Abstract
Objective
While intensity-modulated radiation therapy (IMRT) allows more precise radiation planning, the technology is substantially more costly than conformal radiation and, to date, the benefits of IMRT for uterine cancer are not well defined. We examined the use of IMRT and its effect on late toxicity for uterine cancer.
Methods
Women with uterine cancer treated from 2001-2007 and registered in the SEER-Medicare database were examined. We investigated the extent and predictors of IMRT administration. The incidence of acute and late-radiation toxicities were compared for IMRT and conformal radiation.
Results
We identified a total of 3555 patients including 328 (9.2%) who received IMRT. Use of IMRT increased from 1.5% in 2001 to 23.2% in 2007. In a multivariable model, residence in the western U.S. and receipt of chemotherapy were associated with receipt of IMRT. Women who received IMRT had a higher rate of bowel obstruction (rate ratio=1.41; 95% CI, 1.03-1.93), but other late gastrointestinal and genitourinary toxicities as well as hip fracture rates were similar between the cohorts. After accounting for other characteristics, the cost of IMRT was $14,706 (95% CI, $12,073 to $17,339) greater than conformal radiation.
Conclusion
Use of IMRT for uterine cancer is increasing rapidly. IMRT was not associated with a reduction in radiation toxicity, but was more costly.
Introduction
Despite a number of randomized clinical trials, the role of whole pelvic radiation for uterine cancer remains controversial.[1-3] For women with uterine-confined disease several studies have suggested that adjuvant external beam therapy reduces locoregional recurrences but does not improve overall survival.[1-3] For patients with tumor spread beyond the uterus, the role of radiation is evolving. While chemotherapy is now commonly used for advanced stage disease, pelvic radiotherapy is still often given in combination with cytotoxic therapy.[4, 5]
Traditionally, pelvic radiotherapy is delivered with a four-field box technique. While two-dimensional treatment plans were typically developed using fluoroscopy, three-dimensional conformal radiotherapy with computed tomography guided planning is now widely available.[6] Although conformal therapy provides excellent local tumor control, the normal anatomic structures of the pelvis are also at substantial risk for radiation toxicity. Both acute and late radiation toxicity of the small bowel, rectum, bladder and bone marrow are relatively common.[7]
To more precisely tailor radiation delivery, intensity-modulated radiation therapy (IMRT) has been developed. IMRT allows modulation of the radiation beam over its course to more precisely deliver radiotherapy to target tissues and spare nearby normal structures.[6, 8-11] The outcomes of IMRT compared to conformal therapy have been reported for a number of other tumor sites, but data describing pelvic IMRT for uterine cancer are limited.[8, 9, 12-18] Early studies have shown that IMRT reduces the radiation dose delivered to normal pelvic structures and suggest that IMRT is associated with lower rates of acute toxicity.[8, 9, 11-13, 19-23] Long-term toxicity data and the impact of IMRT on survival are largely lacking. Given the paucity of data describing pelvic IMRT for uterine cancer, we performed a population-based analysis to determine the uptake of IMRT, examine the effect of IMRT on late toxicity, and analyze the cost associated with IMRT compared to conformal pelvic radiotherapy for women with uterine cancer.
Methods
Data Source
We utilized the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.[24] SEER is a population-based cancer registry maintained by the National Cancer Institute that provides data on tumor characteristics, treatment, and survival, as well as demographic and selected census tract-level information. The Medicare database includes information on patients with Medicare part A (inpatient) and part B (outpatient) including diagnoses and billed claims. These two files are linked and provide data on initial services and follow-up. Exemption from the Columbia University Institutional Review Board was obtained. The SEER-Medicare database has been validated and utilized in a number of outcomes studies.[24-26]
Patient Selection
Women ≥65 years of age with primary tumors of the uterus diagnosed between January 1, 2001 and December 31, 2007 were analyzed. Only patients who received radiotherapy as defined below with a first claim within 18 months of diagnosis were included in the cohort. Each patient was classified as having received IMRT or conformal radiation using ICD-9 and CPT codes as previously described (Supplementary Table).[14, 15] We excluded patients with any radiation claims >6 months before the recorded diagnosis of uterine cancer as well as women with any claims for potential late radiation toxicities prior to initiation of radiation. We excluded patients who were enrolled in a non-Medicare health maintenance organization because the billing claims for these patients are not submitted to Medicare for reimbursement.[27] Patients diagnosed at autopsy or by death certificate were also excluded and only patients who survived for more than 6 months after diagnosis were included in the analysis.
