Abstract
Successful cancer gene therapy depends on the development of non-toxic, efficient, tumor cell- specific systemic gene delivery systems. Our laboratory has developed a systemically administered, ligand–liposome complex that can effectively and preferentially deliver its therapeutic payload to both primary and metastatic tumors. To further improve the transfection efficiency of this targeting complex, a synthetic pH-sensitive histidylated oligolysine K[K(H)KKK]5-K(H)KKC (HoKC), designed to aid in endosomal escape and condensation of DNA, was included in the complex. The presence of HoKC increased the in vitro transfection efficiency over that of the original complex. Moreover, no increase in cytotoxicity was observed due to the presence of the HoKC peptide. In a DU145 human prostate cancer xenograft tumor model in athymic nude mice, inclusion of the HoKC peptide did not interfere with the tumor targeting specificity of the i.v. administered ligand/liposome/DNA complex. Most importantly, the level of transgene expression was significantly elevated in the tumors, but not in the normal tissue in those animals receiving the complex incorporating HoKC. The in vivo enhancement of transfection efficiency by this modified gene delivery vehicle could lead to a reduction in the number of administrations required for antitumor efficacy.
INTRODUCTION
The primary requirement for the development of effective gene therapy modalities for the treatment of cancer and other diseases is the efficient delivery of the therapeutic molecule of interest to the site(s) in the body where it is needed. Our laboratory has developed systemically administered, ligand–liposome complexes that can effectively and preferentially deliver their therapeutic payload to both primary and metastatic tumors (1–9). Delivery of the wild-type p53 (wtp53) gene via these complexes resulted not only in tumor growth inhibition and/or increased survival, but also, in complete, long-term elimination of some tumors in mouse models of breast, prostate and head and neck cancers (2–4). These results demonstrate the promise of targeted gene therapy for cancer. However, from the standpoint of patient quality of life and economics, any reduction in the frequency of administration and effective dose of the therapeutic would be advantageous. Thus, we sought to improve the transfection efficiency of our efficacious, systemically delivered, tumor-targeting complex.
The mechanism of liposome-mediated gene delivery is not fully understood. However, the process, from the entrance of the lipid/DNA complex into the cell to protein expression, can be broken down into three stages: (i) binding and internalization of the lipid/DNA complex; (ii) release of DNA from the endosome; (iii) entry of DNA into the nucleus. Many methods aimed at these three stages are being developed in an effort to improve the efficiency of gene delivery. These include (a) enhancing cellular uptake by increasing DNA condensation via the addition of polycationic molecules such as polylysine (10–12) or protamine (13,14); (b) the incorporation of cell-specific ligands such as transferrin (2,15,16) or folate (17,18); (c) increasing endosomal DNA release by the addition of a pH-sensitive peptide or lipid, whose structure is determined by the pH of the surrounding medium, such as GALA (19), dioleoyl-melittin (20) or C-DOPE (21). All of these methods markedly increase the transfection efficiency of liposome-based gene delivery systems. Here, we have modified our previously developed gene delivery system that already incorporates a targeting ligand to include a pH-sensitive synthetic peptide, thus exploiting two of these approaches. Based on the Arg-Gly-Asp (cRGD) histidylated oligolysine described by Aoki et al. (22), a novel pH-sensitive histidylated oligolysine peptide, K[K(H)KKK]5K(H)KKC (HoKC), which has DNA-condensing and pH-sensitive properties, was designed. The linear lysine chain is thought to provide positive charges to condense DNA and thus increase cellular uptake. Histidyl residues (histidylated at ∼25% of the lysine content), the imidazole of which has a pK ≈ 6.0, giving it a positive charge in the lysosome, is thought to aid in the release of plasmid DNA from pre-lysosomal vesicles (23). The cysteine residue at the end of the polymer is designed to enable conjugation of HoKC to the liposome. This novel HoKC peptide was incorporated into our established targeted gene delivery system and its gene delivery efficiency was evaluated in vitro and in vivo.
As high as a 39-fold increase in transfection efficiency in prostate and pancreatic cancer cell lines, which is dependent on the liposome formulation, was observed when HoKC was conjugated to the liposome. Moreover, in vitro cytotoxicity assays demonstrated that the incorporation of HoKC did not result in any increase in toxicity of the delivery system. Most significantly, using a human prostate cancer (DU145) xenograft tumor model in athymic nude mice, the systemically delivered, optimized TfRscFv/LipA-HoKC/DNA complex was shown to still effectively and preferentially target the tumor.
MATERIALS AND METHODS
Chemicals
1,2-Dioleoyl-3-trimethylammonium propane (DOTAP), dioleoylphosphatidylethanolamine (DOPE), dimethyldioctadecyl ammonium bromide (DDAB), N-maleimido-phenylbutyrate-DOPE (MPB-DOPE) and DOPE-rhodamine were purchased from Avanti Polar Lipids (Alabaster, AL). The K[K(H)KKK]5-K(H)KKC (HoKC) peptide was manufactured by Sigma-Genosys (The Woodlands, TX).
Cell culture
Human prostate cancer cell line DU145 (ATCC HTB-81) was maintained in minimum essential medium with Earle’s salts (MEM) supplemented with 10% fetal bovine serum (FBS) plus 50 µg/ml each penicillin, streptomycin and neomycin and 2 mM l-glutamine. Human pancreatic cancer cell line PANC-1 (ATCC CRL-1469) was maintained in Iscove’s minimum essential medium with phenol red (IM) supplemented with 10% FBS plus 50 µg/ml each penicillin, streptomycin and neomycin and 2 mM l-glutamine.
Plasmids
pLuc or pGFP, the firefly luciferase and green fluorescent protein (GFP) genes, respectively, driven by the cytomegalovirus (CMV) promoter, were employed as reporter genes (Promega, Madison, WI). The p53 expression plasmid pCMVp53 contains the full-length 1.7 kb human wtp53 cDNA under the control of the CMV promoter, followed by the SV40 polyadenylation signal. All of the plasmids were propagated in Escherichia coli bacterial strain DH5α and purified using the Qiagen Mega/Giga plasmid purification kits (Valencia, CA). The plasmid was quantified spectrophotometrically (A260/A280 values ∼1.95).
Preparation of LipA-HoKC, TfRscFv/LipA-HoKC/DNA and TfRscFv/LipA/DNA complexes
Cationic liposomal formulations A (DOTAP:DOPE at a 1:1 molar ratio), B (DDAB:DOPE at 1:1), G (DOTAP:DOPE: cholesterol at 1:1:1) and H (DDAB:DOPE:cholesterol at 1:1:1) were prepared using the ethanol injection method as previously described (6). Each liposome formulation also included MPB-DOPE at 5 molar percent of total lipid. Since the HoKC peptide {K[K(H)KKK]5-K(H)KKC} carries a terminal cysteine, MPB-DOPE was included in all of the liposome compositions to allow conjugation of peptide to the liposome. The Lip-HoKC liposomes were prepared using the coupling reaction (described below) between the cationic liposomes carrying the maleimide group (Lip-MPB) and the peptide. An aliquot of 0.1 mmol of the peptide with a free thiol group on cysteine was added to 2 mmol of Lip-MPB in 10 mM HEPES, pH 7.4, solution and rotated at room temperature (20–30 r.p.m.) for 2 h. The resulting Lip-HoKC has a lipid concentration of 1.4 mM.
