Abstract
Background: In cases of severe subversion of the morphology of calcaneal fractures with trabecular defects, bone graft is often necessary to provide a mechanical buttress. The mineralized collagen (MC) is a novel bone substitute that is developed by biomimetic synthesis strategy that mimics the extracellular matrix (ECM) of natural bone in structure and chemical composition. It can avoid donor site morbidity and complications associated with harvesting autologous bone graft.
Objective: In this study, we conducted a retrospective matched-pair analysis to assess the clinical and radiological performances of MC as a bone graft substitute in intra-articular calcaneal fractures with trabecular defects.
Methods: 24 pairs of intra-articular calcaneal fractures with trabecular defects were treated with open reduction, internal fixation, and grafting either with MC or autograft. Patient demographics, medical history, and CT fracture classification were matched. Fractures were monitored 6 weeks, 12 weeks, 6 months, and 1 year postoperatively for healing and postoperative complications and results were analyzed.
Results: All patients had follow-up at a minimum of 12 months after surgery with a mean follow-up time of 17 months. All fractures were healed; there were no significant differences in the meantime to union and clinical between the two groups. The radiographic evaluation confirmed that a significant improvement in the mean Böhler’s angle, Gissane’s angle and the calcaneus height was observed in all patients in both treatment groups. A total of 29% (7/24) of patients suffered from harvest-site morbidity at 12 months in the autograft group. In contrast, all patients were free from postoperative local complications in the iliac region and no patient developed adverse reactions attributable to MC in the MC group.
Conclusion: These results justify and favor the use of MC as a good autograft alternative in displaced intra-articular calcaneal fractures with trabecular defects.
Keywords: calcaneal fracture, trabecular defect, mineralized collagen, bone substitute, autograft alternative
Introduction
Displaced intra-articular fractures of the calcaneus remain a therapeutic dilemma for most surgeons. The current trend point for the treatment of intra-articular calcaneal fractures is open reduction and internal fixation (ORIF), but the most appropriate management still remains in dispute.1-4 From the view of the biomechanical, the posterior facet is an important part of the calcaneal arc, a large cancellous defect in this area requires bone graft to provide a mechanical buttress and to avoid secondary loss of reduction or alignment. Among available graft materials, autologous bone graft is considered the gold standard and is the choice of most surgeons currently. However, the morbidity and complications of associated with autologous bone graft have prompted the development of bone graft substitutes as alternatives.5-7
Ideally, a synthetic bone graft substitute should mimic the nature bone in macro/micro- structure, mechanical strength, bioactivity such as bone conduction and induction. Most importantly, At the nanostructural level, nature bone is comprised mainly of collagen fibers and nanocrystals of bone minerals, particularly hydroxyapatite (HA).8 According to the principle of biomimetic strategy, an engineered HA-collagen nanocomposite (mineralized collagen, MC) has been developed.9,10 It is a novel bone substitute that has similar composition and hierarchical structure to natural bone. MC has a porous structure with porosity of 90% and pore size of 100–300 μm. The compressive strength of MC is about 3 MPa, which is comparable to human cancellous bone. Previous in vivo preclinical and clinical trials have shown MC is a safe and promising substitute for autologous iliac crest bone graft.10-12 It has now been approved by the China Food and Drug Administration as a class III medical device.
The purpose of the present study was to assess the efficacy of MC as a bone graft substitute compared with the use of autologous iliac crest bone graft for treating displaced intra-articular calcaneal fractures.
Results
Patient disposition
According to the inclusion and exclusion criteria, we performed 1:1 matching on the basis of age (within 3 y), sex, and fracture classification.13,14 Finally, we matched 24 pairs of patients. There were 32 men and 16 women, with a mean age of 46 y. Follow-up time frame was ranged from 12 to 24 mo with mean follow-up at 17 mo. The injuries of mechanism were falls (n = 26) or motor vehicle accidents (n = 22). According to the Sanders classification, there were 12 type II, 20 type III, and 16 type IV fractures. ORIF was performed at a mean of 7 d after injury (range, 5–17 d).
