Abstract
Caffeine inhibits cell cycle checkpoints, sensitizes cells to ionizing radiation-induced cell killing and inhibits the protein kinase activity of two cell cycle checkpoint regulators, Ataxia-Telangiectasia mutated (ATM) and ATM- and Rad3-related (ATR). In contrast, caffeine has been reported to have little effect on the protein kinase activity of the DNA-dependent protein kinase (DNA-PK), which is essential for the repair of DNA double-strand breaks. Previously, we reported that DNA-PK phosphorylates Thr21 of the 32 kDa subunit of replication protein A (RPA32) in response to camptothecin. In this report we demonstrate that the camptothecin-induced phosphorylation of RPA32 on Thr21 is inhibited by 2 mM caffeine. In addition, we show that caffeine inhibits immunoprecipitated and purified DNA-PK, as well as DNA-PK in cell extracts, with an IC50 of 0.2–0.6 mM. Caffeine inhibited DNA-PK activity through a mixed non-competitive mechanism with respect to ATP. In contrast, 10-fold higher concentrations of caffeine were required to inhibit DNA-PK autophosphorylation in vitro and caffeine failed to inhibit DNA-PKcs dependent double-strand break repair in vivo. These data suggest that while DNA-PK does not appear to be the target of caffeine-induced radiosensitization, caffeine cannot be used to differentiate between ATM, ATR and DNA- PK-dependent substrate phosphorylation in vivo.
INTRODUCTION
Various biological stresses activate cell cycle checkpoints, which halt cell cycle progression and allow for either DNA repair or apoptotic pathways to function (1,2). Two protein kinases important for cell cycle checkpoint induction after DNA damage are Ataxia-Telangiectasia mutated (ATM) and ATM- and Rad3-related (ATR), both of which belong to the phosphatidyl inositol 3-kinase like serine/threonine protein kinase (PIKK) family (reviewed in 3,4). Caffeine inhibits the in vitro protein kinase activity of ATM and ATR, with an IC50 of 0.2 and 1.1 mM, respectively (5). Treatment of cells with 1 or 3 mM caffeine is sufficient to inhibit 50% of either ATM- or ATR-dependent substrate phosphorylation (5), respectively, and caffeine inhibits various IR and UV-induced cell cycle checkpoints that are ATM and/or ATR-dependent (reviewed in 6). A recent study has suggested that caffeine inhibits cell cycle checkpoints without inhibiting ATM and/or ATR (7) and precisely how caffeine induces radiation sensitivity remains unclear.
Another member of the PIKK family is catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs), which, together with a heterodimeric regulatory subunit (Ku70/80), forms the DNA-dependent protein kinase holoenzyme, DNA-PK (reviewed in 8). Cells deficient in any component of DNA-PK are radiosensitive due a defect in the primary DNA double-strand break (DSB) repair pathway called non-homologous end joining (NHEJ). We have recently shown that autophosphorylation of a cluster of residues on DNA-PKcs is essential for DNA DSB repair in vivo (9). In addition, numerous reports demonstrate that small molecule inhibitors of DNA-PK protein kinase activity radiosensitize cells (10–12), suggesting that the primary physiological substrate of DNA-PK in response to DNA damage may in fact be DNA-PKcs. However, DNA-PK has been reported to retain >50% of its activity in in vitro kinase assays in the presence of caffeine concentrations as high as 10 mM (5) and caffeine has no effect on the rate of IR-induced DSB repair in vivo (13). It is therefore likely that caffeine radiosensitizes cells through a mechanism other than by inhibition of NHEJ. One possibility is that caffeine acts on the homologous recombination (HR) pathway for repair of DNA DSBs, since HR-defective cells display reduced radiosensitization in the presence of caffeine, where as ATM or DNA-PK (NHEJ)-deficient cells are radiosensitized by caffeine comparable to wild-type cells (13–15). However, it is certainly possible that caffeine has other effects on DNA damage response pathways.
Recently, using a phosphospecific antibody that only recognizes the 32-kDa subunit of replication protein A (RPA32) phosphorylated on Thr21, we unambiguously identified Thr21 of RPA32 as an in vitro phosphorylation site of DNA-PK (16). Treatment of cells with camptothecin (CPT), a topoisomerase I inhibitor, induced RPA32 Thr21 phosphorylation in a DNA-PK-dependent manner (16). Herein we report that the CPT-induced phosphorylation of RPA32 on Thr21 is inhibited by caffeine. Furthermore, we demonstrate that DNA-PK substrate phosphorylation is directly inhibited by caffeine in in vitro protein kinase assays, indicating that caffeine cannot be used to differentiate between ATM, ATR and DNA-PK-dependent substrate phosphorylation in cells.
