Abstract
Background
Lymph node metastasis has a significant impact on laryngeal cancer prognosis. The role of lymph node ratio (LNR, ratio of metastatic to examined nodes) in the staging of laryngeal cancer was not reported.
Patients and Methods
Records of laryngeal cancer patients with lymph node involvement from Surveillance, Epidemiology, and End Results database (SEER, training set, N = 1963) and Fudan University Shanghai Cancer Center (FDSCC, validating set, N = 27) were analyzed for the prognostic value of LNR. Kaplan–Meier survival estimates, the Log-rank χ2 test and Cox proportional hazards model were used for univariate and multivariate analysis. Optimal LNR cutoff points were identified by X-tile.
Results
Optimal LNR cutoff points classified patients into three risk groups R1 (≤0.09), R2 (0.09–0.20) and R3 (>0.20), corresponding to 5-year cause-specific survival and overall survival in SEER patients of 55.1%, 40.2%, 28.8% and 43.1%, 31.5%, 21.8%, 2-year disease free survival and disease specific survival in FDSCC patients of 74.1%, 62.5%, 50.0%, and 67.7%, 43.2%, 25.0%, respectively. R3 stratified more high risk patients than N3 with the same survival rate, and R classification clearly separated N2 patients to 3 risk groups and N1 patients to 2 risk groups (R1–2 and R3).
Conclusions
R classification is a significant prognostic factor of laryngeal cancer and should be used as a complementary staging system of N classification.
Introduction
As with other cancers of the head and neck, lymph node (LN) involvement decreases survival rates of laryngeal cancer by approximately 50%. [1] The treatment choice of laryngeal cancer depends on functional outcome, the patient’s wishes, reliability of follow-up, and general medical condition. For early-stage glottic or supraglottic cancers, surgery (partial laryngectomy through either endoscopic or open approaches) and radiotherapy seem to be equally effective. [1], [2], [3] For patients with T4a tumors, the standard approach is a total laryngectomy with ipsilateral thyroidectomy and neck dissection. [1] For managing other locally advanced, resectable glottic and supraglottic cancers, the NCCN guidelines recommended concurrent chemoradiation, surgery or induction chemotherapy as the primary care for individual choice. [1].
Postoperative radiation with or without concurrent chemotherapy were recommended by NCCN for patients with adverse features which included extracapsular nodal spread, positive margin, pT4 primary, N2 or N3 nodal disease, perineural invasion and vasular embolism. [1] Over the past 3 decades, more attention has been paid to the identification of factors that might help the surgeon to assess the precise risk of failure and benefit from more intensified therapy in individual patients. The lymph node ratio (LNR) was found to improve prognostic information in breast cancer, gastric cancer, colorectal cancer, melanoma and others. [4], [5], [6], [7] Although the prevalence of overall metastasis and occult metastasis of lymph nodes were found for 40%–75% and 26%–36% of laryngeal cancer patients, there was still no report about LNR on the survival of laryngeal cancer. [8], [9], [10].
To discuss the role of LNR for the postoperative staging of laryngeal cancer, the SEER (Surveillance, Epidemiology and End Results)-registered laryngeal cancer patients with lymph node metastasis were analyzed and the cutoff points for the LNR in defining patients as high, medium or low risk groups were also identified in current study.
Patients and Methods
Patients
The SEER laryngeal cancer dataset was extracted from SEER database (SEER*Stat 7.0.5). [11] Cancers were limited to the larynx, which were defined as the glottis (C32.0, vocal cord), supraglottis (C32.1, false cord, posterior surface of epiglottis, aryepiglottic fold), subglottis (C32.2), laryngeal cartilage (C32.3), overlapping lesion of larynx (C32.8) and larynx, NOS (C32.9). Histology was limited to squamous cell carcinoma (histology recode - broad groupings 8050–8090). Only laryngeal carcinomas as a single primary tumor or the first of two or more primary tumors were included. The LNR was calculated as the number of positive lymph nodes divided by the number of lymph nodes examined.
The cases with dis-concordant N classification information and the number of positive regional lymph nodes recorded in SEER database were rejected. Because the aim of current study was to identify the role of LNR staging for laryngeal cancer, the cases with unclassified T classification, M classification, grade and other variables were also enrolled in the analysis set and were defined as Tx, Mx and unknown group to avoid losing information and select bias. Finally, a total of 4183 cases of laryngeal carcinoma were collected as the analysis set, and among them 1963 cases had pathological lymph node involvement (pN+).