Radiographic outcomes
The radiographic evaluation confirmed that all the fractures were healed without secondary loss of reduction.15 Preoperative lateral X-ray film showed intra-articular calcaneal fracture (Fig. 1A). At 6 mo postoperatively, the MC group lateral X-ray film showed satisfactory healing and clinical union (Fig. 1B).The mean time to union in autograft group was shorter than MC group (7.9 vs. 8.3 wk), but this finding was not statistically significant (P > 0.05). At 6 mo and 12 mo postoperatively, there were no statistically significant difference that existed in the radiological assessment between the two groups P > 0.05 (Table 1).
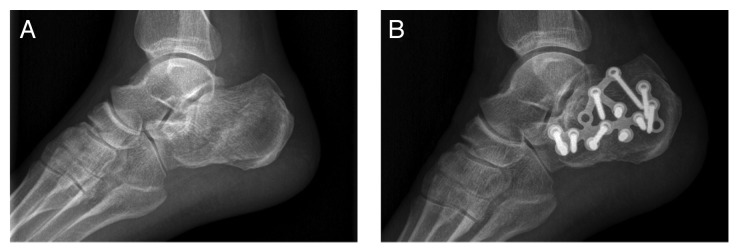
Figure 1. CT imaging of the treatment site. (A) preoperative three-dimensional CT imaging; (B) 6 mo postoperative CT imaging.
Table 1. Treatment effects of the MC group and the autograft group.
| Group | Parameter | preoperatively | 6 mo postoperatively | 12 mo postoperatively |
|---|---|---|---|---|
| A | Böhler’s angle (°) | 6.2 ± 7.1 | 30.6 ± 7.5* | 30.4 ± 6. 8* |
| Gissane’s angle (°) | 138.1 ± 15 | 117.6 ± 9.4* | 117.2 ± 9.4* | |
| calcaneus height (mm) | 37.2 ± 5.6 | 48.5 ± 6.5* | 48.2 ± 2.6* | |
| B | Böhler’s angle (°) | 6.4 ± 4.2 | 30.5 ± 8.5* | 30.3 ± 7.6* |
| Gissane’s angle (°) | 136.2 ± 12 | 119.5 ± 9.2* | 118.2 ± 10* | |
| calcaneus height (mm) | 38.2 ± 4.6 | 48.3 ± 5.4* | 48.6 ± 6.3* |
A (Autograft group n = 24), B (MC group n = 24). Data are presented as mean ± standard deviation (*P > 0.05).
In the MC group, postoperative CT scan imaging was available for review on 8 patients. Preoperative three-dimensional CT image showed the fracture configuration (Fig. 2A).At 6 mo postoperatively, CT imaging demonstrated the radiolucent zones between implanted MC and the surrounding bone immediately postoperatively, which gradually disappeared, all areas of implanted MC exhibited intensity that was similar to the surrounding of the cancellous bone, with evidence of graft incorporation (Fig. 2B). At 1 y follow-up all 8 patients demonstrated radiological bone healing and the MC graft was completely replaced by new formed bone.
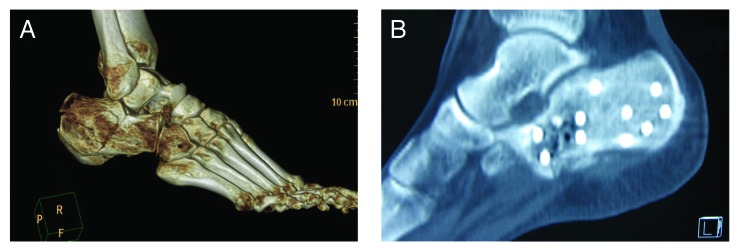
Figure 2. X-ray examination of the healing effect. (A) Preoperative lateral X-ray film; (B) 6 mo postoperative lateral X-ray film.