Patient Characteristics
Age at diagnosis was categorized into 5-year intervals. We recoded the SEER marital status variable as married, not married, and unknown. Socioeconomic status (SES) was generated from an aggregate score derived from education, poverty level, and income from the 2000 census tract data, as described previously.[28] Patients' scores were ranked on a scale of 1-5 with 1 being the lowest value. To estimate the prevalence of comorbid disease in our cohort, we used the Klabunde adaptation of the Charlson comorbidity index.[29, 30] Area of residence was categorized as metropolitan or nonmetropolitan. Tumor grade was grouped as well, moderately, or poorly differentiated or unknown.[31] The SEER registries in which patients were treated were categorized as: eastern (Connecticut, New Jersey), Midwestern (Detroit, Iowa, Utah, Atlanta, rural Georgia, Kentucky, Louisiana), and western (San Francisco, Hawaii, New Mexico, San Jose, Seattle, Los Angeles, greater California). Tumor histology was grouped as: endometrioid, serous, clear cell, carcinosarcoma, leiomyosarcoma, and other/unknown.[32] Performance of hysterectomy and lymphadenectomy, defined as removal of any lymph nodes, were recorded for each patient. Likewise, use of chemotherapy (any cytotoxic agent) and vaginal brachytherapy within 18 months of diagnosis was noted for each subject. Stage was recorded based on extent of disease and reported according to the 1988 FIGO classification system.
Outcomes
We examined late radiation toxicities reported with pelvic radiotherapy. Late radiation toxicity included radiation enteritis, bowel obstruction, other gastrointestinal complications (colonic stricture, vascular insufficiency of the bowel, rectovaginal fistula), genitourinary complications (radiation cystitis, genitourinary stricture, vesico-vaginal fistula), and hip fracture (Supplemental Table).
The cost of radiation was determined using Medicare reimbursement data for all claims associated with a code for conformal radiation or IMRT. Cost data was adjusted for inflation using the Prospective Pricing Index for part A claims and the Medicare Economic Index for part B claims and reported in 2010 dollars using previously described methodology.[33, 34] To adjust for geographic variation, we used the geographic adjustment factor for part A claims and the geographic practice cost index for part B claims.[33, 34] The total costs for all inpatient and outpatient claims associated with a billing code for radiation or IMRT were summed to report an aggregate, per patient radiation cost. Radiation cost data is reported as median costs with interquartile ranges.
Statistical Analysis
Clinical and demographic characteristics for women receiving conformal radiation therapy and IMRT were compared using χ2 tests. Multivariabe logistic regression models were developed to examine predictors of IMRT use while controlling for other clinical variables. Results are reported as odds ratios with 95% confidence intervals (CI). [35] Given that cost data is not normally distributed, multivariable adjustment of costs based on the type of radiation administered were analyzed using quantile (median) regression methods.[36] This method directly estimates the adjusted median costs and 95% confidence intervals were derived based on bootstrap resampling methods. To estimate toxicities, we calculated the rate of each radiation-associated toxicity per 100 person-years of follow-up as previously described.[14] Follow-up was calculated as the time from the first radiation claim to the date of last contact or death. Toxicity was compared between the conformal radiation therapy and IMRT groups using rate ratios with 95% confidence intervals.[14] The cumulative incidence of late radiation toxicity was further examined using Kaplan-Meier analyses. Separate analyses were reported for late gastrointestinal toxicity (gastrointestinal fistula or stricture), late genitourinary toxicity (genitourinary fistula, stricture, or radiation cystitis) and hip fracture. For each analysis the results were compared using the log-rank test. All analyses were conducted with SAS, version 9.13 (SAS Institute, Cary, NC). All statistical tests were two-sided.