The FDSCC (Fudan Univesity Shanghai Cancer Center) laryngeal cancer dataset was built prospectively and recorded the layngeal cancer patients treated at Cancer Hospital, Fudan University, Shanghai, China from January, 2003 to January 2012. To avoid the bias caused by pre-operative radiotherapy and/or chemotherapy, and occult LNs metastasis, only patients primarily operated with neck dissection were enrolled in current research. The Pathologic reviews showed that 27 patients were pN+ which included 5 pN1 and 22 pN2 according to AJCC staging system. The 2-year disease specific survival (DSS, laryngeal cancer specific) and disease free survival (DFS, no local recurrence and distant metastasis) for pN+ laryngeal cancer patients were 66.3% and 51.0%, respectively.
Ethics Statement
For SEER cases, this study was based on public use de-identified data from the U.S. SEER database and did not include interaction with human subjects or use personal identifying information. The study did not require informed consent from the SEER registried cases and the authors obtained Limited-Use Data Agreements from SEER. For FDSCC patients, written informed consent were obtained. The study was approved by the Review Board of Fudan Univesity Shanghai Cancer Center, Shanghai, China.
Statistical Analysis
Firstly, we evaluated the prognostic value of LNR as a continuous variable, adjusting for other covariates associated with survival of 1963 SEER pN+ laryngeal cancer cases. Furtherly, we proceeded to determine the most appropriate cutoff points for categorizing LNR as high, medium, and low risk groups. Two pairs of cutoff points were identified using different methods and compared with LNR as a continuous variable to identify the optimal cutoff points. The first pair of cutoff points were identified by tertiles to split the patients into equal sized groups. [12], [13] The second pair cutoff points were calculated by X-tile using the minimum P values from log-rank χ2 statistics. [14] Finally, the prognostic significance of LNR staging was validated in FDSCC patients.
The survival rate and curves was calculated using the Kaplan–Meier method. Statistical comparisons of different factors with mortality were made with the Log-rank χ2 test. In multivariate analysis, forward stepwise regression analysis was carried out with a Cox proportional hazards model. Harrell’s concordance index (C-index) and AIC (Akaike information criterion) value related to the Cox regression model were analyzed to compare the predictive ability of the staging system. A smaller AIC value and a higher C-index value indicated a more desirable model for predicting outcome. A P value of 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS software version 17.0 (SPSS Inc., Chicago, IL) and R2.14.0 software with packages (MASS and Survival).
Results
LNR is a Prognostic Factor of Laryngeal Cancer Survival
The clinical characteristics, 5-year cause specific survival (CSS) and overall survival (OS) estimates, and Log-rank χ2 test of univariate variables of the 1963 SEER patients with pN+ laryngeal cancer were shown in Table 1. Using multivariate Cox regression analysis, we found that race, radiation sequence, T classification, N classification, M classification, continuous LNR and age were all independent variables for predicting survival (Table 2).
Table 1. Clinicopathological characteristics, cause specific survival (CSS) and overall survival (OS) of SEER laryngeal cancer cases with pathological lymph node involvement.