The full complex was formed in a manner identical to that used to produce our original TfRscFv/Lip/DNA complex (6). Here also the anti-transferrin receptor single chain antibody fragment (TfRscFv) (6) is mixed with Lip-HoKC at a specific ratio (see Results), and incubated at room temperature for 10 min. DNA is then added to the TfRscFv/Lip-HoKC solution, mixed and again incubated at room temperature for 15 min, after which dextrose was added to a final concentration of 5%. The ratio of TfRscFv to liposome to DNA can be varied, resulting in complexes with different transfection efficiencies. A / within the complex indicates mixing of the components, while a - indicates conjugation.
The sizes the complexes were determined by dynamic light scattering at 25°C with a Zetasizer 3000HS system (Malvern UK).
Flow cytometry
The TfRscFv/LipA(Rd)/DNA and TfRscFv/LipA(Rd)-HoKC/DNA complexes were prepared as described above except that rhodamine-DOPE, at 0.05 molar percent of total lipid, was included in the liposome preparation. Aliquots of 1 × 106 PANC-1 cells were incubated with the florescent labeled complexes carrying the pLuc vector DNA for 1.5 h on ice. The cells were then washed twice, with 10 ml of phosphate-buffered saline (PBS) each wash, at room temperature for 5 min with gentle rocking. Paraformaldehyde (0.4%, 2 ml) was then added to the cells. After 15 min at room temperature without rocking, they were again washed twice with 10 ml of PBS as above.
In vitro transfection
5 × 104 DU145 or 3 × 104 PANC-1 cells were seeded per well of a 24-well plate. Twenty-four hours later, the medium containing 10% FBS was replaced with serum-free medium and the cells transfected with the complexes carrying the luciferase gene (at 0.05 µg pLuc/well) as previously described (6). After 4 h incubation, serum was added to a final concentration of 10%. In the bafilomycin A experiments, transfection was performed in the presence of 200 nM bafilomycin A in serum-free medium. After 3.5 h incubation, the medium was replaced with fresh medium containing 10% FBS. Twenty-four hours post-transfection the luciferase activity was assessed using a Luciferase Assay System (Promega, Madison, WI) and protein concentration determined using a micro BCA™ protein assay kit (Pierce, Rockford, IL) according to the manufacturers’ protocols. The enzyme activity, reported as relative light units (RLU)/µg total protein, is indicative of the level of expression of the luciferase gene in the cells and reflects the transfection efficiency.
In vitro chemosensitization and cell viability of LipA-HoKC
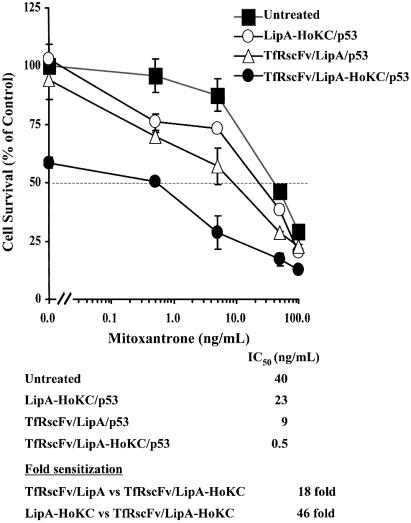
For chemosensitization studies, 5 × 103 DU145 cells/well were plated in a 96-well plate. After 24 h, the cells were transfected, at 0.06 µg p53/well, either with the full complex (TfRscFv/LipA-HoKC/p53), the complex minus the HoKC peptide (TfRscFv/LipA/p53) or the complex with the peptide but minus the targeting ligand (LipA-HoKC/p53). One day post-transfection, mitoxantrone (Immunerx®, Seattle, WA) was added in increasing concentrations (in triplicate). The XTT assay (Boehringer Mannheim, Indianapolis, IN) (24) was performed ∼4 days later and IC50 values, the mitoxantrone concentration (ng/ml) yielding 50% growth inhibition, were calculated.
For the cell viability studies, 5 × 103 PANC-1 cells/well were seeded in a 96-well plate. Twenty-four hours later, TfRscFv/LipA-HoKC, LipA alone or HoKC alone were added at HoKC concentrations of 0.008–0.25 µM. After 24 h, cell survival was assessed by XTT assay.
In vivo tumor selective targeting ability of TfRscFv/LipA-HoKC/DNA
Human prostate cancer cells (DU145) (6 × 106) suspended in MatriGel collagen basement membrane (Collaborative Biomedical Products) were injected s.c. into the lower back above the tail of female athymic NCR nu/nu mice. The tumors were allowed to develop to a size of ∼100 mm3. The TfRscFv/LipA-HoKC, transferrin/LipA-HoKC, LipA-HoKC and TfRscFv/LipA complexes (in 5% dextrose) carrying the GFP gene (40 µg DNA/injection), prepared as described above, were i.v. injected either once or three times within 24 h. Twenty-four hours after the last or single injection the animals were killed, the tumor, liver and lung excised and protein isolated for western analysis (6). Protein isolated from the tumor, liver and lung of an untreated animal was also included as a control. Protein concentration was determined using the micro BCA™ protein assay. Samples of 40 µg total protein/lane was loaded and run on a 12.5% Criterion™ precast gel (Bio-Rad). The GFP protein band was detected using a monoclonal anti-GFP antibody (BAbCO) and an ECL western blot kit. The same membrane was subsequently treated with an anti-GAPDH antibody to establish equal protein loading per lane.
RESULTS
In vitro optimization of TfRscFv/LipA-HoKC/DNA complex
We have previously found that transfection efficiency can vary greatly with the composition and ratio of the constituents in the complex (3,4,6). For these initial optimization studies, liposome formulation A (DOTAP:DOPE, 1:1) (LipA) was used since this formulation was previously shown to be effective, when used in our ligand/liposome/DNA complexes, for a number of different tumor cell types (1–6). Therefore, the ratio of the three components, TfRscFv, LipA-HoKC and DNA, was optimized to yield maximal in vitro transfection efficiency using the luciferase assay.
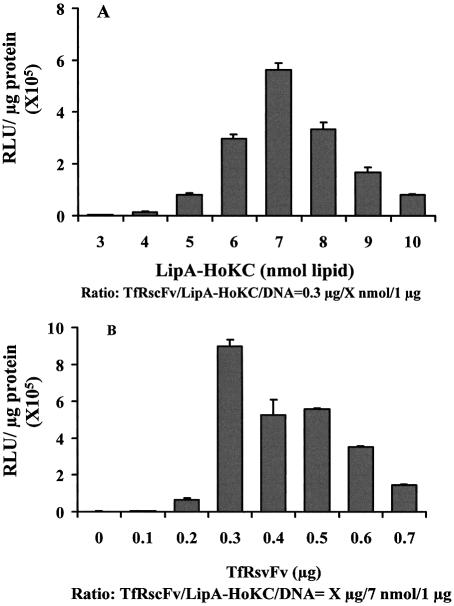
First, based on the optimal ratio of our previously developed TfRscFv/LipA/DNA complex (0.3 µg:14 nmol:1 µg) (6), the ratio of TfRscFv to DNA was fixed at 0.3:1 (w/w) while the ratio of LipA-HoKC to DNA was varied from 3:1 to 10:1 (nmol:µg). As shown in Figure 1A, a LipA-HoKC:DNA ratio of 7:1 (nmol:µg) was found to give the highest transfection activity in PANC-1 cells. Similar results were observed in DU145 cells (data not shown).
Figure 1.
