Abstract
The mevalonate-based isoprenoid biosynthetic pathway is responsible for producing cholesterol in humans and is used commercially to produce drugs, chemicals, and fuels. Heterologous expression of this pathway in Escherichia coli has enabled high-level production of the antimalarial drug artemisinin and the proposed biofuel bisabolane. Understanding the kinetics of the enzymes in the biosynthetic pathway is critical to optimize the pathway for high flux. We have characterized the kinetic parameters of phosphomevalonate kinase (PMK, EC 2.7.4.2) from Saccharomyces cerevisiae, a previously unstudied enzyme. An E. coli codon-optimized version of the S. cerevisiae gene was cloned into pET-52b+, then the C-terminal 6X His-tagged protein was expressed in E. coli BL21(DE3) and purified on a Ni2+ column. The KM of the ATP binding site was determined to be 98.3 µM at 30°C, the optimal growth temperature for S. cerevisiae, and 74.3 µM at 37°C, the optimal growth temperature for E. coli. The KM of the mevalonate-5-phosphate binding site was determined to be 885 µM at 30°C and 880 µM at 37°C. The Vmax was determined to be 4.51 µmol/min/mg enzyme at 30°C and 5.33 µmol/min/mg enzyme at 37°C. PMK is Mg2+ dependent, with maximal activity achieved at concentrations of 10 mM or greater. Maximum activity was observed at pH = 7.2. PMK was not found to be substrate inhibited, nor feedback inhibited by FPP at concentrations up to 10 µM FPP.
Introduction
The mevalonate pathway is an important conduit for the production of crucial metabolites with a wide array of functions, including terpenoids [1], [2], hormones and steroids [3]. The heterologous expression of this pathway in Escherichia coli has enabled high-level production of the antimalarial drug artemisinin [4]–[6], but the chemical structures of these metabolites also make them interesting targets for solving some of the most crucial problems in the energy market [7], [8]. With only slight modifications to mevalonate pathway intermediates and products, either in vivo or through traditional chemical engineering processes post cell culture extraction, these molecules can be transformed into biofuels that, depending on our ability to scale-up, could offset or replace traditional liquid fuels [9]. This would allow us to replace petroleum-based, CO2 producing fuels with fuels that are carbon neutral. Although industrial-scale corn-based ethanol production is already a reality in the energy market, ethanol is a less than desirable biofuel because not only does it divert crops from the food supply, it is not compatible with our current distribution infrastructure or vehicle fleet [10].
Whether these fuel alternatives are five-carbon alcohols derived from the mevalonate pathway intermediates isopentenyl pyrophosphate and dimethylallyl pyrophosphate [11], or downstream, terpene-based molecules like bisabolene [8], further improvement of titers may be realized through a more robust understanding of the enzymes in the mevalonate pathway and the ways in which those enzymes are regulated by metabolic intermediates. In particular, proteomics data has previously shown that the fourth and fifth enzymes in the pathway—mevalonate kinase (MK) and phosphomevalonate kinase (PMK), respectively—are expressed at relatively low levels and may be targets for increasing overall isoprenoid production [12], [13]. Previous work has also shown that substrate inhibition and feedback inhibition of MK may be responsible for limiting flux through the pathway [14]. Because MK—a phosphotransferase that acts on mevalonate and ATP to yield mevalonate-5-phosphate—and PMK—a phosphotransferase that acts on mevalonate-5-phosphate and ATP to yield mevalonate-5-diphosphate—both require ATP to function and downstream prenyl phosphates might act as general ATP binding site inhibitors, PMK was identified as another potential source of pathway regulation.
PMKs from other sources have been studied revealing implications for pathway engineering. For example, PMK from E. faecalis is Mn2+ dependent rather than Mg2+ dependent [15]. Pig-derived PMK is substrate inhibited by ATP under high ATP, low mevalonate phosphate concentrations [16]. If S. cerevisiae PMK is similarly dependent or inhibited it would make an ideal target for protein engineering. Furthermore, S. cerevisiae prefers to grow at 30°C, but much of our production takes place in E. coli, which necessitates understanding how PMK activity is affected by a change in growth temperature from 30°C to 37°C. Herein we report cloning a codon-optimized sequence of S. cerevisiae PMK into an expression vector, the expression and purification of PMK in E. coli, and the kinetic characterization of the purified enzyme.