| Categorical variables | No. of patients | 5-year CSS | 5-year OS | ||||
| (n = 1963) | Rate (%) | Log-rank χ2 | P value | Rate (%) | Log-rank χ2 | P value | |
| Race | 3.965 | 0.138 | 5.289 | 0.071 | |||
| White | 1480 (75.4%) | 43.7 | 33.9 | ||||
| Black | 397 (20.2%) | 38.8 | 29.7 | ||||
| Other | 86 (4.4%) | 48.1 | 40.9 | ||||
| Gender | 2.795 | 0.095 | 1.545 | 0.214 | |||
| Male | 1526 (77.7%) | 42.1 | 32.4 | ||||
| Female | 437 (22.3%) | 45.9 | 36.8 | ||||
| Year of diagnosis | 0.390 | 0.823 | 0.228 | 0.892 | |||
| 1988–1994 | 429 (21.9%) | 43.3 | 33.1 | ||||
| 1995–2001 | 637 (32.5%) | 42.4 | 34.0 | ||||
| 2002–2008 | 897 (45.7%) | 43.4 | 32.3 | ||||
| Primary site | 15.271 | 0.009 | 15.803 | 0.007 | |||
| Glottis | 327 (16.7%) | 40.9 | 32.5 | ||||
| Supraglottis | 1285 (65.5%) | 44.8 | 34.7 | ||||
| Subglottis | 36 (1.8%) | 0 | 32.1 | ||||
| Laryngeal cartilage | 11 (0.6%) | 32.7 | 24.2 | ||||
| Overlapping lesion | 133 (6.8%) | 41.0 | 29.6 | ||||
| Larynx, NOS | 171 (8.7%) | 36.0 | 28.7 | ||||
| Histological grade | 17.037 | 0.002 | 21.628 | <0.001 | |||
| I | 89 (4.5%) | 44.4 | 34.3 | ||||
| II | 937 (47.7%) | 45.3 | 36.5 | ||||
| III | 783 (39.9%) | 42.2 | 31.6 | ||||
| IV | 20 (1.0%) | 46.1 | 20.0 | ||||
| Unknown | 134 (6.8%) | 27.9 | 22.3 | ||||
| Radiation sequence | 33.328 | <0.001 | 56.831 | <0.001 | |||
| No radiation | 504 (25.7%) | 35.4 | 23.7 | ||||
| Pre-operative | 72 (3.7%) | 38.3 | 28.9 | ||||
| Post-operative | 1363 (69.4%) | 45.9 | 37.2 | ||||
| Other | 24 (1.2%) | 38.3 | 27.3 | ||||
| Cancer directed surgery | 26.251 | <0.001 | 27.370 | <0.001 | |||
| Yes | 1761 (89.7%) | 44.5 | 34.8 | ||||
| Other | 202 (10.3%) | 26.1 | 18.6 | ||||
| T staging | 59.303 | <0.001 | 54.620 | <0.001 | |||
| T1 | 182 (9.3%) | 57.7 | 46.2 | ||||
| T2 | 526 (26.8%) | 48.5 | 36.1 | ||||
| T3 | 295 (15.0%) | 42.1 | 34.7 | ||||
| T4a | 809 (41.2%) | 39.3 | 31.2 | ||||
| T4b | 55 (2.8%) | 29.6 | 20.3 | ||||
| Tx | 96 (4.9%) | 24.5 | 17.6 | ||||
| N staging | 61.415 | <0.001 | 47.574 | <0.001 | |||
| N1 | 546 (27.8%) | 59.7 | 47.3 | ||||
| N2 | 1313 (66.9%) | 37.1 | 28.8 | ||||
| N3 | 104 (5.3%) | 31.9 | 20.6 | ||||
| M staging | 55.169 | <0.001 | 45.658 | <0.001 | |||
| M0 | 1845 (94.0%) | 44.1 | 34.3 | ||||
| M1 | 89 (4.5%) | 19.2 | 15.4 | ||||
| Mx | 29 (1.5%) | 40.8 | 29.4 | ||||
| Continuous variables | Median (range) | ||||||
| Age | 60 years (17–91) | ||||||
| No. of LN examined | 28(1–90) | ||||||
| No. of positive LNs | 2(1–90) | ||||||
| Lymph node ratio | 0.11(0.01–1.00) | ||||||
Table 2. Multivariate analysis of the lymph node ratio (LNR) and covariates associated with survival of laryngeal cancer cases with lymph node metastasis.