MATERIALS AND METHODS
Cell cultures
Human (ATM-deficient) lymphoblastoid cells (L3) were grown as described previously (17–19). V3 (DNA-PKcs-deficient) hamster cells and V3 cells complemented with the PRKDC cDNA were grown as previously reported (9).
Reagents
Caffeine (BDH and Sigma) and pentoxifylline (Sigma) were dissolved in water and stored at –20°C.
Treatment of cell cultures, Affi-gel blue pulldowns and western immunoblot analysis
Cells were treated with CPT and analyzed for RPA32 Thr21 phosphorylation as described previously (16). Briefly, cells were treated with CPT for 2 h, washed in phosphate buffered saline, lysed in buffer containing Nonidet P-40 and extracts were enriched for RPA using Affi-gel blue resin (Bio-Rad). Samples were eluted off the Affi-gel blue resin using SDS sample buffer, fractionated on SDS polyacrylamide gels and immunoblotted with either a mouse monoclonal antibody to RPA32 (Ab-3, Oncogene) or a phospho-specific rabbit polyclonal to RPA32 Thr21 [as described by Block et al. (16)].
Cell extract assay for DNA-PK activity
V3 (DNA-PKcs-deficient) hamster cells or V3 cells complemented with the cDNA corresponding to human DNA-PKcs were collected, washed in phosphate buffered saline, and then washed in a hypotonic buffer [LSB: 10 mM HEPES pH 7.2, 25 mM KCl, 10 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, 0.1mM dithiothreitol (DTT), 0.2 mM phenylmethyl sulphonyl fluoride, 0.2 µg/ml pepstatin A, 0.2 µg/ml aprotinin, 0.2 µg/ml leupeptin]. Cell pellets were re-suspended in two packed cell volumes of LSB and frozen in liquid nitrogen. Lysates were thawed and adjusted to contain 500 mM NaCl, 10 mM MgCl2 and 0.5 mM DTT. Samples were centrifuged at 10 000 g for 10 min. Cell extracts (15 µg) were incubated with either the DNA-PK substrate peptide (PESQEAFADLWKK-NH2) or a mock peptide (PESEQAFADLWKK-NH2) in a 20 µl assay containing 10 mM HEPES–HCl pH 7.4, 50 mM NaCl, 10 mM MgCl2, 100 µg/ml sonicated calf thymus DNA, 0.4 mM EDTA, 0.1 mM DTT, 250 µM ATP, 2 µCi [γ-32P]ATP and 250 µM of the indicated peptide for 5 min at 30°C. Assays were then processed as described previously (20). Protein concentrations were determined using the Detergent Compatible (DC) protein assay (Bio-Rad) using BSA as the standard.
Assays using purified DNA-PK
DNA-PK subunits (DNA-PKcs and Ku70/80) were purified from HeLa cells as described previously (21) with modifications as described elsewhere (22). The protein kinase activity of purified DNA-PK was determined as described previously (21). DNA-PK autophosphorylation assays were as described previously (23). PHAS-I was purchased from Stratagene. Heterotrimeric RPA was expressed and purified from Escherichia coli as described previously (24).
DNA-PK immunoprecipitation kinase assays
Extracts from human lymphoblastoid (L3) cells were prepared as described previously (16), and 1 mg of extract was incubated with 4 µl of a rabbit polyclonal antibody recognizing DNA-PKcs (DPK-1) for 16 h at 4°C with end over end rotation. Protein A sepharose (PAS) was added, the incubation was continued for 45 min, and then the PAS beads were extensively washed as described previously (16). PAS beads were then resuspended in kinase buffer (10 mM HEPES–HCl pH 7.4, 50 mM NaCl, 10 mM MgCl2) containing 1 µM microcystin-LR, 100 µM ATP, 10 µCi [γ-32P]ATP and 0.5 µg PHAS-I, and then incubated for 10 min at 30°C. Reactions were analyzed using SDS–polyacrylamide gel electrophoresis followed by autoradiography.