Optimization of the TfRscFv/LipA-HoKC/DNA complex. The ratio of LipA-HoKC to DNA and TfRscFv to DNA were optimized for transfection activity of the TfRscFv/LipA-HoKC/DNA complex by varying the concentration of LipA-HoKC (A) or TfRscFv (B) in the complex used to deliver the pLuc reporter gene to PANC-1 cells in vitro. Aliquots of 4 × 104 PANC-1 cells/well were plated in 24-well plates in complete IM medium. Twenty-four hours later, the medium was replaced with IM minus serum and transfected with complex (at 0.05 µg pLuc/well) containing different amounts of LipA-HoKC or TfRscFv. After 4 h incubation serum was added to a final concentration of 10%. Twenty-four hours later the luciferase assay was performed. The results are given as relative light units (RLU)/µg protein. All data are shown as means ± SD (n = 3).
The ratio of LipA-HoKC to DNA was subsequently fixed at 7:1 (nmol:µg) while the ratio of TfRscFv to DNA was varied from 0.1:1 to 0.7:1 (µg:µg). For comparison, the cells were also transfected with the LipA-HoKC/DNA complex minus the TfRscFv targeting molecule. Incorporating the HoKC peptide in the complex (Fig. 1B) at a TfRscFv:DNA ratio of 0.3:1 (µg:µg) gave the highest transfection activity. This is the same optimal ratio previously found for our original TfRscFv targeted complex (5,6), indicating that the presence of the peptide may not be interfering with complex formation and/or targeting. Thus, the optimal ratio for the TfRscFv/LipA-HoKC/DNA complex is 0.3 µg:7 nmol:1 µg. As the aim of this study was to develop a more effective tumor targeting gene delivery vehicle, the complexes with HoKC (TfRscFv/LipA-HoKC/DNA) or without HoKC (TfRscFv/LipA/DNA) were used at their own optimal ratios in the subsequent experiments.
Previous reports (25–27) have indicated that the non-chemical conjugation of an HK peptide to a liposome (simple mixing) could increase transfection efficiency. Here we compared the transfection activities (via luciferase gene expression) between complexes incorporating the HoKC peptide either by conjugation to LipA-Mal or by simple mixing with LipA. Table 1 show the relative transfection efficiencies normalized to our standard complex without HoKC (TfRscFv/LipA/DNA). Both conjugation and mixing increased transfection efficiency in DU145 and PANC-1 cells. However, conjugation of HoKC to LipA-Mal resulted in a significantly higher level of transfection efficiency in both cell lines, 13.3 ± 2.3-fold in DU145 and 34.1 ± 14.2-fold in PANC-1 versus ∼3-fold for both by simple mixing. Thus, in the remainder of these studies the peptide was always added to the complex via conjugation. All of the above experiments were repeated at least once with similar results.
Table 1. Transfection activity relative to the TfRscFv/LipA/DNA complex.
| Complex | Cell line | |
|---|---|---|
| DU145 | PANC-1 | |
| TfRscFv/LipA/DNAa | 1b | 1c |
| TfRscFv/LipA-HoKC/DNAd (LipA conjugated to HoKC) | 13.25 ± 2.24 | 34.07 ± 14.17 |
| TfRscFv/LipA/HoKC/DNAd (LipA mixed with HoKC) | 2.22 ± 0.36 | 2.84 ± 0.46 |
| LipA-HoKC/DNAd | 0.017 ± 0.009 | 0.011 ± 0.005 |
| LipA/HoKC/DNAd | 0.013 ± 0.011 | 0.013 ± 0.006 |
| TfRscFv/HoKC/DNAd | 0.001 ± 0.0001 | 0.43 ± 0.54 |
A/ within the complex indicates mixing, while A- indicates conjugation. The data represent means ± SD (n = 3).
aThe ratio of LipA to DNA is 14 nmol to 1 µg.
bThis represents a value of 55111 ± 23698 RLU/µg protein.
cThis represents a value of 10459 ± 4497 RLU/µg protein.
dThe ratio of LipA to DNA is 7 nmol to 1 µg.
Optimizing the TfRscFv/Lip-HoKC/DNA complex for liposome formulation
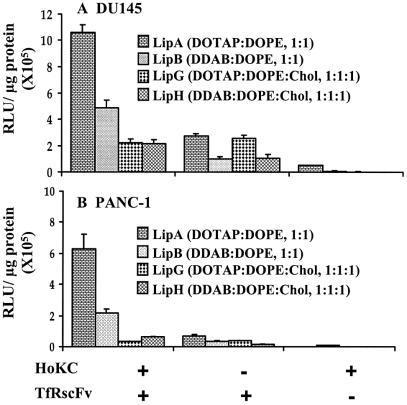
Although, in our original ligand/liposome/DNA complex, LipA was found in general to be the most efficient formula for transfection, there is the possibility that conjugation of HoKC to the lipids could affect the interaction between lipids and thus the structure of the complex and the transfection efficiency. Therefore, the transfection efficiency of a series of HoKC conjugated formulations (TfRscFv/ Lip-HoKC/pLuc) was assessed in two human tumor cell lines, prostate cancer (DU145) and pancreatic cancer (PANC-1). Through comparison with the complexes minus HoKC (TfRscFv/Lip/pLuc), the general transfection-enhancing nature of this peptide could also be studied. The formulations used were LipA, LipB, LipG and LipH. These four formulations were selected because they all include DOPE, a necessity since DOPE-MPB is required for peptide conjugation.
The complexes without the targeting molecule (Lip-HoKC/pLuc) were used as controls. The results are shown in Figure 2A and B. The transfection efficiency was higher in DU145 cells than in PANC-1 cells, both with and without the HoKC peptide. With the exception of LipG, conjugation of the HoKC peptide to the liposomes resulted in an increased level of transfection in both cell lines. In DU145 cells, an ∼4- (LipA), 5- (LipB) and 2-fold (LipH) increase was observed, while in PANC-1, the transfection efficiency was improved by ∼9-, 6- and 4-fold (LipA, LipB and LipH, respectively). It is of interest to note that inclusion of cholesterol as a helper lipid decreased or even eliminated the enhancing effect of the peptide. As described in Materials and Methods, the difference between liposome formulations A and G and formulations B and H is the presence of cholesterol. The most dramatic diminution of the peptide effect was seen in the DOTAP:DOPE formulations (A and G). These results clearly demonstrate that, while generally improving transgene expression, the transfection-enhancing effect of HoKC is dependent on the liposome formulation and cell line. Moreover, these experiments demonstrate the importance of the targeting moiety (TfRscFv) in obtaining high transfection efficiency, even with inclusion of the pH-sensitive peptide, and further support our previous findings (1–6). The transfection efficiency of the TfRscFv/Lip/pLuc complex was significantly higher with all four liposome compositions in both cell lines when compared to that of the unliganded, peptide-conjugated liposome complexes (Lip-HoKC/pLuc) (Fig. 2A and B). Repeat experiments showed similar results.
Figure 2.
Comparison of different liposome formulations. Aliquots of 5 × 104 DU145 or 4 × 104 PANC-1 cells/well were plated in 24-well plates in their appropriate medium. Twenty-four hours later, the medium containing 10% FBS was replaced with serum-free medium and the cells were transfected with the full complexes (TfRscFv/Lip-HoKC/pLuc) containing different liposome compositions (A, B, G and H) all carrying the luciferase gene at 0.05 µg DNA/well. For comparison, cells were also transfected with the complex without HoKC (TfRscFv/Lip/pLuc) or the peptide containing complex minus the targeting moiety (Lip-HoKC/pLuc). After 4 h incubation, serum was added to a final concentration of 10%. Twenty-four hours later, the luciferase assay was performed. The results are given as relative light units (RLU)/µg protein. All data are shown as means ± SD (n = 3).