| Variables | Cause specific survival | Overall survival | ||
| HR(95% CI) | P value | HR(95% CI) | P value | |
| Race (White as reference) | ||||
| Black | 1.212(1.041–1.412) | 0.013 | 1.238(1.084–1.413) | 0.001 |
| Other | 0.920(0.674–1.252) | 0.592 | 0.858(0.652–1.123) | 0.261 |
| Radiation sequence (no radiation as reference) | ||||
| Pre-operative | 0.846(0.606–1.182) | 0.328 | 0.839(0.627–1.123) | 0.238 |
| Post-operative | 0.694(0.599–0.805) | <0.001 | 0.654(0.577–0.743) | <0.001 |
| Other | 0.976(0.556–1.714) | 0.932 | 0.952(0.574–1.579) | 0.848 |
| T classification (T1 as reference) | ||||
| T2 | 1.308(1.013–1.689) | 0.040 | 1.277(1.033–1.577) | 0.024 |
| T3 | 1.740(1.316–2.301) | <0.001 | 1.540(1.216–1.951) | <0.001 |
| T4a | 1.856(1.450–2.375) | <0.001 | 1.630(1.327–2.002) | <0.001 |
| T4b | 2.365(1.574–3.552) | <0.001 | 2.157(1.526–3.048) | <0.001 |
| Tx | 1.963(1.285–3.001) | 0.002 | 1.725(1.186–2.511) | 0.004 |
| N classification (N1 as reference) | ||||
| N2 | 1.948(1.668–2.274) | <0.001 | 1.642(1.445–1.865) | <0.001 |
| N3 | 1.806(1.358–2.402) | <0.001 | 1.668(1.309–2.127) | <0.001 |
| M classification (M0 as reference) | ||||
| M1 | 1.700(1.194–2.420) | 0.003 | 1.579(1.137–2.195) | 0.006 |
| MX | 0.722(0.386–1.349) | 0.307 | 0.864(0.510–1.466) | 0.588 |
| LNR as continuous variable | 2.299(1.905–2.775) | <0.001 | 1.810(1.532–2.138) | <0.001 |
| Age | 1.013(1.006–1.019) | <0.001 | 1.020(1.014–1.025) | <0.001 |
Cutoff Points Identification of LNR
To stratify the patients with lymph node metastasis as high, medium and low risk groups associated with CSS, the upper and lower tertiles of continuous LNR that corresponded to 0.06 and 0.23 were defined as the first pair of cutoff points. The X-tile, which can control the inflated type I error problem and minimize the loss of information due to multiple testing through cross-validation, identified 0.09/0.20 as the second pair of cutoff points. [6], [14] The SEER cases with lymph node metastasis were stratified as high, medium and low risk groups according to the two pairs of cutoff points identified above. The case numbers, the 5-year CSS and 5-year OS of the different risk groups were summarized in Table 3. To compare the predictive ability of the categorical LNR and the continuous LNR, the C-index and AIC value of the Cox regression model (Table 2) with substitution of the continuous LNR with the categorical LNR were calculated. As listed in Table 3, the models of categorical LNRs defined by cutoff points 0.09/0.20 showed superior predictive ability to that of the continuous LNR and another categorical LNR, with the lowest AIC value and highest C-index value associated with the Cox regression model. The SEER laryngeal cancer patients with lymph node metastasis were classified as R1 (LNR ≤0.09), R2 (LNR 0.10–0.20) and R3 (LNR >0.20) three risk groups (R classification).
Table 3. Univariate and multivariate analysis of the categorical and continuous LNR with cause-specific survival (CSS) and overall survival (OS) of SEER laryngeal cancer patients with lymph node metastasis.
| LNRclassification | CaseNo. | 5-yearCSS(%) | Log-rankχ2 (P ) | Multivariate analysis of CSS† | 5-yearOS(%) | Log-rankχ2 (P ) | Multivariate analysis of OS† | ||||
| HR (95% CI) | C-index | AIC | HR (95% CIs) | C-index | AIC | ||||||
| Continuous LNR | 2.299(1.905–2.775) | 0.658 | 14037.85 | 1.810(1.532–2.138) | 0.642 | 18430.78 | |||||
| Cutpoints 0.06/0.23 | 109.197 | 0.658 | 14042.83 | 87.729 | 0.642 | 18432.54 | |||||
| R1: 0–0.06 | 637 | 56.3 | (<0.001) | Reference | 43.9 | (<0.001) | Reference | ||||
| R2: 0.06–0.23 | 737 | 42.4 | 1.270(1.074–1.502) | 33.4 | 1.233(1.070–1.421) | ||||||
| R3: >0.23 | 589 | 29.4 | 1.928(1.635–2.273) | 22.2 | 1.624(1.409–1.871) | ||||||
| Cutpoints 0.09/0.20 | 125.228 | 0.661 | 14027.14 | 94.673 | 0.645 | 18424.27 | |||||
| R1: 0–0.09 | 860 | 55.1 | (<0.001) | Reference | 43.1 | (<0.001) | Reference | ||||
| R2: 0.09–0.20 | 458 | 40.2 | 1.421(1.202–1.680) | 31.5 | 1.308(1.133–1.509) | ||||||
| R3: >0.20 | 645 | 28.8 | 1.962(1.695–2.271) | 21.8 | 1.607(1.416–1.825) | ||||||
The multivariate analysis was adjusted using the same Cox regression model at Table 2.