Results
A total of 3555 patients with uterine cancer who received pelvic radiotherapy were identified. Overall, 3227 (90.8%) women received conformal radiation while 328 (9.2%) received IMRT (Table 1). Use of IMRT increased over time from 3.3% in 2002 to 23.2% by 2007 (Figure 1). IMRT was used for 9.0% of white women compared to 11.1% of black women (P=0.46) and in 9.4% of residents of metropolitan areas vs. 7.8% of those residing in non-metropolitan areas (P=0.35). Patients in the western U.S. were more likely to receive IMRT than women in the eastern and Midwestern regions (P<0.0001). Women who received chemotherapy (P<0.0001) and those who did not receive brachytherapy (P=0.006) were more likely to receive IMRT.
Table 1.
Clinical and demographic characteristics of the cohort stratified by radiation.
| Conformal radiation | IMRT | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | (%) | N | (%) | P-value | |
| 3227 | (90.8) | 328 | (9.2) | ||
| Age (years) | 0.13 | ||||
| 65-69 | 733 | (90.2) | 80 | (9.8) | |
| 70-74 | 870 | (91.3) | 83 | (8.7) | |
| 75-79 | 796 | (92.3) | 66 | (7.7) | |
| ≥80 | 828 | (89.3) | 99 | (10.7) | |
| Race | 0.46 | ||||
| White | 2810 | (91.0) | 278 | (9.0) | |
| Black | 248 | (88.9) | 31 | (11.1) | |
| Other/Hispanic/missing | 169 | (89.9) | 19 | (10.1) | |
| Year of diagnosis | <0.0001 | ||||
| 2001/2002 | 1036 | (97.6) | 25 | (2.4) | |
| 2003 | 505 | (95.1) | 26 | (4.9) | |
| 2004 | 460 | (91.5) | 43 | (8.6) | |
| 2005 | 440 | (91.9) | 39 | (8.1) | |
| 2006 | 422 | (83.2) | 85 | (16.8) | |
| 2007 | 364 | (76.8) | 110 | (23.2) | |
| Marital status | 0.62 | ||||
| Married | 1385 | (90.4) | 147 | (9.6) | |
| Unmarried | 1744 | (91.2) | 199 | (8.8) | |
| Unknown | 98 | (89.1) | 12 | (10.9) | |
| Area of residence | 0.35 | ||||
| Metropolitan | 2931 | (90.6) | 303 | (9.4) | |
| Non-metropolitan | 296 | (92.2) | 25 | (7.8) | |
| SEER registry | <0.0001 | ||||
| Eastern | 1087 | (94.7) | 61 | (5.3) | |
| Midwest | 1110 | (90.7) | 114 | (9.3) | |
| West | 1030 | (87.1) | 153 | (12.9) | |
| Socioeconomic status | 0.12 | ||||
| Lowest (first) quintile/unknown | 390 | (88.4) | 51 | (11.6) | |
| Second quintile | 623 | (89.0) | 77 | (11.0) | |
| Third quintile | 762 | (91.5) | 71 | (8.5) | |
| Fourth quintile | 731 | (92.0) | 64 | (8.1) | |
| Highest (fifth) quintile | 721 | (91.7) | 65 | (8.3) | |
| Comorbidity score | 0.68 | ||||
| 0 | 1577 | (91.0) | 156 | (9.0) | |
| 1 | 913 | (91.0) | 90 | (9.0) | |
| ≥2 | 737 | (90.0) | 82 | (10.0) | |
| Tumor histology | 0.65 | ||||
| Endometrioid | 2207 | (90.5) | 233 | (9.6) | |
| Serous/clear cell | 340 | (90.9) | 34 | (9.1) | |
| Carcinosarcoma/leiomyosarcoma | 303 | (91.0) | 30 | (9.0) | |
| Other/unknown | 377 | (92.4) | 31 | (7.6) | |
| Stage1 | <0.0001 | ||||
| IA/IB | 250 | (88.0) | 34 | (12.0) | |
| IC | 396 | (89.8) | 45 | (10.2) | |
| INOS | 815 | (95.0) | 43 | (5.0) | |
| II | 491 | (92.0) | 43 | (8.1) | |
| III | 695 | (87.4) | 100 | (12.6) | |
| IV | 339 | (94.2) | 21 | (5.8) | |
| Unknown | 241 | (85.2) | 42 | (14.8) | |
| Tumor grade | 0.47 | ||||
| 1 | 526 | (90.5) | 55 | (9.5) | |
| 2 | 1004 | (91.9) | 89 | (8.1) | |
| 3 | 1255 | (90.4) | 133 | (9.6) | |
| Unknown | 442 | (89.7) | 51 | (10.3) | |
| Hysterectomy | 0.25 | ||||
| No | 474 | (89.4) | 56 | (10.6) | |
| Yes | 2753 | (91.0) | 272 | (9.0) | |
| Lymphadenectomy | 0.31 | ||||
| No | 1223 | (91.4) | 115 | (8.6) | |
| Yes | 2004 | (90.4) | 213 | (9.6) | |
| Brachytherapy | 0.006 | ||||
| No | 1593 | (89.4) | 188 | (10.6) | |
| Yes | 1634 | (92.1) | 140 | (7.9) | |
| Chemotherapy | <0.0001 | ||||
| No | 2306 | (92.5) | 187 | (7.5) | |
| Yes | 921 | (86.7) | 141 | (13.3) | |
| Location of radiation administration | <0.0001 | ||||
| Hospital-based | 52 | (100) | - | - | |
| Free standing | 883 | (87.4) | 127 | (12.6) | |
| Combination | 2292 | (91.9) | 201 | (8.1) | |
FIGO (International Federation of Obstetrics and Gynecology) 1998 staging criteria.