Pulsed-field gel electrophoresis-based DSB repair assay
DNA repair assays were carried out basically as described elsewhere (25,26). Briefly, cells were harvested and resuspended in serum free media (containing a 2× concentration of caffeine where required), and then mixed with an equal part of 2% agarose. Agarose plugs (50 µl) were cast containing ∼1 × 108 cells. Once solidified, plugs were put into 2 ml serum free media with the indicated concentration of caffeine and irradiated with 40 Gy at a dose rate of 3.7 Gy/min using a 137Cs Gamma Cell Irradiator (Nordion). Plugs were returned to 37°C with 5% CO2 for the indicated period of time and incubated with lysis buffer (50 mM Tris–HCl pH 8.0, 50 mM EDTA, 50 mM NaCl, 2% N-lauryl sarcosyl, 0.1 mg/ml proteinase K) for 1 h at 4°C followed by 15 h at 50°C. Plugs were then washed in TE buffer (10 mM Tris–HCl pH 8.0, 0.1 mM EDTA) and incubated in TE buffer containing 0.1 mg/ml bovine pancreas RNase I A (Roche). Plugs were inserted into wells of a 0.5% agarose gel containing 0.5 µg/ml ethidium bromide and were subjected to pulsed-field gel electrophoresis using a CHEF-DRIII (Bio-Rad) apparatus at 2 V/cm for 22 h with a switch time of 1.8 s. Gels were visualized using a Gel Doc 2000 system (Bio-Rad), images were captured and saved in TIFF format using Quantity One software version 4.0.4 (1998), and ethidium bromide staining bands were quantitated using Image Quant (version 5.2). The percent of DSBs unrepaired were estimated as follows:
[A/(A + B)]/[Ao/(Ao + Bo)] × 100
where, A = DNA migrating into the gel, B = DNA retained within the plug, Ao = DNA migrating into the gel when no repair was allowed and Bo = DNA retained within the plug when no repair was allowed.
RESULTS
Caffeine inhibits CPT-induced RPA32 Thr21 phosphorylation
Previously, we confirmed reports that Thr21 was the major in vitro phosphorylation site of DNA-PK within RPA32 and, using a phospho-specific antibody, demonstrated that this site is phosphorylated in a DNA-PK-dependent manner in response to CPT in human cells (16). In order to confirm this finding, human lymphoblastoid L3 cells (which express DNA-PKcs but lack ATM) were treated with 1 µM CPT, with or without pretreatment with various concentrations of caffeine or wortmannin. As expected (27), wortmannin concentrations as low as 20 µM inhibited the CPT-induced phosphorylation of RPA32 on Thr21 (Fig. 1). However, caffeine concentrations as low as 2 mM were also found inhibit CPT-induced phosphorylation of RPA32 on Thr21 (Fig. 1). Inhibition of CPT-induced RPA32 phosphorylation by caffeine was also observed in experiments where cells were labeled with 32P-labeled orthophosphate, treated with CPT and analyzed for RPA32 phosphorylation by autoradiography of immunoprecipitated RPA32 (data not shown). These results were unexpected given that a previous report indicated that >10 mM caffeine was required to inhibit 50% of the protein kinase activity of immunoprecipitated DNA-PK (5).
Figure 1.
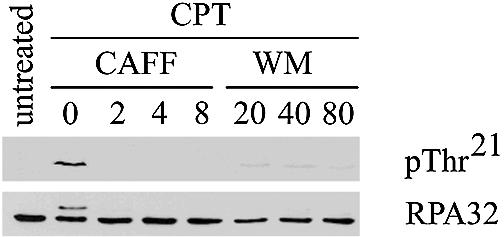
CPT-induced RPA32 phosphorylation is inhibited by wortmannin and caffeine. DNA-PKcs-proficient ATM-deficient lymphoblastoid cells (L3) were either untreated or treated with 1 µM CPT, after pretreatment with caffeine [CAFF] (mM) or wortmannin [WM] (µM) as indicated. Extracts were enriched for RPA using Affi-gel blue pulldowns and RPA32 was visualized by western blotting with either a RPA32 Thr21-phospho- specific antibody [pThr21] or an RPA32 antibody [RPA32].
DNA-PK protein kinase activity in cell extracts is inhibited by caffeine
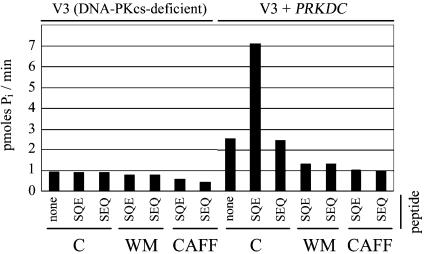
In order to further investigate the effect of caffeine on DNA-PK protein kinase activity, cell extracts from either DNA-PKcs-deficient (V3) hamster cells or V3 cells complemented with the PRKDC cDNA (encoding human DNA-PKcs) were analyzed for DNA-PK activity using either a DNA-PK substrate (SQE) peptide or a mock substrate (SEQ) peptide. Only the PRKDC-complemented V3 cell extracts showed DNA-PK kinase activity, as judged by the difference between phosphorylation of the SQE and SEQ peptides (Fig. 2). In addition, phosphorylation of the SQE peptide in the PRKDC-complemented V3 cell extracts was inhibited by both 1 µM wortmannin and 10 mM caffeine (Fig. 2). The IC50 for the inhibition of DNA-PK activity in cell extracts by caffeine was determined to be 0.6 mM (as judged by phosphorylation of the SQE peptide with extracts from PRKDC-complemented V3 cells in the presence of increasing concentrations of caffeine, Fig. 3A). Thus, caffeine appears to potently inhibit the in vitro protein kinase activity of DNA-PK.