Selecting High Risk Patients by R classification
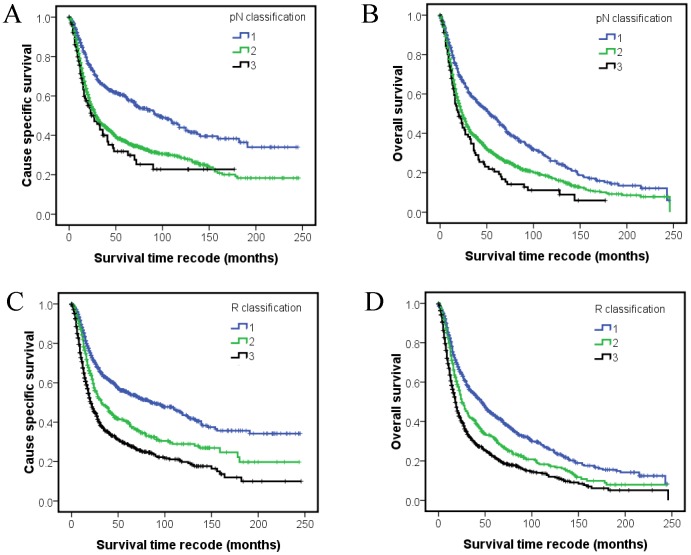
N classification was widely used for postoperative staging of lymph node of laryngeal cancer, while the cause specific survival curves of N3 crossed with N2 after 150 months follow-up (Figure 1A). N1, N2 and N3 accounted for 27.8%, 66.9% and 5.3% of all pN+ patients (Table 1). Compared with pN classification, the survival curves of individual R classification separated clearly even after 20 years follow-up (Figure 1C and 1D). The R classification also showed homogenous patients grouping which stratified the patients to 43.8% (R1), 23.3% (R2) and 32.9% (R3) of all pN+ patients (Table 3). The 5 and 10-year CSS and OS of N3 patients were and 31.9%, 22.7% and 20.6%, 11.1%, individually. The 5 and 10-year CSS and OS of R3 patients were and 28.8%, 19.8% and 21.8%, 12.0%, respectively. R3 stratified more high risk patients than N3 with the same survival rate.
Figure 1. Kaplan–Meier survival estimates according to pN classification and R classification of SEER laryngeal cancer patients with lymph node metastasis: cause-specific survival (A) and overall survival (B) of the SEER set with different pN classification; cause-specific survival (C) and overall survival (D) of the SEER set with different R classification.