Clinical outcomes
The mean duration of surgery in autograft group was significantly longer, 106 min (range, 70–120 min) compared with 80 min (range, 50–100 min) in the MC group (P > 0.05). In the autograft group, patients had total score of the Maryland foot averaged 86 ± 10 points at 12 mo postoperative follow-up. This translated to a functional result rating 10 excellent cases, 10 good cases, and 4 fair cases. In comparison, the averaged total score of the Maryland foot in MC group was 90 ± 12 points with 9 excellent cases, 12 good cases, and 3 fair cases. The clinical outcome was not significantly different between the two groups (P > 0.05). At final follow-up, 16 patients reported pain with ambulation, 10 patients exhibited ankle swelling > 1 cm compared with the uninjured foot.
Postoperative complications
Postoperative complications included: three patients developed a secondary wound infection successfully treated with debridements and intravenous antibiotics; four patients experienced delayed wound healing. None of the patients developed material rejection, wound necrosis, or osteonecrosis.
A total of 29% (7/24) of patients suffered from harvest-site morbidity at 12 mo postoperatively in the autograft group, including three patients reported donor site pain, and four patients were dissatisfied with the aesthetics of the iliac crest incision. In contrast, all patients were free from postoperative local complications in the iliac region and no patient developed adverse reactions attributable to MC in the MC group.
Discussion
Bone graft is necessary to achieve a successful reconstruction for the treatment of intra-articular calcaneal fractures with trabecular defects. Autologous bone harvested from iliac crest bone graft has been considered as the gold standard in calcaneal trauma surgery for a long time. However, because of lack of enough amount of autologous bone and related local complications, and the risk of disease transmission with allograft, there has been sustained interest in developing alternative synthesis artificial bone graft materials to fill osseous defects.16-18 Natural bone has good biocompatibility, structure compatibility, and excellent biomechanical strength. It is considered as an assemblage of hierarchical building blocks, elegantly designed with HA and collagen in nanodimensions. Therefore, processing the scaffolding system that mimic the natural bone mainly in terms of structure and chemical composition, is a very important part for the success of bone tissue engineering.19-21
According to the principle of biomimetic strategy, a new bone-resembling composite was developed: mineralized collagen (MC), which has nanostructural and compositional similarity with natural bone tissue. This composite not only possesses high porosity to approximately 90% with pore sizes ranging from 100–300 μm, but also has necessary mechanical strength to support the space. In turn, the cells can undergo proliferation, migration, and differentiation to specific tissue as they would have naturally. Extensive vitro and animal studies have that demonstrated MC has excellent biocompatibility, biodegradability, high osteoconducitive activity, and promotes bone repair and regeneration.10-12 Recently MC has been evaluated in spinal fusion. In a series of 91 patients who underwent two- or three-level ACDF with rigid anterior plate fixation with either autologous or MC, the results demonstrated 95.7% fusion rate for MC with results comparable to those with autologous bone which was 100%.10
Perioperative measures indicated that the operative time and blood loss were significantly less in MC group than in autograft group. There were no differences in postoperative clinical results between the studies groups, however, 29% suffered from harvest-site morbidity in autograft group at 12 mo. The patients in our study were under continuous radiographic observation throughout the evaluation period, specifically compared Böhler’s angle, Gissane’s angle and calcaneus height obtained immediately postoperatively and those obtained after fracture healing as well as 1 y after surgery. In follow-up, there was no significant difference between the two treatment groups in resorted radiographic anatomy parameters. Finally, there were no adverse events directly attributable to the use of MC.
Rapid vascularization of bone graft materials is an important process for prompt and long-term successful osteogenesis.22,23 In the previous study by Liao et al., the new bone-like tissue was found to grow into the implanted nHAC/PLA early in the 2 wk after surgery when this composite was implanted in a rabbit posterolateral spinal fusion model. The authors suggested that the high biocompatibility and similarity to natural bone made this synthetic mineralized collagen composite (MC) and autogenous bone material reaction positive.24,25
In our study, the mean time to union in autograft group was shorter than MC group (7.9 vs. 8.3 wk), but this finding was not statistically significant (P > 0.05). Meanwhile, CT imaging demonstrated that the MC integrates surrounding cancellous bone within six to 12 mo; with evidence of radiological bone healing and MC graft completely reaplaced by new formed bone at 1 y follow-up.