Figure 2.
Inhibition of DNA-PK substrate phosphorylation in cell extracts by caffeine. Extracts from V3 [DNA-PKcs-deficient] cells or V3 cells complemented with a copy of the human PRKDC cDNA [V3 + PRKDC] were assayed for DNA-PK protein kinase activity towards a DNA-PK substrate peptide [SQE] or a mock substrate peptide [SEQ] in the presence or absence of 1 µM WM or 10 mM CAFF. DNA-PK protein kinase activity was expressed as the picomoles of phosphate incorporated into the peptide substrate per minute.
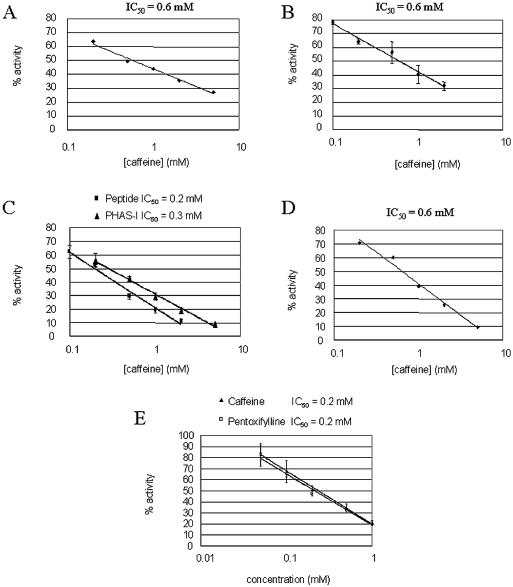
Figure 3.
Inhibition of DNA-PK protein kinase activity in vitro. (A) Extracts from PRKDC-complemented V3 cells were assayed for DNA-PK protein kinase activity towards a DNA-PK substrate peptide [SQE] in the presence of increasing concentrations of caffeine. Assays were done in duplicate and averaged. IC50s are as indicated. (B) Highly purified DNA-PK was assayed towards heterotrimeric RPA (tRPA) in the presence of increasing concentrations of caffeine. Samples were fractionated on SDS–polyacrylamide gels and RPA32 was excised from the gel and 32P was quantitated by Cerenkov counting. Assays were done in triplicate and averaged. Error bars are ± 1 standard error of the mean (SEM). (C) Highly purified DNA-PK was assayed towards either the DNA-PK substrate peptide or PHAS-I in the presence of increasing concentrations of caffeine. Assays were done in triplicate and averaged. (D) DNA-PKcs was immunoprecipitated from L3 cells using a rabbit polyclonal DNA-PKcs antibody (DPK-1) and assayed for activity towards PHAS-I in the presence of increasing concentrations of caffeine. Assays were done in duplicate and averaged. (E) Highly purified DNA-PK was assayed towards the DNA-PK substrate peptide in the presence of increasing concentration of caffeine (second supplier) and pentoxifylline.
Direct inhibition of in vitro DNA-PK substrate phosphorylation by caffeine
Since it was possible that another caffeine-inhibitable protein kinase was contributing to the detected DNA-PK activity in cell extracts, we next determined whether caffeine directly inhibited the protein kinase activity of DNA-PK. Highly purified DNA-PK was used to phosphorylate heterotrimeric RPA (tRPA) in the presence of increasing concentrations of caffeine. Similar to results in cell extracts, the IC50 for the inhibition of DNA-PK kinase activity was found to be 0.6 mM (Fig. 3B). Western blots confirmed that no ATM was present in the purified DNA-PK preparations (data not shown), and the absence of manganese [which is essential for in vitro substrate phosphorylation by ATM and ATR (28–30)] in the kinase assays, provides further evidence that neither ATM nor ATR contributed to the observed kinase activity.