SEER=Surveillance, Epidemiology, and End Results. IMRT=intensity-modulated radiation therapy.
Figure 1.
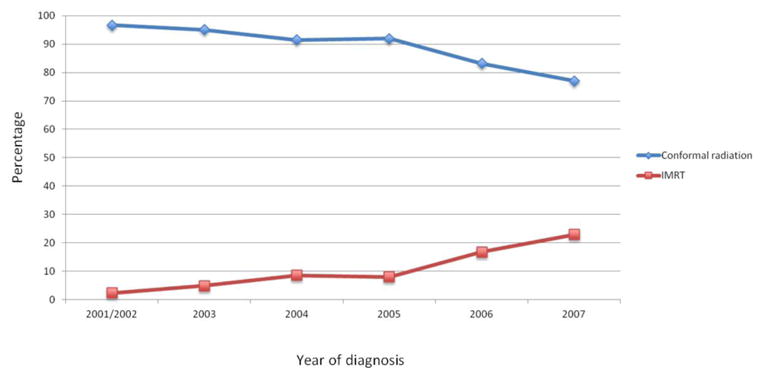
Pelvic radiotherapy stratified by year of treatment for women with uterine cancer.
In a multivariable model, year of diagnosis remained the strongest predictor of IMRT use; compared to women treated in 2001 the odds ratio for receiving IMRT in 2007 was 15.26 (95% CI, 7.26-32.08) (Table 2). Compared to women in the eastern U.S., those in the Midwest (OR=1.74; 95% CI, 1.25-2.41) and those in the western U.S. (OR=2.23; 95% CI, 1.63-3.04) were more likely to receive IMRT. Patients who received chemotherapy were 74% more likely to have IMRT than those who did not receive chemotherapy.
Table 2.
Multivariable logistic regression model of predictors of IMRT use.