As the best results were obtained with LipA, this formulation was consequently employed for the remainder of the studies described in this report.
Evaluation of the cytotoxicity of LipA-HoKC
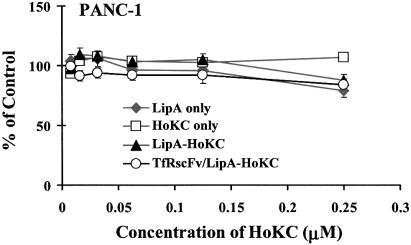
Throughout our previous studies with Tf/liposome/DNA, F-liposome/DNA and TfRscFv-liposome/DNA complexes (2,4,5,6), we have seen no indication that, at effective concentrations, our liposomes cause significant toxicity. The design of the HoKC peptide used here is based on the information that biodegradable peptide linkers and low molecule weight peptides appear to have less toxicity in vivo than higher molecular weight peptides (28). To establish that inclusion of the HoKC peptide in the complex does not increase its toxicity, HoKC peptide alone, LipA alone, LipA conjugated to the HoKC peptide (LipA-HoKC) or TfRscFv/LipA-HoKC were added to PANC-1 cells at increasing peptide concentrations. Twenty-four hours later, an XTT assay was performed to ascertain the percent of cells surviving (Fig. 3). At concentrations up to 0.25 µM, well beyond that used in in vitro studies, there was no indication of cytotoxicity due to presence of the HoKC peptide. Moreover, no statistically significant difference in toxicity after conjugation of the HoKC peptide to LipA (LipA-HoKC) or inclusion of TfRscFv as the targeting moiety (TfRscFv/LipA-HoKC) was observed. Similar results were found in repeat experiments. Thus, it does not appear that inclusion of the pH-sensitive HoKC peptide in our ligand/liposome/DNA complex should adversely affect its use as a therapeutic agent.
Figure 3.
Cell viability in the presence of LipA-HoKC. Aliquots of 5 × 103 PANC-1 cells/well were plated in 96-well plates the day before addition of different concentrations of LipA alone, HoKC peptide alone, LipA-HoKC or TfRscFv/LipA-HoKC. Twenty-four hours later, XTT assays was performed to determine cell survival. The results are given as the percent of untreated cells. All data are shown as means ± SD (n = 3).
Peptide effect on cell binding
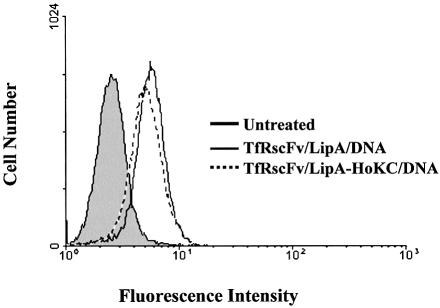
Based upon the fact that, as shown above in Figure 1, the optimal TfRscFv:DNA ratio for the peptide-containing complex was the same as that of the original TfRscFv/LipA/DNA complex, it appears that presence of the HoKC peptide in the complex did not interfere with complex formation and/or targeting. Along these same lines, we wished to examine the effect of peptide inclusion on binding of the complex to cells to assess whether the presence of peptide enhanced or reduced binding. Complexes with [TfRscFv/LipA(Rd)/DNA] and without [TfRscFv/LipA(Rd)-HoKC/DNA] HoKC, prepared using LipA that included 0.05 molar percent rhodamine-labeled DOPE, were incubated with 1 × 106 PANC-1 cells for 1.5 h, a long enough period of time to allow the complex to bind to the cells, but not long enough to permit internalization. After washing and paraformaldehyde fixation the cells were analyzed by flow cytometry using excitation/emission wavelengths of 488 and 575 nm, respectively. As shown in Figure 4, the curves produced by both complexes were vertically superimposable. Therefore, the shift in fluorescence produced by binding of the complex to the cells is not affected either positively or negatively by inclusion of the peptide.
Figure 4.
The effect of peptide inclusion on the binding of TfRscFv/LipA/DNA complexes to the cell surface. Fluorescent liposomes were prepared by inclusion of rhodamine-labeled DOPE (0.05 molar percent) during preparation of the liposome. PANC-1 cells (1 × 106) were incubated with the complexes, with or without HoKC, on ice for 1.5 h, washed and binding to the cells (fluorescent intensity) analyzed by flow cytometry.
Effect of bafilomycin A on transfection activity
The above results suggested that the increase in transfection activity observed after incorporation of the HoKC peptide into the ligand/liposome/DNA complex was not due to increased cell binding as a result, for example, of enhanced projection of the TfRscFv targeting moiety if it binds to the HoKC peptide. It has been well established that, under acidic conditions, such as in the endosome, histidylated peptides contribute to destabilization of the endosomal membrane (23,25,27,29), resulting in improved DNA release. To evaluate the effect of endosomal pH, and thus histidine’s contribution to the transfection activity of the HoKC-bearing complex, DU145 cells were transfected with TfRscFv/LipA-HoKC/pLuc (0.05 µg/well) in the absence or presence of 200 nM bafilomycin A, an inhibitor of the proton pump ATPase that is involved in endosome acidification. Twenty-four hours later, the transfection activity was assessed via luciferase activity. In the presence of bafilomycin A, transfection activity was reduced to half of that with the HoKC-containing complex without the drug (Fig. 5). This therefore suggests that low endosomal pH plays a role in the enhancement in transfection activity due to the incorporation of HoKC in the complex. Moreover, this effect may be through protonization of the imidazole group of histidine in the acidic environment of the endosome resulting in destabilization of the endosomal membrane and thus an increase in DNA release. These experiments have been performed twice with the same results.
Figure 5.
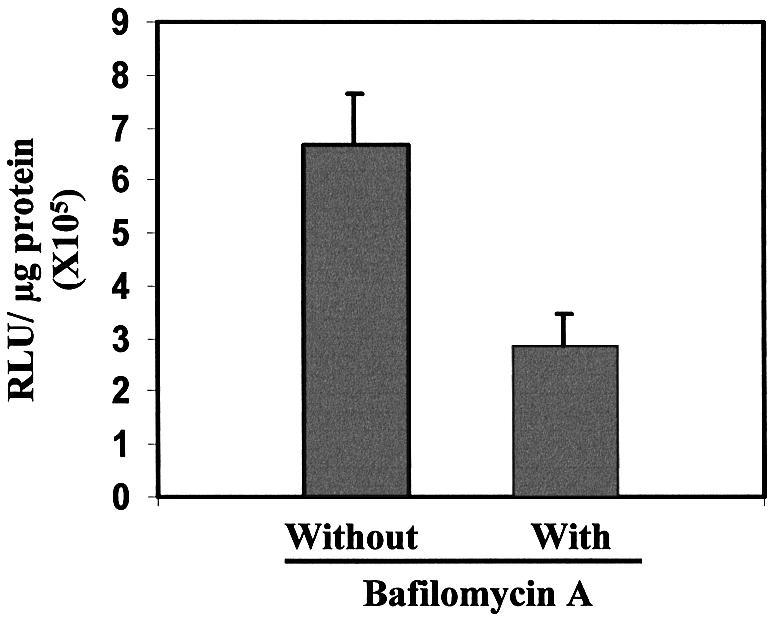
The effect of endocytic pH on the transfection activity of complexes containing HoKC. Aliquots of 5 × 104 DU145 cells/well were plated in 24-well plates in their appropriate medium. Twenty-four hours later, the medium containing 10% FBS was replaced with serum-free medium or serum-free medium containing 200 nM bafilomycin A and transfected with the TfRscFv/LipA-HoKC/pLuc complex (0.05 µg DNA/well). After 3.5 h, the medium was replaced with complete medium. Twenty-four hours later, luciferase assays were performed. The results are given as relative light units (RLU)/µg protein. All data are shown as means ± SD (n = 3) of three wells.