To eliminate the possibility that the inhibition of DNA-PK by caffeine was substrate-dependent, the SQE peptide and PHAS-I were used as substrates in kinase assays using highly purified DNA-PK in the presence of increasing concentrations of caffeine. Caffeine was found to inhibit DNA-PK protein kinase activity towards the SQE peptide and PHAS-I with an IC50 of 0.2 and 0.3 mM, respectively (Fig. 3C). In addition, we sought to rule out the possibility that the immunoprecipitation of DNA-PKcs may alter the ability of caffeine to inhibit DNA-PK activity, so DNA-PKcs was immunoprecipitated from ATM-deficient (L3) cell extracts using a rabbit polyclonal antibody (DPK-1) and used to phosphorylate PHAS-I in the presence of increasing concentrations of caffeine. Once again, caffeine inhibited DNA-PK kinase activity with an IC50 of 0.6 mM (Fig. 3D). Similar results were obtained when DNA-PK was immunoprecipitated using a monoclonal antibody that recognizes DNA-PKcs (monoclonal antibody 42–27) (data not shown). Lastly, another methylxanthine, pentoxifylline, was determined to inhibit DNA-PK activity towards the SQE peptide with an IC50 of 0.2 mM and caffeine purchased from a second supplier inhibited DNA-PK as previously determined (Fig. 3E).
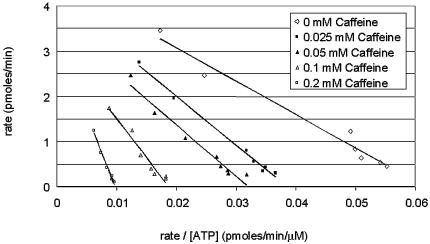
In order to understand the mechanism of DNA-PK inhibition by caffeine, DNA-PK activity was determined in the presence of various ATP concentrations at various caffeine concentrations. DNA-PK activity was recorded as the picomoles of phosphate incorporated into the SQE substrate peptide per minute, and values of DNA-PK activity and DNA-PK activity divided by the concentration of ATP were plotted on an Eadie-Hofstee graph (Fig. 4). As the concentration of caffeine increased, the slope (equal to Km) increased and the y-intercept (equal to Vmax) decreased (Table 1), which is characteristic of mixed non-competitive inhibition of DNA-PK with respect to ATP.
Figure 4.
Mixed non-competitive inhibition of DNA-PK by caffeine. Purified DNA-PK was used to phosphorylate the SQE peptide in the presence of [γ-32P]ATP and varied ATP concentrations (8.3, 10, 12.5, 16.7, 25, 50, 100 or 200 µM) with varied concentrations of caffeine (0, 0.025, 0.05, 0.1 or 0.2 mM). The 32P:(pmol of unlabeled ATP) ratio for each ATP concentration was calculated and used to quantitate the rate of phosphate incorporated into the SQE peptide. Each point represents an average of duplicates.
Table 1. Eadie–Hofstee equation values for the inhibition of DNA-PK by caffeine.
| Caffeine (mM) | Vmax (y-intercept) | Km (slope) | Vmax/Km (×10–2) |
|---|---|---|---|
| 0 | 4.54 | 73.7 | 6.17 |
| 0.025 | 4.01 | 107.7 | 3.86 |
| 0.05 | 3.65 | 113.6 | 3.21 |
| 0.1 | 3.28 | 170.6 | 1.88 |
| 0.2 | 3.22 | 334.9 | 0.98 |
Caffeine has no effect on DNA-PKcs autophosphorylation
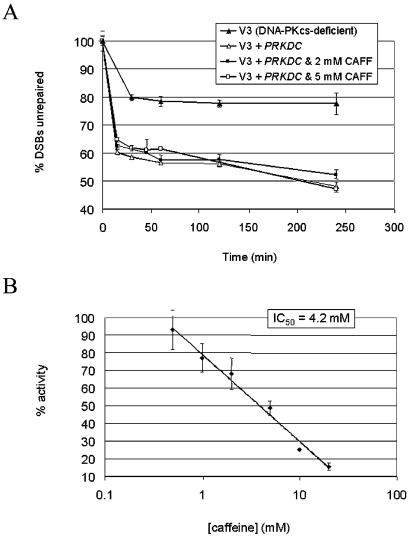
The inhibition of DNA-PK by caffeine was difficult to reconcile with previous reports stating the DNA-PK-proficient and deficient cells were equally radiosensitized by caffeine and that caffeine has no effect on the rate of IR-induced DSB repair, as well as our own findings that DNA-PKcs autophosphorylation is required for cell survival after ionizing radiation. To address this issue we used the FAR assay to examine the rates of IR-induced DSB repair in cells treated with or without caffeine. As previously reported (13), we did not observe caffeine-induced inhibition of DSB repair (Fig. 5A). Interestingly, when we assayed for the ability of caffeine to inhibit DNA-PKcs autophosphorylation, 10 times more caffeine was required to inhibit DNA-PKcs autophosphorylation than DNA-PK substrate phosphorylation (Fig. 5B). Even in the presence of 10 mM caffeine DNA-PK still readily underwent autophosphorylation (25% as compared to no caffeine). Together, these data suggest that inhibition of DNA-PK autophosphorylation likely does not contribute to caffeine induced radiosensitization.