| IMRT | |
|---|---|
| Age (years) | |
| 65-69 | Referent |
| 70-74 | 0.93 (0.68.1.27) |
| 75-79 | 0.94 (0.67-1.31) |
| ≥80 | 1.27 (0.92-1.75) |
| Race | |
| White | Referent |
| Black | 1.07 (0.71-1.61) |
| Hispanic | 0.28 (0.07-1.12) |
| Other | 0.88 (0.53-1.46) |
| Year of diagnosis | |
| 2001 | Referent |
| 2002 | 2.36 (1.02-5.47)* |
| 2003 | 3.49 (1.58-7.72)* |
| 2004 | 6.50 (2.98-14.19)* |
| 2005 | 5.91 (2.69-12.98)* |
| 2006 | 11.60 (5.48-24.58)* |
| 2007 | 15.26 (7.26-32.08)* |
| Marital status | |
| Married | Referent |
| Unmarried | 0.87 (0.69-1.10) |
| Unknown | 1.23 (0.67-2.26) |
| Area of residence | |
| Non-metropolitan | Referent |
| Metropolitan | 1.50 (0.97-2.33) |
| SEER registry | |
| Eastern | Referent |
| Midwest | 1.74 (1.25-2.41)* |
| West | 2.23 (1.63-3.04)* |
| Socioeconomic status | |
| Lowest (first) quintile | Referent |
| Second quintile | 1.05 (0.73-1.52) |
| Third quintile | 0.79 (0.54-1.15) |
| Fourth quintile | 0.76 (0.51-1.14) |
| Highest (fifth) quintile | 0.78 (0.52-1.16) |
| Comorbidity score | |
| 0 | Referent |
| 1 | 0.95 (0.73-1.23) |
| ≥2 | 1.06 (0.80-1.41) |
| Tumor histology | |
| Endometrioid | Referent |
| Serous | 0.71 (0.46-1.10) |
| Clear cell | 0.70 (0.32-1.52) |
| Carcinosarcoma | 0.70 (0.44-1.11) |
| Leiomyosarcoma | 0.61 (0.23-1.62) |
| Other/unknown | 0.62 (0.42-0.92)* |
| Stage1 | |
| IA | Referent |
| IB | 0.91 (0.39-2.11) |
| IC | 0.79 (0.35-1.78) |
| INOS | 1.17 (0.50-2.70) |
| II | 0.97 (0.43-2.18) |
| III | 1.13 (0.52-2.46) |
| IV | 0.68 (0.28-1.63) |
| Unknown | |
| Tumor grade | |
| 1 | Referent |
| 2 | 0.85 (0.61-1.20) |
| 3 | 0.89 (0.63-1.24) |
| Unknown | 1.02 (0.67-1.54) |
| Hysterectomy | |
| No | Referent |
| Yes | 0.93 (0.62-1.39) |
| Lymphadenectomy | |
| No | Referent |
| Yes | 1.03 (0.76-1.39) |
| Brachytherapy | |
| No | Referent |
| Yes | 0.82 (0.65-1.03) |
| Chemotherapy | |
| No | Referent |
| Yes | 1.75 (1.36-2.25)* |
FIGO (International Federation of Obstetrics and Gynecology) 1998 staging criteria.
SEER=Surveillance, Epidemiology, and End Results. IMRT=intensity-modulated radiation therapy.
P<0.005
Table 3 displays late radiation-related toxicities and toxicity rates per 100 person-years of follow-up. Compared to women who received conformal RT, those who received IMRT had a higher rate of bowel obstruction (rate ratio=1.41; 95% CI, 1.03-1.93). The rates of other late gastrointestinal toxicities were similar between the cohorts (Table 3). Likewise, there was no statistically significant difference in late genitourinary toxicity (RR=1.24; 95% CI, 0.85-1.81). The 100 person-year rate of hip fractures was 32.4 in women who received IMRT compared to 23.4 in those who received conformal therapy (rate ratio=1.38; 95% CI, 0.96-1.99).
Table 3.
Radiation-related toxicity stratified by type of radiation administered.
| Conformal Radiation | IMRT | IMRT vs. Conformal RT | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | (%) | 100 Person -Years | Rate | N | (%) | 100 Person -Years | Rate | Rate ratio | |
| Late toxicities | |||||||||
| Radiation enteritis | 275 | 8.5% | 11.55 | 23.8 | 19 | 5.8% | 0.65 | 29.1 | 1.22 (0.77-1.94) |
| Bowel obstruction | 494 | 15.3% | 18.68 | 26.4 | 42 | 12.8% | 1.12 | 37.3 | 1.41 (1.03-1.93)* |
| Other gastrointestinal | 176 | 5.5% | 7.18 | 24.5 | 12 | 3.7% | 0.35 | 34.4 | 1,49 (0.78-2.52) |
| complications | |||||||||
| Genitourinary | 271 | 8.4% | 10.61 | 25.5 | 30 | 9.2% | 0.95 | 31.7 | 1.24 (0.85-1.81) |
| complication | |||||||||
| Hip fracture | 376 | 11.7% | 16.06 | 23.4 | 32 | 9.8% | 0.99 | 32.4 | 1.38 (0.96-1.99) |
IMRT=intensity-modulated radiation therapy
A series of Kaplan-Meier analyses were performed to explore differences in the time to development of late radiation toxicity. There were no differences in the rates of gastrointestinal toxicity (P=0.66), genitourinary toxicity (P=0.46) or hip fracture (P=0.46) (Figure 2). The median cost of a course of conformal radiotherapy was $14,644 (IQR, $6226, $23,587) compared to $26,149 (IQR, $20,078, $38,642) for IMRT (P<0.0001). In a quantile regression model accounting for other patient, tumor, and treatment characteristics, the cost of IMRT remained $14,706 (95% CI, $12,073 to $17,339) greater than conformal radiation.