In vitro chemosensitization of prostate cancer cells by the TfRscFv/LipA-HoKC/DNA complex
Our previous studies have demonstrated a pronounced synergistic effect of the combination of wtp53 gene therapy and chemotherapeutic agents (4). Here we examined the ability of the TfRscFv/LipA-HoKC/p53 complex to sensitize DU145 cells to mitoxantrone, a first line chemotherapeutic agent for treatment of prostate cancer. As controls, DU145 cells were also transfected with the complex minus the HoKC peptide (TfRscFv/LipA/p53) or the complex minus the targeting molecule (LipA-HoKC/p53). As we have already demonstrated that LipA-HoKC without p53 is not toxic to the cells (Fig. 3), a control complex without p53 was not included in the experiment. Two days post-transfection, mitoxantrone was added at increasing concentrations (in triplicate). The XTT assay was performed ∼4 days later and IC50 values, the drug concentration yielding 50% growth inhibition, were calculated. A significant (∼18-fold) increase in sensitization by the peptide-containing complex (TfRscFv/LipA-HoKC/p53) over the complex without the HoKC peptide was observed, reducing the IC50 value from 9 to 0.5 ng/ml (Fig. 6). The level of response to mitoxantrone was even more enhanced between the full complex and the unliganded LipA-HoKC/p53 (∼46-fold sensitization). We have previously demonstrated that p53 treatment could induce apoptosis in a p53 dose-dependent manner (2). The hypothesis behind this study is that inclusion of the HoKC peptide in the complex would increase endosomal release of the p53 DNA, thus enhancing nuclear uptake, gene expression and therapeutic effect. Thus, a low dose of p53 (0.06 µg) was used in these studies to permit us to more clearly evaluate the increased efficiency of the complex carrying HoKC. While our standard TfRscFV/LipA/p53 complex alone (at 0 ng/ml mitoxantrone) shows only minimal cell killing effect (10%) with this low dose of p53, the 40% cell kill without the chemotherapeutic agent evident in the cells treated with TfRscFV/LipA-HoKC/p53 represents cell death (apoptosis) induced by the much more efficient transfection and expression of exogenous wtp53 in these tumor cells. Thus, these in vitro studies support the theory that the presence of HoKC peptide may facilitate the release of more of the exogenous wtp53 into the cytosol with an increased amount possibly reaching the nucleus to produce its therapeutic effect, i.e. chemosensitization. It should be noted that none of the curves in this XTT assay have been normalized. However, even if normalized to eliminate the effect of p53 without the chemotherapeutic agent, there is still a 3- to 8-fold increase in chemosensitization by the complex with HoKC compared to the controls (data not shown). These experiment were repeated several times with virtually identical results.
Figure 6.
In vitro chemosensitization of DU145 cells to mitoxantrone by TfRscFv/LipA-HoKC/p53. Aliquots of 5 × 103 DU145 cells/well were seeded in a 96-well plate and transfected 24 h later with the optimized TfRscFv/LipA-HoKC/p53 complex (0.06 µg p53/well). The complex without HoKC (TfRscFv/LipA/p53) or the peptide containing complex minus the targeting moiety (LipA-HoKC/p53) were used for comparison. After 4 days, the XTT assay was performed in triplicate. IC50 values are the drug concentration yielding 50% growth inhibition. All data are shown as means ± SD (n = 3).
TfRscFv/LipA-HoKC/DNA-mediated tumor-targeted in vivo gene expression
We have previously shown (5,6) that the TfRscFv/liposome/DNA complex can preferentially target tumors after systemic (i.v.) administrations. In these studies we have routinely administered a total of three injections over 24–36 h to achieve an expression level easily detected by western analysis. However, if, due to the presence of the HoKC peptide, there is an increase in transfection efficiency/available cytosolic DNA, then a single injection might be sufficient to reach these high expression levels. Therefore, in addition to confirming that conjugation of the HoKC peptide to the liposome does not inhibit binding of TfRscFv to the Tf receptor and thus tumor specificity, we evaluated the level of expression of a GFP reporter gene in DU145 xenograft tumors, normal liver and lung after either one or three i.v. tail vein injections. These experiments have been performed twice with similar results. Athymic nude mice bearing s.c. xenograft tumors of ∼100 mm3 were systemically (i.v.) injected once only with the TfRscFv/LipA-HoKC/GFP, the TfRscFv/LipA/GFP (no HoKC) or the liposome–peptide complex without targeting ligand (LipA-HoKC/GFP) at 40 µg DNA/injection. For comparison to our previous findings, mice were also injected three times over 24 h with the standard TfRscFv/LipA/GFP (no HoKC) complex.
After one injection of our standard TfRscFv/LipA/GFP complex, a low level of expression is observed in the DU145 tumor (Fig. 7). This level is approximately the same as that found with the unliganded LipA-HoKC. In contrast, there is an ∼3-fold increase in expression in the tumors from mice that received one injection of TfRscFv/LipA-HoKC/GFP complex. Most significantly, this level is similar to that achieved after three injections of the complex minus the HoKC peptide (TfRscFv/LipA/GFP). In all cases, there was very little expression evident in either the liver or lung of these animals. It should be noted that we have previously found that GAPDH levels differ between human xenograft tumors and normal mouse organs. However, staining with Ponceau S demonstrates approximately equal amounts of protein per lane (7). With the exception of the lung from the animal that received the single injection with the complex minus peptide, the levels of GAPDH are relatively consistent within each tissue. These results demonstrate, first, the tumor specificity of the complex and, second, that inclusion of the HoKC peptide did not interfere with this tumor targeting capability. These in vivo targeting experiments were performed twice with similar results. It is of interest to note that when Tf itself was used as the targeting ligand in place of TfRscFv with the liposome-HoKC complex, a level of GFP expression virtually identical to that with TfRscFv was observed. Therefore, these findings are an indication of the positive effect of the HoKC peptide on endosomal release. Administration of the same amount of DNA via this ligand/liposome-HoKC/DNA complex can result in a significantly higher level of tumor-specific expression, demonstrating the potential of this improved complex as a therapeutic delivery system.
Figure 7.