Porosity is an important factor that influences the speed of degradability, high-porosity materials have a high speed of degradability.26 The MC used in the current study is characterized by a homogeneous porosity of 90%, with spherical pores of 100–300 μm in diameter and the pores are well interconnected with each other and open into the outer surface. This macroporosity similar to that of spongious bone is beneficial to facilitate the growth of vasculature into the material and provide an ideal environment for bone formation.
Ideally a bone graft substitute should provide the combination of osteoinductive, osteoconductive and osteogenetic properties. MC has a limitation as a graft material in which it acts purely as an osteoconductive scaffold and contains neither osteogenic cell nor osteoinductive factor. Hence, we have conducted a series of animal experiments using MC in combination with adjuvant osteoinductive materials (P24) to make up for its limitation. The published preliminarily results are encouraging.27-29
Patient and Methods
Materials
MC is prepared in two main steps.9,10 First, mineralized type I collagen fibrils were synthesized by self-assembly of collagen triple helices and hydroxyapatite (HA). During the biomineralization process, HA crystals nucleate and grow within collagen helices and such nucleation and growth are regulated by collagen fibers. Second, the mineralized type I collagen fibrils were mixed with polylactic acid solution and then MC was obtained after thorough freeze-drying. The product was cut into small granules and sterilized by irradiation of60Co before clinical use.
Patients
Hospital records from January 2008 through June 2012 were retrospectively reviewed to identify all patients who suffered from intra-articular calcaneal fractures with trabecular defects who were treated with open reduction, internal fixation, and bone grafting. Patients were divided into two groups: treated group who were grafted with MC (managed between January 2010 and June 2012) and control group who were grafted with autograft (managed between January 2008 and December 2010). The inclusion criteria were (1) closed displaced intra-articular fracture, (2) bone defects greater than 3cm3 after operative reduction, (3) the follow-up period was > 12 mo. The exclusion criteria included (1) cigarette smoking, (2) diabetes, (3) open fractures, (4) an active infection, (5) insufficient soft tissue coverage, (6) pathologic fracture. To minimize other variables such as changes in surgical techniques, all case was performed by the same senior surgeon. This study was approved by the Committee of Medical Ethics and the Institutional Review Board of our hospital and performed with the informed consent of each patient and his or her family.
Surgical technique
Surgical exposure was gained via the extended lateral approach. The skin incision is L-shaped over the lateral aspect of the heel with the horizontal arm and vertical arm continued approximately at the mid-point between the tip of the lateral malleolus and the sole. The incision goes straight down to the bone and a full thickness flap is developed. The peroneal sheath is minimally opened, just sufficient to detach it from the bone and retracted. The posterior facet and the angle of Gissane were meticulously restored and K wires were used for provisional stabilization. After reduction, a bony defect was present beneath the reduced posterior facet. Depending on the group, the bony defect was filled with MC or autograft. Afterward, the osteosynthesis with a standard AO, a calcaneal plate was performed (Fig. 3). For the purpose of autologous grafting, the autograft was obtained from the anterior iliac crest. After reduction final checking with C-Arm fluoroscopy, the wound was closed over a drain without tension.
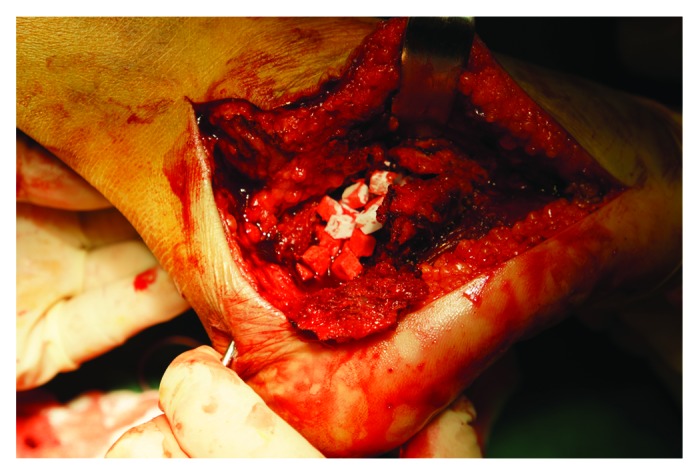
Figure 3. Mineralized collagen implanted in the void.