Figure 2.
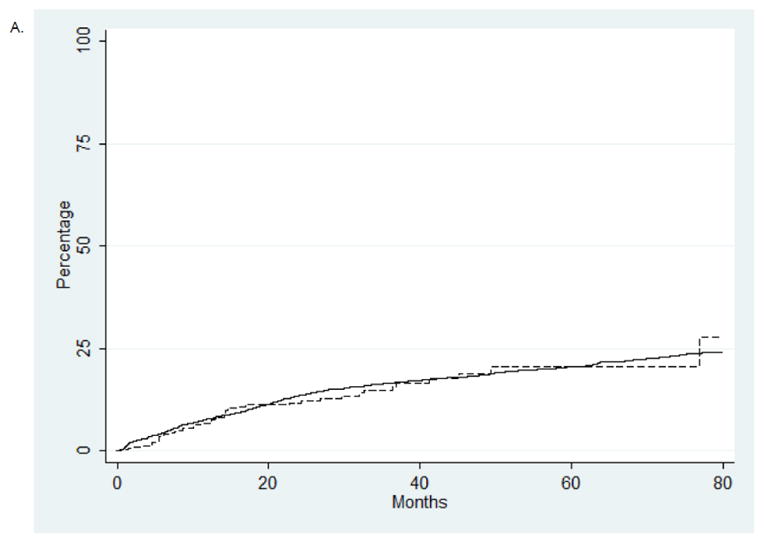
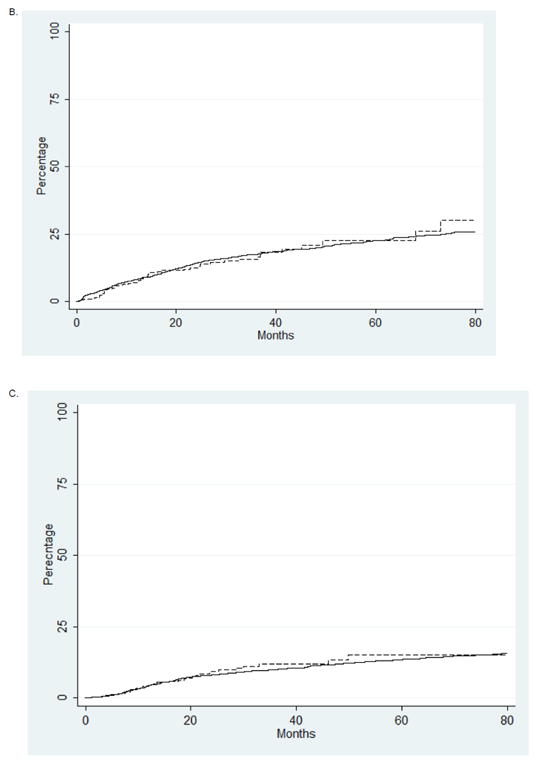
Kaplan-Meier analysis of late radiation toxicity stratified by type of radiation administered. (A) Gastroinstestinal toxicity (P=0.66). B. Genitourinary toxicity (P=0.46). C. Hip fracture (P=0.24).
Discussion
Data describing the effectiveness of pelvic IMRT for uterine cancer is limited and predominately consists of small studies comparing IMRT to historical controls. Compared to conformal pelvic radiotherapy, IMRT appears to spare normal tissues and has been associated with lower rates of acute toxicity.[6, 8, 9, 11-13, 19, 23] One series noted gastrointestinal toxicity in 11% of women who received IMRT compared to 50% in those who had whole pelvic radiation while a second study noted a reduction from 20% to 10% in grade 2 or greater genitourinary toxicity with IMRT.[9, 13] A recently reported phase II feasibility trial by the Radiation Therapy Oncology Group (RTOG) reported a non-significant 12% reduction in grade ≥2 bowel adverse events compared to historic controls and concluded that a phase III trial was warranted.[8]
Our findings are somewhat surprising in that we found little difference in the rates of late toxicity between the IMRT cohort compared to those who received conformal radiotherapy. One hypothesis for these findings is that the radiation dose delivered to surrounding tissues for those who received IMRT was greater than planned and expected. The difficulty in treatment planning for IMRT was demonstrated by the RTOG's feasibility trial in which 68% of the treatment plans delivered a higher than specified dose to the bladder, 76% of subjects received a higher than planned rectal dose and the femoral head constraints were exceeded in 33% of women.[8] Given that these results are from highly experienced centers it appears likely that field planning for IMRT, and probably outcome, are highly variable. Our data suggests that the potential benefits of IMRT for patients with uterine cancer have not yet been realized.