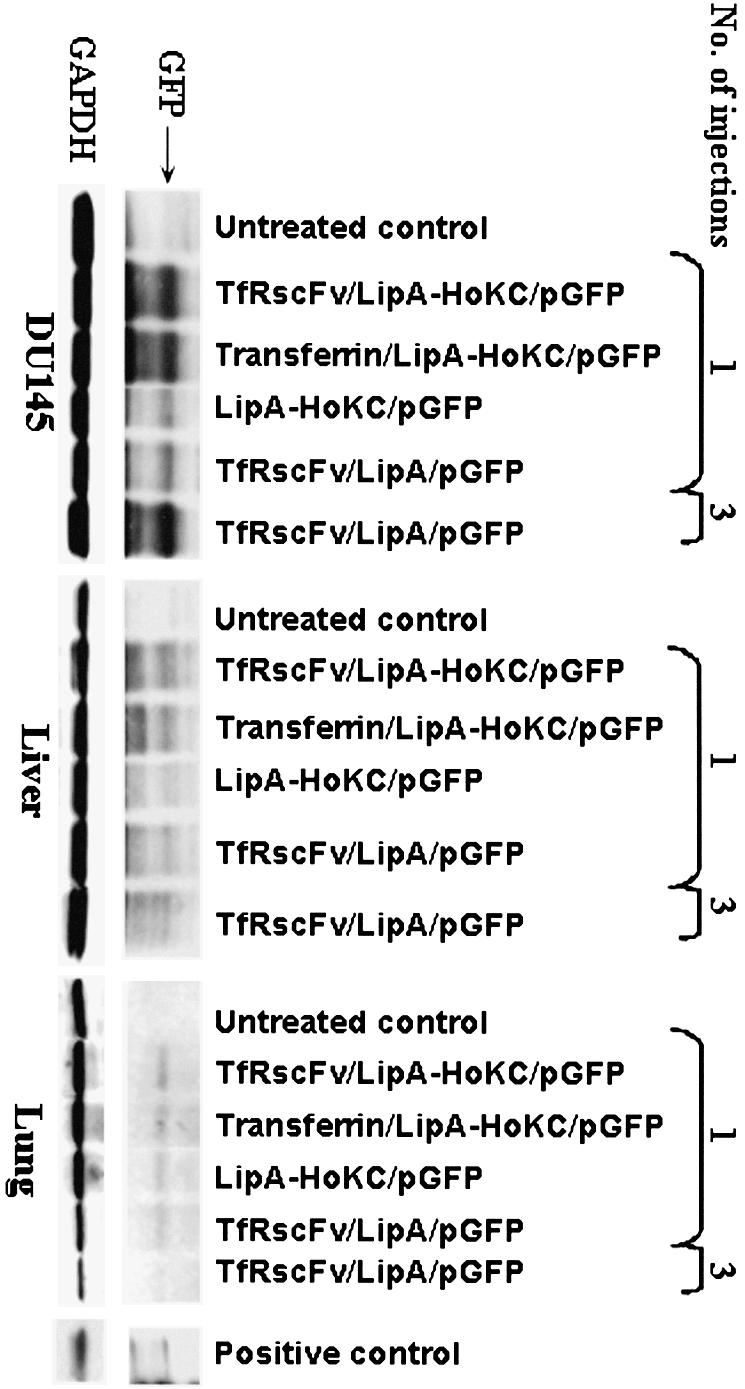
In vivo tumor targeting by systemically delivered TfRscFv/LipA-HoKC/DNA. Athymic nude mice carrying DU145 subcutaneous xenograft tumors were i.v. injected with various complexes. The animals were injected either once or three times within 24 h. Twenty-four hours after the last or single injection the tumors, liver and lung were excised and protein isolated for western analysis. An aliquot of 40 µg protein/lane was loaded. The GFP protein band was detected using a monoclonal anti-GFP antibody (BAbCO) and an ECL western blot kit. The same membrane was subsequently probed with an anti-GAPDH antibody to establish equal protein loading per lane.
DISCUSSION
Our previous reports of in vitro and in vivo experiments have clearly demonstrated that when compared with non-targeting gene delivery systems, association of a tumor-targeting ligand (Tf, folate or TfRscFv) with the lipoplex improved transgene expression in tumors (1–3,5,6). In those studies we have shown histologically, using β-galactosidase as the reporter gene, that a high percentage of target cells are transfected using our ligand-targeted lipoplex. The percent of transfected tumor cells is ∼70–80% in vitro and 30–50% after one i.v. injection in vivo (2,3). Although this complex has shown in vivo tumor specificity with resultant antitumor efficacy, there is still room for improvement. One way to accomplish this is to enhance release of the gene from the endosome. Thus, more of the DNA that enters the tumor cell should be free to reach the nucleus rather than being trapped in the endosome and ultimately undergoing lysosomal enzymatic degradation.
Endosomal compartments are generally acidic in nature. Various methods have been developed to enhance the efficiency of liposomal payload delivery by exploiting this fact (30,31). These include incorporation of pH-sensitive components into liposomes. The selective destabilization of liposomes following acidification of the surrounding media, with resultant release of drug or DNA from the liposome, has been enhanced by inclusion of specific lipids, many based on phosphatidylethanolamine or modifications thereof [e.g. dioleoylphosphatidylethanolamine (DOPE)]. The well-characterized lamellar to hexagonal phase transition that phosphatidylethanolamine undergoes at low pH, thus releasing the liposomal contents, is the most common mechanism employed to produce pH-triggered release. A number of pH-sensitive synthetic peptides have also been designed in an attempt to develop molecules that can attach to, but do not perturb, the surface of the liposome at neutral pH, yet subsequently fuse adjacent bilayers at acidic pH. These include GALA, a 30 amino acid amphipathic peptide with a glutamic acid, alanine, leucine, alanine repeat unit (19,30).
The established membrane fusion ability of histidine in weakly acidic conditions, such as the endosome, and the DNA condensation ability of lysine have been utilized to increase gene delivery efficiency (25–27). Changing the histidine location and the ratio of histidine to lysine, a series of histidine/lysine (HK) peptides have been examined for their potential as gene delivery vehicles. Mixson and co-workers (25–27) have designed a series of linear and branched HK peptides of varying lengths. The lysine in these co-polymer peptides can complex with and partially neutralize the negative charge of the plasmid DNA, while the histidine is believed to buffer and disrupt the endosomes. These co-polymer peptides contain a metabolizable peptide bond that, when combined with liposomes, can enhance transfection efficiency. They found that the inclusion of linear and branched co-polymer peptides (without conjugation) significantly increased the transfection efficiency over cationic liposomes alone (25–27). Moreover, when used with liposomes, a 2:1 ratio of histidine to lysine was superior to a 1:1 ratio with respect to increases in transfection efficiency (27).
Aoki et al. (22) employed a different type of histidylated lysine peptide for use in gene delivery. Here, in place of the large branched co-polymer peptide (26,27), the molecule is a 30 amino acid linear peptide, [K(H)K-KK]6, linked to a purported tumor vasculature targeting peptide containing an Arg-Gly-Asp (RGD) motif (CRGDCF) (32). In this histidylated oligolysine, DNA binds directly to and is carried by the oligolysine, while the histidyl residues facilitate endosomal release and delivery to the cytosol. Systemic administration of the luciferase gene via this peptide vector resulted in significantly higher levels of luciferase activity in tumor versus some normal tissues (22). However, he found no statistical difference between uptake by tumor and liver. Midoux and Monsigny (29) and Bello and Midoux (33) similarly found that a large (190 amino acids), linear histidylated polylysine (without a targeting moiety) could effectively transfect plasmid DNA in vitro into various tumor cell lines. This transfection efficiency was optimal when the polypeptide contained 38% histidyl residues.