Radiographic and clinical assessment
A standard X-rays and CT (CT) scan was conducted pre-operatively, immediately post-operatively and then at 3 wk, 12 wk, 6 mo and 1 y postoperatively on all calcaneus fractures. Three radiographical parameters were compared between the two groups: Gissane’s angle, Böhler’s angle, and the calcaneal height using the lateral view. For MC group, CT was reviewed to evaluate the presence of graft incorporation, and new bone regeneration within the defect. The fractures were classified according to the classification systems proposed by Sanders and Zwipp using preoperative CT images.13,14
Clinical follow-up was performed by our research group at 3 wk, 12 wk, 6 mo and 1 y postoperatively, using the Maryland foot score. According to Sanders R et al., the total score on this scale is interpreted as follows: excellent, 90 to 100 points; good, 75 to 89 points; fair, 50 to 74 points; failure, less than 50 points.15
Statistical analysis
Distributions of variables were given as the mean and the standard deviation. The Student t test was used to assess the difference of continuous measures between the groups. The Fisher exact test was used for dichotomous data analysis. The level of significance was set at P < 0.05.
Conclusions
This study demonstrated promising result regarding the efficacy of MC as an extender in displaced intra-articular calcaneal fractures with successful healing rate and clinical scores equivalent to those of autograft graft. MC may be a good autograft alternative in displaced intra-articular calcaneal fractures with trabecular defects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NO. 81171684, 81370134), the International Science and Technology Cooperation Program of China, NO.2013DFG32690), and the Natural Science Foundation of Hubei Province (NO.2012FFB03601).
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/27250
References
- 1.Epstein N, Chandran S, Chou L. Current concepts review: intra-articular fractures of the calcaneus. Foot Ankle Int. 2012;33:79–86. doi: 10.3113/FAI.2012.0079. [DOI] [PubMed] [Google Scholar]
- 2.Lesić AR, Atkinson HD, Bumbasirević V, Bumbasirević MZ. Calcaneal fractures - the orthopaedic challenge. Acta Chir Iugosl. 2012;59:33–9. doi: 10.2298/ACI1203033L. [DOI] [PubMed] [Google Scholar]
- 3.Rammelt S, Zwipp H. Ankle Fractures. In: Bentley G, ed. European Instructional Lectures. Berlin: Springer, 2012:205-19. [Google Scholar]
- 4.Guerado E, Bertrand ML, Cano JR. Management of calcaneal fractures: what have we learnt over the years? Injury. 2012;43:1640–50. doi: 10.1016/j.injury.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Hak DJ. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg. 2007;15:525–36. doi: 10.5435/00124635-200709000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dinopoulos H, Dimitriou R, Giannoudis PV. Bone graft substitutes: What are the options? Surgeon. 2012;10:230–9. doi: 10.1016/j.surge.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Panchbhavi VK. Synthetic bone grafting in foot and ankle surgery. Foot Ankle Clin. 2010;15:559–76. doi: 10.1016/j.fcl.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Faour O, Dimitriou R, Cousins CA, Giannoudis PV. The use of bone graft substitutes in large cancellous voids: any specific needs? Injury. 2011;42(Suppl 2):S87–90. doi: 10.1016/j.injury.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004;69:158–65. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Hong J, Zheng Q, Guo X, Lan S, Cui F, Pan H, Zou Z, Chen C. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J Orthop Res. 2011;29:1745–52. doi: 10.1002/jor.21439. [DOI] [PubMed] [Google Scholar]