A number of studies have examined the uptake and utilization of IMRT for other tumor sites.[10, 14, 15, 37] From 2001 to 2005 use of IMRT for breast cancer increased from <1% to 11% and from 4% to 46% for patients with head and neck tumors.[15, 37] Perhaps most dramatically, use of IMRT for men with localized prostate cancer increased from 0.2% in 2000 to 96% by 2008.[14] Although prospective data for the effectiveness of IMRT for both breast and head and neck cancer have been reported, the data for prostate cancer is mainly derived from observational series.[16-18] Similar to our findings, many of these studies also identified significant regional variation in use of IMRT and hypothesized that these differences were due to differential Medicare reimbursement policies across regions.[10, 15, 37]
We were unable to demonstrate a decrease in late radiation toxicity with IMRT compared to conformal radiotherapy. Long-term analysis of women with stage I endometrial cancer who received whole pelvic radiation in the PORTEC trial noted 5-year actuarial toxicity rates of 20% for gastrointestinal side effects and 8% for genitourinary complications. Severe (grade 3 and 4) late toxicity was less common, and occurred in only 3% of patients. Notably, all of the severe late toxicities in the PORTEC study were gastrointestinal.[7] A number of treatment factors including field volume, daily fractionation, and use of brachytherapy as well as patient and tumor characteristics influence the incidence of radiation-related toxicity.[7] Given that adjuvant pelvic radiation for endometrial cancer is delivered to a large tissue volume and uses a relatively low dose of radiation (45-55 Gy), the potential toxicity benefits of IMRT for uterine cancer may be less significant than when pelvic radiotherapy is given for cervical cancer in which the total dose of radiation delivered (>80 Gy) is often much higher.
A major concern surrounding the use of IMRT is cost. For breast cancer, IMRT is more than twice as expensive as conformal therapy ($15,230 vs. $7179).[15] Our findings for uterine cancer were similar; compared to conformal therapy, the median cost for IMRT was nearly $12,000 greater. Given the substantial cost of IMRT, reimbursement appears to play an important role in allocation of the technology. Smith an colleagues noted that among Medicare beneficiaries with breast cancer, those women residing in regions with the most favorable reimbursement coverage were much more likely to receive IMRT than those patients who resided in regions with less favorable remuneration. Likewise, patients treated at freestanding radiation centers were more likely to receive IMRT than those managed at hospital-associated centers.[15] We also identified substantial regional variation in use of IMRT that may have been influenced by reimbursement policies.
We recognize a number of important limitations in our analysis. Perhaps most importantly, we lack details on the actual delivery of radiation including the total dose delivered, fractionation schedule, and total treatment time. All of these parameters are known to influence toxicity and certainly warrant further study. Our study encompasses the years in which pelvic IMRT disseminated into practice. As with any new procedure, results might be expected to improve as physicians gain experience with the technique. The phase II RTOG trial demonstrates the difficulties with treatment planning for IMRT.[8] We recognize that not all toxicities may have been captured. To limit this bias we analyzed only major toxicities that were likely to generate a claim. Any underreporting of toxicities should have been balanced between the two groups. Finally, we are unable to record individual patient and physician preferences as well as subtle unmeasured patient and tumor characteristics that likely influenced both the allocation of treatment as well as outcomes.
In conclusion, our findings suggest that the use of IMRT has increased over time for women with uterine cancer undergoing pelvic radiotherapy. Despite the theoretic benefits of IMRT, we were unable to demonstrate a reduction in the rate of late radiation-related toxicities. Given the increased cost of IMRT, further randomized trials to examine the effectiveness and quality of treatment planning for women with uterine cancer undergoing pelvic radiotherapy are warranted.