It is interesting to note that, unlike Mixson’s HK copolymer, the use of Aoki’s RGD peptide for liposome delivery was not reported. Therefore, we wished to evaluate the gene delivery efficiency of our ligand/liposome/DNA complex in combination with this type of peptide. We adapted the histidylated oligolysine described by Aoki et al. (22) for use with our TfRscFv/liposome/DNA complex. This peptide was designed to have a limited size and the potential to be modified for conjugation to liposomes. The linear pH-sensitive peptide we designed, K[K(H)KKK]5K(H)KKC, contains a cysteine residue at the end, enabling conjugation of this peptide to the liposome through a maleimide group. This type of histidylated oligolysine (HoKC) was also attractive as low molecular weight peptides are potentially less toxic in vivo than high molecular weight peptides (28). Similar to Mixon’s HK co-polymer, we found that mixing HoKC with the TfRscFv/LipA/DNA complex, without conjugation, also increased transfection efficiency. However, the increase was significantly lower than that with conjugated HoKC (Table 1).
The increase in transfection efficiency observed here after incorporation of HoKC in the complex is likely not a result of an increase in the association between the cells and complex, as the results of the flow cytometry analysis of the TfRscFv/LipA(Rd)/DNA and the TfRscFv/LipA(Rd)-HoKC/DNA complexes were almost superimposable (Fig. 4). At the same time, as shown in Figure 5, it was found that the transfection activity of TfRscFv/LipA-HoKC/DNA was reduced by half in the presence of bafilomycin A, an inhibitor of the proton pump ATPase that is involved in endosome acidification. These findings support the hypothesis that pH-sensitive, histidine-related endosomal destabilization and DNA release may be responsible for the observed improvement in transfection efficiency.
There are two characteristics that can be used to describe these low molecular weight histidylated oligolysine peptides. One is the number of amino acid residues in the peptide (degree of polymerization, DP) and the other is the number of histidyl residues (degree of histidylation, DH). It was reported that although histidylated oligolysine (DP = 19, DH = 12 or 15) by itself was efficient for oligonucleotide delivery, it was not useful for delivery of plasmid DNA (23). Similarly, luciferase expression was unmeasurable when our HoKC (DP = 25, DH = 6) alone was used as plasmid carrier. This result indicated that histidylated oligolysines themselves could not efficiently compact plasmid DNA without the aid of the cationic liposome.
For clinical application, no or low toxicity is a primary consideration. Liposomes have been widely used as drug delivery vehicles because of their low toxicity. Thus, it is important that addition of the HoKC peptide does not result in increased cytotoxicity. Careful consideration has gone into the design of our HoKC. It has been reported that low molecular weight peptides are potentially less toxic in vivo (28). Thus, a small peptide of 31 amino acids was designed. There are two advantages to this HoKC. First, the peptide linker is biodegradable and thus should not cause cytotoxicity. Second, the oligo sequence is well defined, which is beneficial for large quantity synthesis and quality control. Using the XTT assay, the cytotoxicities of LipA-HoKC, TfRscFv/LipA-HoKC, LipA and HoKC alone were investigated. As theorized, there was no cytotoxicity associated with this peptide either alone or when complexed with TfRscFv/LipA (Fig. 3).
We have previously shown that delivery of wtp53 via the ligand/liposome/p53 complex could sensitize various tumor cell lines, including prostate, to a number of different chemotherapeutic agents both in vitro and in vivo (1,2,4,6) and that expression of exogenous wtp53 could induce apoptosis (2). If incorporation of the HoKC peptide into the lipoplex does indeed increase gene delivery, the effect of p53 on the response of a tumor cell to a conventional chemotherapeutic agent should also be enhanced. The results of the XTT assay shown in Figure 6 demonstrate a clear and significant increase in chemosensitization (18-fold) by the complex containing the HoKC peptide, even over that found with our parental TfRscFv/LipA/p53 complex (no HoKC). The enhanced p53 effect due to incorporation of HoKC into the liposome is further supported by the dramatic level of cell kill (60% survival, 40% cell death) evident even without mitoxantrone (0 mg/ml) induced by the p53 transfected via the TfRscFv/LipA-HoKC/p53 complex in Figure 6. As the same amount of DNA was transfected per well by both complexes, the increased effect of the p53 gene seen with the complex incorporating HoKC lends credence to the presumption of the effect of the HoKC peptide on endosomal release. It is important to note that this response to TfRscFv/LipA-HoKC/p53 is not due to non-specific cytotoxicity of TfRscFv/LipA-HoKC, since no cytotoxicity of the TfRscFv/LipA-HoKC minus p53 was observed in Figure 3.
Interestingly, there was virtually no difference in the size of the complex with HoKC peptide (TfRscFv/LipA-HoKC/DNA) or without HoKC peptide (TfRscFv/LipA/DNA). The number average values, as measured on the Malvern Zetasizer 3000 were 364 ± 16 and 329 ± 43 nm, respectively. One possible explanation for not seeing a decreased size of the complex after addition of the HoKC peptide is that in our original TfRscFv/LipA/DNA complex, the liposome composition and the ratio of the three components had already been optimized to result in DNA condensation and small size, similar to that demonstrated in Xu et al. (7).
We have previously shown that the TfRscFv/liposome/DNA complex can preferentially target tumors after systemic (i.v.) administrations (5,6), where we administered a total of three injections over 24–36 h to achieve an expression level easily detected by western analysis. However, if, due to the presence of the HoKC peptide, there is an increase in transfection efficiency, then a single injection might be sufficient to reach this same expression level. Using a GFP reporter gene, the levels of expression in DU145 xenograft tumors and normal liver and lung after either one or three i.v. tail vein injections were evaluated (Fig. 7). The high level of tumor preferential expression observed after a single i.v. injection of the complex incorporating the peptide once again demonstrates the positive effect of the HoKC peptide. Administration of the same amount of DNA via this ligand/liposome-HoKC/DNA complex resulted in a significantly higher level of expression than that from the complex without peptide. Moreover, the low level of expression of EGFP in both the liver and lung after systemic administration of either complex (with or without HoKC) once again shows the preferential targeting of our liganded lipoplex and also demonstrates that inclusion of the HoKC peptide does not interfere with this crucial characteristic. This is particularly important with regard to lung, since many non-tumor-targeted cationic liposome preparations have a larger size than our complex and aggregate in the lung. Thus, these proof of principle studies demonstrate the potential of this complex as a therapeutic modality.
In conclusion, the specific and efficient targeted gene delivery previously observed with our ligand/liposome/DNA vector system was increased by incorporating a novel pH-sensitive cationic peptide, HoKC, designed to aid in endosomal escape and condensation of DNA, into the complex. Most significantly, in vivo experiments demonstrated that inclusion of the peptide reduced the requirement for repeated administration of this gene delivery vehicle.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Ms Amy Marshall and Ms Brianna Kalk for aid in preparation of this manuscript and the LCC Animal Research Resource, Macro Molecular Synthesis Shared Resource, Flow Cytometry Shared Resource and Tissue Culture Shared Resource Facilities for their assistance in these studies. This work was supported in part by grants from NIH (DE13151) to E.H.C. and SynerGene Therapeutics Inc. to K.F.P.