Figure 5.
Caffeine has minimal effect on DNA-PK autophosphorylation in vitro and DNA-PK-dependent DSB repair. (A) V3 (DNA-PKcs-deficient) or PRKDC-complemented V3 cells were embedded in agarose plugs (containing caffeine where indicated), treated with 40 Gy of IR and allowed to recover. Plugs were electrophoresed in the presence of ethidium bromide. The percent of unrepaired DSBs was calculated as described in Materials and Methods. (B) Purified DNA-PK was incubated under autophosphorylation conditions in the presence of increasing concentrations of caffeine. Phosphorylation was quantitated as in Figure 3B.
DISCUSSION
In contrast to a previous report (5), we provide evidence that caffeine is an efficient inhibitor of the in vitro protein kinase activity of DNA-PK, regardless of whether DNA-PK activity was assayed in whole cell extracts, in immunoprecipitates from whole cell extracts, or using highly purified reconstituted holoenzyme. Furthermore, caffeine inhibited DNA-PK-dependent substrate phosphorylation in tissue culture cells. The IC50 for caffeine inhibition of DNA-PK was intermediate to the values reported for the inhibition of immunoprecipitated or purified ATM (5,22) and ATR (5), indicating that treatment of cells with caffeine cannot be used to differentiate between the potentially overlapping roles of DNA-PK, ATM and ATR in the regulation of a given substrate phosphorylation event.
Furthermore, caffeine inhibits DNA-PK through a mixed non-competitive mechanism with respect to ATP, which means that caffeine can bind to both DNA-PK and DNA-PK–ATP complexes but with unequal affinity. We can exclude the possibility that caffeine acts as an ATP-competitive inhibitor based on the decrease in Vmax observed at increasing caffeine concentrations, and we can exclude uncompetitive inhibition as a possible mode of action based on the changing Vmax/Km ratio as the concentration of caffeine was increased (Table 1). Thus caffeine appears to inhibit DNA-PK through binding to a site outside of the ATP-binding pocket located within the active site. Two other well-characterized compounds inhibit DNA-PK through different mechanisms; LY294002 inhibits by directly competing with ATP for the ATP-binding site yielding competitive reaction kinetics; wortmannin inhibits by competing with ATP for the ATP-binding site but also by covalently binding to a key lysine residue within the active site to yield classic non-competitive reaction kinetics (31). Thus caffeine appears to inhibit DNA-PK kinase activity through a novel mechanism.
We have also shown that DNA-PK autophosphorylation is only weakly inhibited by caffeine in vitro (Fig. 5B), which likely explains why caffeine has no detectable effect on the rate of DSB repair in vivo (Fig. 5A and 13). Previously, similar results were described for the inhibition ATM protein kinase activity by caffeine, wherein 1 mM caffeine was sufficient to nearly abolish immunoprecipitated ATM protein kinase activity towards a GST-p53 substrate but caffeine concentrations as high as 4 mM failed to effect ATM autophosphorylation-dependent activation in vitro (18). ATM was recently reported to exist as a homodimer in resting cells and DNA damage-induced ATM autophosphorylation on Ser1981 was associated with ATM monomerization (32). In addition, two Ku and two DNA-PKcs molecules (a dimer of holoenzymes) are hypothesized to be present at the broken/free DNA ends to facilitate their synapses (in vitro or in vivo), after which DNA-PK autophosphorylation remodels the DNA-PK complex, possibly dissociating DNA-PKcs from Ku (8,33–35). Perhaps the decreased ability of caffeine to inhibit ATM and DNA-PK autophosphorylation relative to substrate phosphorylation reflects a homodimer-induced change in the inhibitory caffeine binding site when one molecule of the dimer is positioned in the other’s substrate binding (or active) site but not when an exogenous substrate occupies the substrate binding site. It will be interesting to determine whether caffeine has similar effects on other PIKKs, such as ATR and mTOR.