Supplementary Material
Research Highlights.
Use of IMRT for uterine cancer is increasing rapidly.
IMRT is not associated with a reduction in radiation toxicity, but was more costly.
Acknowledgments
Dr. Hershman is the recipient of a grant from the National Cancer Institute (NCI R01CA134964).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Information Services, and the Office of Strategic Planning, HCFA; Information Management Services (IMS), Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflicts of Interest Notification: There are no financial disclosures, conflicts of interest or acknowledgements.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keys HM, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Creutzberg CL, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355(9213):1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 3.Blake P, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137–46. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homesley HD, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112(3):543–52. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez Secord A, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107(2):285–91. doi: 10.1016/j.ygyno.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Small W, Jr, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):428–34. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creutzberg CL, et al. The morbidity of treatment for patients with Stage I endometrial cancer: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2001;51(5):1246–55. doi: 10.1016/s0360-3016(01)01765-5. [DOI] [PubMed] [Google Scholar]
- 8.Jhingran A, et al. A Phase II Study of Intensity Modulated Radiation Therapy to the Pelvis for Postoperative Patients With Endometrial Carcinoma: Radiation Therapy Oncology Group Trial 0418. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56(5):1354–60. doi: 10.1016/s0360-3016(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 10.Sher DJ, et al. Predictors of IMRT and conformal radiotherapy use in head and neck squamous cell carcinoma: a SEER-Medicare analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):e197–206. doi: 10.1016/j.ijrobp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Roeske JC, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69(2):201–7. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Brixey CJ, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54(5):1388–96. doi: 10.1016/s0360-3016(02)03801-4. [DOI] [PubMed] [Google Scholar]
- 13.Mundt AJ, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52(5):1330–7. doi: 10.1016/s0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 14.Sheets NC, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Jama. 2012;307(15):1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith BD, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103(10):798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 16.Pignol JP, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–92. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 17.Kam MK, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–9. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 18.Pow EH, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–91. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Ahamad A, et al. Intensity-modulated radiation therapy after hysterectomy: comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys. 2005;62(4):1117–24. doi: 10.1016/j.ijrobp.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Portelance L, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51(1):261–6. doi: 10.1016/s0360-3016(01)01664-9. [DOI] [PubMed] [Google Scholar]
- 21.Wong E, et al. Intensity-modulated arc therapy for treatment of high-risk endometrial malignancies. Int J Radiat Oncol Biol Phys. 2005;61(13):830–41. doi: 10.1016/j.ijrobp.2004.06.253. [DOI] [PubMed] [Google Scholar]
- 22.Lujan AE, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57(2):516–21. doi: 10.1016/s0360-3016(03)00521-2. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, et al. Prospective Study of Functional Bone Marrow-Sparing Intensity Modulated Radiation Therapy With Concurrent Chemotherapy for Pelvic Malignancies. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Potosky AL, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–48. [PubMed] [Google Scholar]
- 25.Du X, et al. Accuracy and completeness of Medicare claims data for surgical treatment of breast cancer. Med Care. 2000;38(7):719–27. doi: 10.1097/00005650-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Warren JL, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 27.Hershman DL, et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst. 2009;101(23):1633–41. doi: 10.1093/jnci/djp387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du XL, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110(3):660–9. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, et al. Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39(6):439–52. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 31.Wright JD, et al. Use and benefits of laparoscopic hysterectomy for stage I endometrial cancer among medicare beneficiaries. J Oncol Pract. 2012;8(5):e89–99. doi: 10.1200/JOP.2011.000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JD, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–85. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
- 33.Brown ML, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV-104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 34.Warren JL, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–97. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison PD. Logistic regression using SAS: theory and application. Cary, North Carolina: SAS Institute Inc; 2012. [Google Scholar]
- 36.Koenker R. Quantile Regression. New York: Canbridge University Press; 2005. [Google Scholar]
- 37.Guadagnolo BA, et al. Evaluation of trends in the use of intensity-modulated radiotherapy for head and neck cancer from 2000 through 2005: socioeconomic disparity and geographic variation in a large population-based cohort. Cancer. 2010;116(14):3505–12. doi: 10.1002/cncr.25205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


