated PNECs (p = 0.003) and PBECs (p = 0.049). Additionally unstimulated PNECs secreted significantly higher levels of RANTES compared with unstimulated PBECs (p = 0.047). B - Analysis of VEGF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. C - Analysis of GM-CSF secreted apically using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted lower levels of GM-CSF compared with unstimulated PNECs (p = 0.009). There was no significant change in GM-CSF between IL-13 stimulated and unstimulated PBECs. Additionally unstimulated PNECs secreted significantly higher levels of GM-CSF compared with unstimulated PBECs (p = 0.01).
(TIF)
Cytokine analysis of IL-1ra, IL-6, IL-7 & IL-8 secreted basolaterally. A - Analysis of IL-1ra secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly less IL-1ra compared with unstimulated PNECs (p = 0.04). There was no significant difference between IL-13 stimulated and unstimulated PBECs. Additionally there was no difference in IL-1ra secretion between PNECs and PBECs. B - Analysis of IL-6 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted less IL-6 compared with unstimulated PNECs however, this did not reach statistical significance. There was a significant difference in IL-6 secretion between IL-13 stimulated and unstimulated PBECs (p = 0.04). Additionally the IL-13 stimulated PNECs secreted higher levels of IL-1ra compared with IL-13 stimulated PBECs (p = 0.04). C - Analysis of IL-7 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs and PBECs secreted less IL-7 compared with unstimulated PNECs (p = 0.06) and PBECs (p = 0.05) but these did not reach statistical significance. Additionally there was no significant difference in IL-7 secretion between PBECs and PNECs. D - Analysis of IL-8 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference in IL-8 secretion between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs.
(TIF)
Cytokine analysis of IL-12p70, G-CSF, IP-10 & MCP-1 secreted basolaterally. A - Analysis of IL-12p70 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs. However unstimulated PNECs secreted higher levels of IL-12p70 compared with unstimulated PBECs (p = 0.009). B - Analysis of G-CSF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs although there was a large decrease in concentration. However IL-13 stimulated PNECs secreted higher levels of IL-12p70 compared with IL-13 stimulated PBECs (p = 0.048). C - Analysis of IP-10 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs. However unstimulated PNECs secreted higher levels of IP-10 compared with unstimulated PBECs (p = 0.009). D - Analysis of MCP-1 secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was lower MCP-1 secretion between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs however this did not reach statistical significance. Unstimulated PNECs secreted significantly higher levels of MCP-1 compared with unstimulated PBECs (p = 0.003) as did IL-13 stimulated PNECs compared with IL-13 stimulated PBECs which was close to reaching significance (p = 0.05).
(TIF)
Cytokine analysis of RANTES, VEGF & GM-CSF secreted basolaterally. A - Analysis of RANTES secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. B - Analysis of VEGF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. There was no significant difference between IL-13 stimulated PNECs and PBECs compared with unstimulated PNECs and PBECs or between PNECs and PBECs. C - Analysis of GM-CSF secreted basolaterally using the Bioplex system. Results were corrected for cell number and absolute concentrations plotted in pg/cell. IL-13 stimulated PNECs secreted significantly more GM-CSF compared with unstimulated PNECs (p = 0.039). Additionally there was no significant difference in GM-CSF secretion between IL-13 stimulated and unstimulated PBECs or between PBECs and PNECs.
(TIF)
Full descriptions of each method used in the study are provided in the supplementary methods section.
(DOC)