Acknowledgments
ACKNOWLEDGEMENTS
Thanks to Katarzyna Kycia for help with the preparation of the manuscript, Ruiqiong Ye for continued assistance with cell culture and Dr Yaping Yu for the purification of DNA-PK. We thank Dr Mark Wold (University of Iowa) for the tRPA expression vector and Dr Yossi Shiloh (Tel Aviv University) for the L3 cell line. This work was supported by grant no. 011053 from the National Cancer Institute of Canada with funds from the Canadian Cancer Foundation. W.D.B. is supported by graduate studentships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). D.M. is supported by a graduate studentship from the Alberta Cancer Board. S.P.L.-M. is a Scientist of the AHFMR, an Investigator of the Canadian Institutes for Health Research, and holds the Alberta Cancer Foundation/Engineered Air Chair in Cancer Research.
REFERENCES
- 1.Zhou B.B. and Elledge,S.J. (2000) The DNA damage response: putting checkpoints in perspective. Nature, 408, 433–439. [DOI] [PubMed] [Google Scholar]
- 2.Jackson S.P. (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis, 23, 687–696. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi A.A., Block,W.D. and Lees-Miller,S.P. (2003) The roles of ATM and ATR in DNA damage-induced cell cycle control. In Meijer,L., Jezequel,A. and Roberge,M. (eds), Progress in Cell Cycle Research. Life in Progress, Roscoff, France, pp. 393–412. [PubMed] [Google Scholar]
- 4.Abraham R.T. (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev., 15, 2177–2196. [DOI] [PubMed] [Google Scholar]
- 5.Sarkaria J.N., Busby,E.C., Tibbetts,R.S., Roos,P., Taya,Y., Karnitz,L.M. and Abraham,R.T. (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res., 59, 4375–4382. [PubMed] [Google Scholar]
- 6.Kaufmann W.K., Heffernan,T.P., Beaulieu,L.M., Doherty,S., Frank,A.R., Zhou,Y., Bryant,M.F., Zhou,T., Luche,D.D., Nikolaishvili-Feinberg,N., Simpson,D.A. and Cordeiro-Stone,M. (2003) Caffeine and human DNA metabolism: the magic and the mystery. Mutat. Res., 532, 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez D. (2003) Caffeine inhibits checkpoint responses without inhibiting the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases. J. Biol. Chem., 278, 37139–37145. [DOI] [PubMed] [Google Scholar]
- 8.Lees-Miller S.P. and Meek,K. (2003) Repair of DNA double-strand breaks by nonhomologous end-joining. Biochimie, 85, 1161–1173. [DOI] [PubMed] [Google Scholar]
- 9.Ding Q., Reddy,Y.V., Wang,W., Woods,T., Douglas,P., Ramsden,D.A., Lees-Miller,S.P. and Meek,K. (2003) Autophosphorylation of the catalytic subunit of the DNA-dependent protein kinase is required for efficient end processing during DNA double-strand break repair. Mol. Cell. Biol., 23, 5836–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernikova S.B., Wells,R.L. and Elkind,M.M. (1999) Wortmannin sensitizes mammalian cells to radiation by inhibiting the DNA-dependent protein kinase-mediated rejoining of double-strand breaks. Radiat. Res., 151, 159–166. [PubMed] [Google Scholar]
- 11.Okayasu R., Suetomi,K. and Ullrich,R.L. (1998) Wortmannin inhibits repair of DNA double-strand breaks in irradiated normal human cells. Radiat. Res., 149, 440–445. [PubMed] [Google Scholar]
- 12.Rosenzweig K.E., Youmell,M.B., Palayoor,S.T. and Price,B.D. (1997) Radiosensitization of human tumor cells by the phosphatidylinositol3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin. Cancer Res., 3, 1149–1156. [PubMed] [Google Scholar]
- 13.Asaad N.A., Zeng,Z.C., Guan,J., Thacker,J. and Iliakis,G. (2000) Homologous recombination as a potential target for caffeine radiosensitization in mammalian cells: reduced caffeine radio sensitization in XRCC2 and XRCC3 mutants. Oncogene, 19, 5788–5800. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Wang,H., Iliakis,G. and Wang,Y. (2003) Caffeine-induced radiosensitization is independent of nonhomologous end joining of DNA double-strand breaks. Radiat. Res., 159, 426–432. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Wang,X., Iliakis,G. and Wang,Y. (2003) Caffeine could not efficiently sensitize homologous recombination repair-deficient cells to ionizing radiation-induced killing. Radiat. Res., 159, 420–425. [DOI] [PubMed] [Google Scholar]