R classification Stratify Individual N Patients to Different Risk Groups
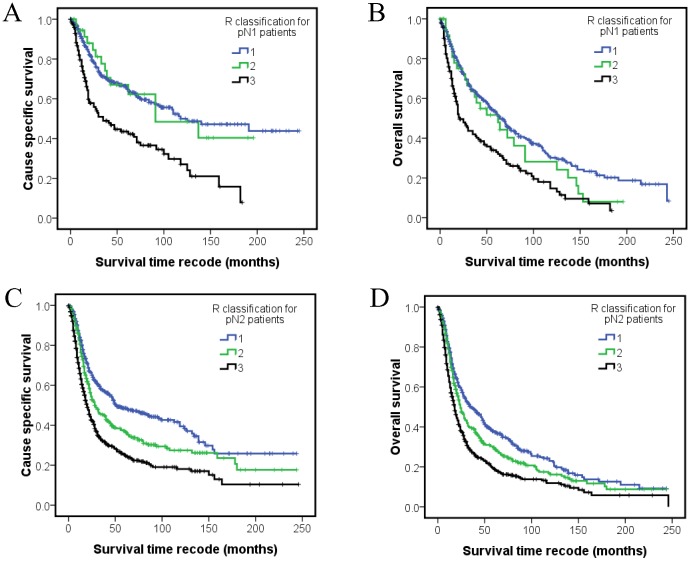
To further analyze the role of R classification for stratify patients to different risk groups, the R classification were defined for patients with individual N classification. For pN1 patients, the 5-year CSS and OS of R1 (N = 361), R2 (N = 40), R3 (N = 145) groups were 65.3%, 67.0%, 43.5% (Log-rank χ2 32.117, P<0.001) and 52.7%, 51.7%, 33.0% (Log-rank χ2 27.654, P<0.001), respectively (Figure 2A and 2B). For pN2 patients, the 5-year CSS and OS of R1 (N = 468), R2 (N = 400), R3 (N = 445) groups were 48.5%, 37.1%, 25.1% (Log-rank χ2 63.523, P<0.001) and 37.6%, 29.6%, 18.9% (Log-rank χ2 45.328, P<0.001), respectively (Figure 2C and 2D). No significant differences of CSS (Log-rank χ2 5.493, P = 0.064) and OS (Log-rank χ2 2.713, P = 0.258) were observed for individual R classification group of pN3 patients (N = 104). R classification clearly separated N2 patients to 3 risk groups and N1 patients to 2 risk groups (R1–2 and R3). The survival of R1 patients of N2 classification is better than the survival of R3 patients of N1 classification. All these results supported that R classification can stratify the patients to different risk groups and complement N classification.
Figure 2. Kaplan–Meier survival estimates of different R classification patients of individual N classification.
The cause-specific survival (A) and overall survival (B) of individual R classification for pN1 patients; cause-specific survival (C) and overall survival (D) of individual R classification for pN2 patients.
Validation of R Classification in FDSCC Patients
To validate R classification in the FDSCC patient set, 27 pN+ cases were analyzed and the median number of LNs examined, positive LNs and LNR were 38 (range, 8–123), 2 (range, 1–28) and 0.09 (range, 0.01–0.70), respectively. The 2-year DSS and DFS of R1 (N = 13), R2 (N = 10), R3 (N = 4) patients were 74.1%, 62.5%, 50.0%, and 67.7%, 43.2%, 25.0%, respectively. The 2-year DSS and DFS of N1 (N = 5) and N2 (N = 22) patients were 50.0%, 69.5% and 50% and 47.6%, respectively. Although no statistical significance were found for survival difference of individual R classification and N classification due to less patients number, the R classification stratify patients to 3 risk groups clearly.
Discussion
When surgery was selected as the primary management of laryngeal cancer, more attention should be paid to identify factors that might help the surgeon to assess the precise risk of failure. The main prognosticators included T classification, quality of surgical resection, positive margins, two or more positive lymph nodes, largest node >3 centimeters in diameter, perineural invasion, and in some studies, age and gender. [15], [16] In 2004, level I evidence was established for the postoperative chemoradiotherapy treatment of patients with selected high risk locally advanced head and neck cancers, with the publication of the results of two trials conducted in EROTC and RTOG. Extracapsular extension and/or microscopically involved surgical margins were the only risk factors for which the impact of chemotherapy enhanced radiation therapy was significant in both trials. [17] Our current results identified that lymph node ratio is as independent risk factors for the postoperative staging of laryngeal cancer. The R classification defined by the cutoff points 0.09/0.20 can clearly stratify patients to different risk groups and complement the pN classification. The R classification should be incorporated into the risk factors mentioned above to stratify patients and assess the treatment benefits. In this exciting and chaotic period in which new chemotherapy agents, new paradigms of treatment, new surgical and radiation technology, and new prognostic factors are rapidly becoming available, a multidisciplinary and collaborative approach for each patient at the start of decision making and planning is a necessity and the absolute standard of medical treatment for excellent patient care. [18].