- 11.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–7. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Xu L, Cui FZ, Qu Y, Lian XJ, Wang XM, et al. Clinical Evaluation of Mineralized Collagen as a Bone Graft Substitute for Anterior Cervical Intersomatic Fusion. Journal of Biomaterials and Tissue Engineering. 2012;2:170–6. doi: 10.1166/jbt.2012.1041. [DOI] [Google Scholar]
- 13.Andermahr J, Jesch AB, Helling HJ, Jubel A, Fischbach R, Rehm KE. [CT morphometry for calcaneal fractures and comparison of the Zwipp and Sanders classifications] Z Orthop Ihre Grenzgeb. 2002;140:339–46. doi: 10.1055/s-2002-32473. [DOI] [PubMed] [Google Scholar]
- 14.Zwipp H, Tscherne H, Thermann H, Weber T. Osteosynthesis of displaced intraarticular fractures of the calcaneus. Results in 123 cases. Clin Orthop Relat Res. 1993:76–86. [PubMed] [Google Scholar]
- 15.Sanders R. Displaced intra-articular fractures of the calcaneus. J Bone Joint Surg Am. 2000;82:225–50. doi: 10.2106/00004623-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M, Heiland M, Wolff KD, Smeets R. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40:706–18. doi: 10.1016/j.jcms.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Li JF, Lin ZY, Zheng QX, Guo XD, Yang SH, Lu HW, et al. Bone formation in ectopic and osteogenic tissue induced by a novel BMP-2 related peptide combined with rat tail collagen. Biotechnology and Bioprocess Engineering. 2010;15:725–32. doi: 10.1007/s12257-009-3130-0. [DOI] [Google Scholar]
- 18.Lin ZY, Duan ZX, Guo XD, Li JF, Lu HW, Zheng QX, Quan DP, Yang SH. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J Control Release. 2010;144:190–5. doi: 10.1016/j.jconrel.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20:60–2. doi: 10.5435/JAAOS-20-01-060. [DOI] [PubMed] [Google Scholar]
- 20.Haitao P, Qixin Z, Xiaodong G. A novel synthetic peptide vector system for optimal gene delivery to bone marrow stromal cells. J Pept Sci. 2007;13:154–63. doi: 10.1002/psc.826. [DOI] [PubMed] [Google Scholar]
- 21.Li JF, Lin ZY, Zheng QX, Guo XD, Lan SH, Liu SN, et al. Repair of rabbit radial bone defects using true bone ceramics combined with BMP-2 related peptide and type I collagen. Mater Sci Eng C. 2010;30:1273–80. doi: 10.1016/j.msec.2010.07.011. [DOI] [Google Scholar]
- 22.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000:10–27. doi: 10.1097/00003086-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Q, Lu HW, Tang S, Liu K, Pan ZQ, Pan HT, et al. Ectopic bone formation in vivo induced by a novel synthetic peptide derived from BMP-2 using porous collagen scaffolds. Journal of Wuhan University Technology. 2007;22:701–5. doi: 10.1007/s11595-006-4701-y. [Materials Science Edition] [DOI] [Google Scholar]
- 24.Liao SS, Guan K, Cui FZ, Shi SS, Sun TS. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine (Phila Pa 1976) 2003;28:1954–60. doi: 10.1097/01.BRS.0000083240.13332.F6. [DOI] [PubMed] [Google Scholar]
- 25.Guo X, Zheng Q, Yang S, Shao Z, Yuan Q, Pan Z, Tang S, Liu K, Quan D. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor β1 gene. Biomed Mater. 2006;1:206–15. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 26.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–64. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Tang S, Zhao J, Xu S, Li J, Teng Y, Quan D, Guo X. Bone induction through controlled release of novel BMP-2-related peptide from PTMC₁₁-F127-PTMC₁₁ hydrogels. Biomed Mater. 2012;7:015008. doi: 10.1088/1748-6041/7/1/015008. [DOI] [PubMed] [Google Scholar]
- 28.Wu B, Zheng Q, Guo X, Wu Y, Wang Y, Cui F. Preparation and ectopic osteogenesis in vivo of scaffold based on mineralized recombinant human-like collagen loaded with synthetic BMP-2-derived peptide. Biomed Mater. 2008;3:044111. doi: 10.1088/1748-6041/3/4/044111. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang S, Shao Z, Wang H, Xiao B, Pan Z, Tang S. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds. Biomed Mater. 2006;1:93–9. doi: 10.1088/1748-6041/1/3/001. [DOI] [PubMed] [Google Scholar]