- 16.Block W.D., Yu,Y. and Lees-Miller,S.P. (2004) Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res., 32, 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye R., Bodero,A., Zhou,B.B., Khanna,K.K., Lavin,M.F. and Lees-Miller,S.P. (2001) The plant isoflavenoid genistein activates p53 and Chk2 in an ATM-dependent manner. J. Biol. Chem., 276, 4828–4833. [DOI] [PubMed] [Google Scholar]
- 18.Kozlov S., Gueven,N., Keating,K., Ramsay,J. and Lavin,M.F. (2003) ATP activates ataxia-telangiectasia mutated (ATM) in vitro. Importance of autophosphorylation. J. Biol. Chem., 278, 9309–9317. [DOI] [PubMed] [Google Scholar]
- 19.Ye R., Goodarzi,A.A., Kurz,E.U., Saito,S., Higashimoto,Y., Lavin,M.F., Appella,E., Anderson,C.W. and Lees-Miller,S.P. (2003) The isoflavonoids genistein and quercetin activate different stress signaling pathways as shown by analysis of site-specific phosphorylation of ATM, p53 and histone H2AX. DNA Repair (Amst.), 3, 235–244. [DOI] [PubMed] [Google Scholar]
- 20.Lees-Miller S.P., Sakaguchi,K., Ullrich,S.J., Appella,E. and Anderson,C.W. (1992) Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol. Cell. Biol., 12, 5041–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan D.W., Mody,C.H., Ting,N.S. and Lees-Miller,S.P. (1996) Purification and characterization of the double-stranded DNA-activated protein kinase, DNA-PK, from human placenta. Biochem. Cell Biol., 74, 67–73. [DOI] [PubMed] [Google Scholar]
- 22.Goodarzi A.A. and Lees-Miller,S.P. (2004) Biochemical characterization of the ataxia-telangiectasia mutated (ATM) protein from human cells. DNA Repair (Amst.), in press. [DOI] [PubMed] [Google Scholar]
- 23.Douglas P., Sapkota,G.P., Morrice,N., Yu,Y., Goodarzi,A.A., Merkle,D., Meek,K., Alessi,D.R. and Lees-Miller,S.P. (2002) Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem. J., 368, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henricksen L.A., Umbricht,C.B. and Wold,M.S. (1994) Recombinant replication protein A: expression, complex formation and functional characterization. J. Biol. Chem., 269, 11121–11132. [PubMed] [Google Scholar]
- 25.Chan D.W., Chen,B.P., Prithivirajsingh,S., Kurimasa,A., Story,M.D., Qin,J. and Chen,D.J. (2002) Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev., 16, 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiBiase S.J., Zeng,Z.C., Chen,R., Hyslop,T., Curran,W.J.,Jr and Iliakis,G. (2000) DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus. Cancer Res., 60, 1245–1253. [PubMed] [Google Scholar]
- 27.Sarkaria J.N., Tibbetts,R.S., Busby,E.C., Kennedy,A.P., Hill,D.E. and Abraham,R.T. (1998) Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res., 58, 4375–4382. [PubMed] [Google Scholar]
- 28.Chan D.W., Son,S.C., Block,W., Ye,R., Khanna,K.K., Wold,M.S., Douglas,P., Goodarzi,A.A., Pelley,J., Taya,Y., Lavin,M.F. and Lees-Miller,S.P. (2000) Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase. J. Biol. Chem., 275, 7803–7810. [DOI] [PubMed] [Google Scholar]
- 29.Canman C.E., Lim,D.S., Cimprich,K.A., Taya,Y., Tamai,K., Sakaguchi,K., Appella,E., Kastan,M.B. and Siliciano,J.D. (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science, 281, 1677–1679. [DOI] [PubMed] [Google Scholar]
- 30.Kim S.T., Lim,D.S., Canman,C.E. and Kastan,M.B. (1999) Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem., 274, 37538–37543. [DOI] [PubMed] [Google Scholar]
- 31.Izzard R.A., Jackson,S.P. and Smith,G.C. (1999) Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res., 59, 2581–2586. [PubMed] [Google Scholar]
- 32.Bakkenist C.J. and Kastan,M.B. (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature, 421, 499–506. [DOI] [PubMed] [Google Scholar]
- 33.DeFazio L.G., Stansel,R.M., Griffith,J.D. and Chu,G. (2002) Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J., 21, 3192–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weterings E., Verkaik,N.S., Bruggenwirth,H.T., Hoeijmakers,J.H. and van Gent,D.C. (2003) The role of DNA dependent protein kinase in synapsis of DNA ends. Nucleic Acids Res., 31, 7238–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merkle D., Douglas,P., Moorhead,G.B., Leonenko,Z., Yu,Y., Cramb,D., Bazett-Jones,D.P. and Lees-Miller,S.P. (2002) The DNA-dependent protein kinase interacts with DNA to form a protein-DNA complex that is disrupted by phosphorylation. Biochemistry, 41, 12706–12714. [DOI] [PubMed] [Google Scholar]