The significant decrease in the number of laryngeal cancer surgical cases and changing use of new drugs and radiation techniques in the chemoradiation era prevent a clear and accurate analysis of risks of postoperative failure based on single institution’s experience. SEER data are abstracted prospectively from registries comprising 26% of U.S. population, which is considered representative of the entire population, and the selection biases, recall biases, treatment fads, influence of loss to follow-up and other oversights associated with single institution’s research were minimized. [11], [19] Current studies use the largest series (SEER) of operated pN+ laryngeal cancer cases to analysis the role of R classification for staging of laryngeal caner and validate the R classification in an independent dataset (FDSCC). The complementary data collection system and the cross-validation of SEER and FDSCC dataset reinforce the conclusion. While limitations still exist for current research, such as the inter-institution differences in patient management, unrecorded details of pathologic reports and medical management covariates of SEER dataset, the less patient number of FDSCC dataset. Integrating R classification with other risk factors to analysis the pooled data of inter-institutional clinical trials will achieve a definitive result of the postoperative staging of laryngeal cancer.
In conclusion, we clearly identify that lymph node ratio was an independent prognostic factor of laryngeal cancer and R classification (LNR 0–0.09, LNR 0.09–0.20 and LNR >0.20)defines laryngeal cancer mortality adequately. R staging is a complementary staging system for N classification. R staging based stratification of patients for postoperative therapy and clinical trials deserved for further research.
Acknowledgments
The authors would like to thank SEER for open access to the database.
Funding Statement
This work was supported by the National Science Foundation of China (81001204 to YLW, 30872958 to QHJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, et al. (2011) National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Head and neck cancers. J Natl Compr Canc Netw 9: 596–650. [DOI] [PubMed] [Google Scholar]
- 2.Suarez C, Rodrigo JP, Silver CE, Hartl DM, Takes RP, et al.. (2012) Laser surgery for early to moderately advanced glottic, supraglottic, and hypopharyngeal cancers. Head Neck. [DOI] [PubMed]
- 3. Weinstein GS, O'Malley BW Jr, Magnuson JS, Carroll WR, Olsen KD, et al. (2012) Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 4. Spillane AJ, Cheung BL, Winstanley J, Thompson JF (2011) Lymph node ratio provides prognostic information in addition to american joint committee on cancer N stage in patients with melanoma, even if quality of surgery is standardized. Ann Surg 253: 109–115. [DOI] [PubMed] [Google Scholar]
- 5. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, et al. (2009) Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 27: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 6. Wang W, Xu DZ, Li YF, Guan YX, Sun XW, et al. (2011) Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection–results of a single-institution study of 1343 Chinese patients. Ann Oncol 22: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 7. Ceelen W, Van Nieuwenhove Y, Pattyn P (2010) Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 17: 2847–2855. [DOI] [PubMed] [Google Scholar]
- 8. Buckley JG, MacLennan K (2000) Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck 22: 380–385. [DOI] [PubMed] [Google Scholar]
- 9. Candela FC, Shah J, Jaques DP, Shah JP (1990) Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch Otolaryngol Head Neck Surg 116: 432–435. [DOI] [PubMed] [Google Scholar]
- 10. Redaelli de Zinis LO, Nicolai P, Tomenzoli D, Ghizzardi D, Trimarchi M, et al. (2002) The distribution of lymph node metastases in supraglottic squamous cell carcinoma: therapeutic implications. Head Neck 24: 913–920. [DOI] [PubMed] [Google Scholar]
- 11.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, et al. (2011) SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site.
- 12. Hu M, Ampil F, Clark C, Sonavane K, Caldito G, et al. (2012) Comorbid predictors of poor response to chemoradiotherapy for laryngeal squamous cell carcinoma. Laryngoscope 122: 565–571. [DOI] [PubMed] [Google Scholar]
- 13. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 14. Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 15. Bernier J, Cooper JS (2005) Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist 10: 215–224. [DOI] [PubMed] [Google Scholar]
- 16. Langendijk JA, Slotman BJ, van der Waal I, Doornaert P, Berkof J, et al. (2005) Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer 104: 1408–1417. [DOI] [PubMed] [Google Scholar]
- 17. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, et al. (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 27: 843–850. [DOI] [PubMed] [Google Scholar]
- 18. Posner MR (2010) Integrating systemic agents into multimodality treatment of locally advanced head and neck cancer. Ann Oncol 21 Suppl 7vii246–251. [DOI] [PubMed] [Google Scholar]
- 19. Vinh-Hung V, Joseph SA, Coutty N, Ly BH, Vlastos G, et al. (2010) Age and axillary lymph node ratio in postmenopausal women with T1–T2 node positive breast cancer. Oncologist 15: 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]