REFERENCES
- 1.Xu L., Pirollo,K.F. and Chang,E.H. (1997) Transferrin-liposome-mediated p53 sensitization of squamous cell carcinoma of the head and neck to radiation in vitro. Hum. Gene Ther., 8, 467–475. [DOI] [PubMed] [Google Scholar]
- 2.Xu L., Pirollo,K.F., Tang,W.H., Rait,A. and Chang,E.H. (1999) Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum. Gene Ther., 10, 2941–2952. [DOI] [PubMed] [Google Scholar]
- 3.Xu L., Pirollo,K.F., Chang,E.H. and Murray,A. (1999) Systemic p53 gene therapy in combination with radiation results in human tumor regression. Tumor Targeting, 4, 92–104. [DOI] [PubMed] [Google Scholar]
- 4.Xu L., Pirollo,K.F. and Chang,E.H. (2001) Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J. Controlled Release, 74, 115–128. [DOI] [PubMed] [Google Scholar]
- 5.Xu L., Tang,W.H., Huang,C.C., Alexander,W., Xiang,L.M., Pirollo,K.F., Rait,A. and Chang,E.H. (2001) Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor. Mol. Med., 10, 723–734. [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L., Huang,C.C., Huang,W.Q., Tang,W.H., Rait,A., Yin,Y., Cruz,I., Xiang,L.M., Pirollo,K.F. and Chang,E.H. (2002) Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol. Cancer Ther., 1, 337–346. [PubMed] [Google Scholar]
- 7.Xu L., Frederick,P., Pirollo,K.F., Tang,W.H., Rait,A., Xiang,L.M., Huang,W.Q. and Chang,E.H. (2002) Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum. Gene Ther., 13, 469–481. [DOI] [PubMed] [Google Scholar]
- 8.Pirollo K.F., Xu,L. and Chang,E.H. (2002) Immunoliposomes: a targeted delivery tool for cancer treatment. In Curiel,D.T. and Douglass,J.T. (eds), Vector Targeting for Therapeutic Gene Delivery. Wiley-Liss, Hoboken, NJ, pp. 33–61. [Google Scholar]
- 9.Chang E.H., Xu,L. and Pirollo,K.F., (1999) Targeted p53 gene therapy-mediated radiosensitization and chemosensitization. In Gutkind,J.S. (ed.), Signaling Networks and Cell Cycle Control: The Molecular Basis of Cancer and Other Diseases. Humana Press, Totowa, NJ, pp. 519–536. [Google Scholar]
- 10.Vitiello L., Chonn,A., Wasserman,J.D., Duff,C. and Worton,R.G. (1996) Condensation of plasmid DNA with polylysine improves liposome-mediated gene transfer into established and primary muscle cells. Gene Ther., 3, 396–404. [PubMed] [Google Scholar]
- 11.Lee R.J. and Huang,L. (1996) Folate-targeted, anionic liposome-entrapped polylysine-condensed dna for tumor cell-specific gene transfer. J. Biol. Chem., 271, 8481–8487. [DOI] [PubMed] [Google Scholar]
- 12.Gao X. and Huang,L. (1996) Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry, 35, 1027–1036. [DOI] [PubMed] [Google Scholar]
- 13.Sorgi F.L., Bhattacharya,S. and Huang,L. (1997) Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther., 4, 961–968. [DOI] [PubMed] [Google Scholar]
- 14.You J., Kamihara,M. and Iijima,S. (1999) Enhancement of transfection efficiency by protamine in DDAB lipid vesicle-mediated gene transfer. J. Biol. Chem. (Tokyo), 125, 1160–1167. [DOI] [PubMed] [Google Scholar]
- 15.Joshee N., Bastola,D.R. and Cheng,P.W. (2002) Transferrin-facilitated lipofection gene delivery strategy: characterization of the transfection complexes and intracellular trafficking. Hum. Gene Ther., 13, 1991–2004. [DOI] [PubMed] [Google Scholar]
- 16.Cheng P.W. (1996) Receptor ligand-facilitated gene transfer: enhancement of liposome-mediated gene transfer and expression by transferrin. Hum. Gene Ther., 7, 275–282. [DOI] [PubMed] [Google Scholar]
- 17.Lee R.J. and Low,P. (1994) Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem., 269, 3198–3204. [PubMed] [Google Scholar]
- 18.Sudimack J. and Lee,R.J. (2000) Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev., 41, 147–162. [DOI] [PubMed] [Google Scholar]
- 19.Turk M.J., Reddy,J.A., Chmielewski,J.A. and Low,P.S. (2002) Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pH. Biochim. Biophys. Acta, 1559, 56–68. [DOI] [PubMed] [Google Scholar]
- 20.Legendre J.Y., Trezciak,A., Bohrmann,B., Deuschle,U., Kitas,E. and Supersaxo,A. (1997) Dioleoylmelittin as a novel serum-insensitive reagent for efficient transfection of mammalian cells. Bioconjugate Chem., 8, 57–63. [DOI] [PubMed] [Google Scholar]
- 21.Reddy J.A. and Low,P.S. (2000) Enhanced folate receptor mediated gene therapy using a novel pH-sensitive lipid formulation. J. Controlled Release, 64, 27–37. [DOI] [PubMed] [Google Scholar]
- 22.Aoki Y., Hosaka,S., Kawa,S. and Kiyosawa,K. (2001) Potential tumor-targeting peptide vector of histidylated oligolysine conjugated to a tumor-homing RGD motif. Cancer Gene Ther., 8, 783–787. [DOI] [PubMed] [Google Scholar]
- 23.Pichon C., Roufai,M.B., Monsigny,M. and Midoux,P. (2000) Histidylated oligolysines increase the transmembrane passage and the biological activity of antisense oligonucleotides. Nucleic Acids Res., 28, 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rait A., Pirollo,K.F., Rait,V., Krygier,J.E., Xiang,L.M. and Chang,E.H. (2001) Inhibitory effects of the combination of HER-2 antisense oligonucleotide and chemotherapeutic agents used for the treatment of human breast cancer. Cancer Gene Ther., 8, 728–739. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q.R., Zhang,L., Stass,S.A. and Mixson,A.J (2000) Co-polymer of histidine and lysine markedly enhances transfection efficiency of liposomes. Gene Ther., 7, 1698–1705. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q.R., Zhang,L., Stass,S.A. and Mixson,A.J. (2001) Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res., 29, 1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q.R., Zhang,L., Luther,P.W. and Mixson,A.J. (2002) Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res., 30, 1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y., Kwok,K.Y., Boukarim,C. and Rice,K.G. (2002) Synthesis of sulfhydryl cross-linking poly(ethylene glycol)-peptides and glycopeptides as carriers for gene delivery. Bioconjugate Chem., 13, 232–239. [DOI] [PubMed] [Google Scholar]
- 29.Midoux P. and Monsigny, M (1999) Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjugate Chem., 10, 406–411. [DOI] [PubMed] [Google Scholar]
- 30.Drummond D.C., Zignani,M. and Leroux,J. (2000) Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res., 39, 409–460. [DOI] [PubMed] [Google Scholar]
- 31.Venugopalan P., Jain,S., Sankar,S., Singh,P., Rawat,A. and Vyas,S.P. (2002) pH-sensitive liposomes: mechanism of triggered release to drug and gene delivery prospects. Pharmazie, 57, 659–671. [PubMed] [Google Scholar]
- 32.Pasqualini R., Koivunen,E. and Ruoslahti,E. (1997) Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol., 15, 542–546. [DOI] [PubMed] [Google Scholar]
- 33.Bello R.M. and Midoux,P. (2001) Histidylated polylysine as DNA vector: elevation of the imidazole protonation and reduced cellular uptake without change in the polyfection efficiency of serum stabilized negative polyplexes. Bioconjugate Chem., 12, 92–99. [DOI] [PubMed] [Google Scholar]