Results and Discussion
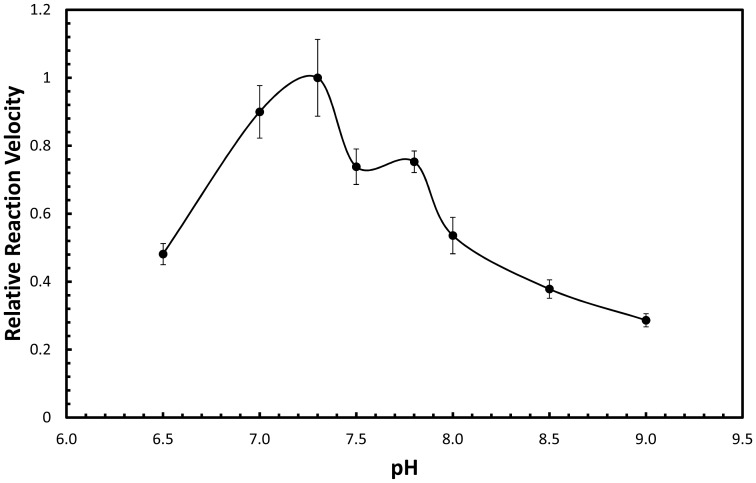
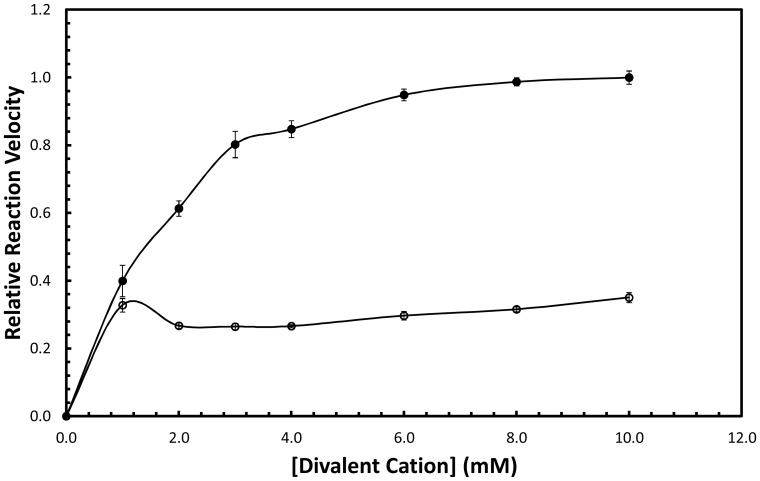
Although phosphomevalonate kinase (PMK) from S. cerevisiae has previously been studied in partially purified lysates [17], and even utilized to study the kinetics of another enzyme [18], this is the first time PMK from S. cerevisiae has been kinetically characterized in isolation. In a study of the partially purified enzyme it was reported that pH did not affect PMK activity, but we found that PMK does have an optimal activity at pH = 7.2, and its activity drops off below pH = 6.5 and above pH = 8.0 (Figure 1). Although at first glance there is an apparent “shoulder” in the pH profile, careful consideration of the profile shows that the shoulder is within error and therefore cannot be considered to conclusively exist. Although we did not test a wide array of storage conditions, solutions with high PMK concentrations were found to be stable long term only at pH = 8.0 with 800 mM NaCl. As found previously S. cerevisiae PMK shows a cation dependence on Mg2+, with 10 mM corresponding to maximal activity (Figure 2).
Figure 1. pH dependence of S. cerevisiae phosphomevalonate kinase.
Figure 2. Divalent cation dependence.
Closed circles are data for Mg2+ and open circles are data for Mn2+.
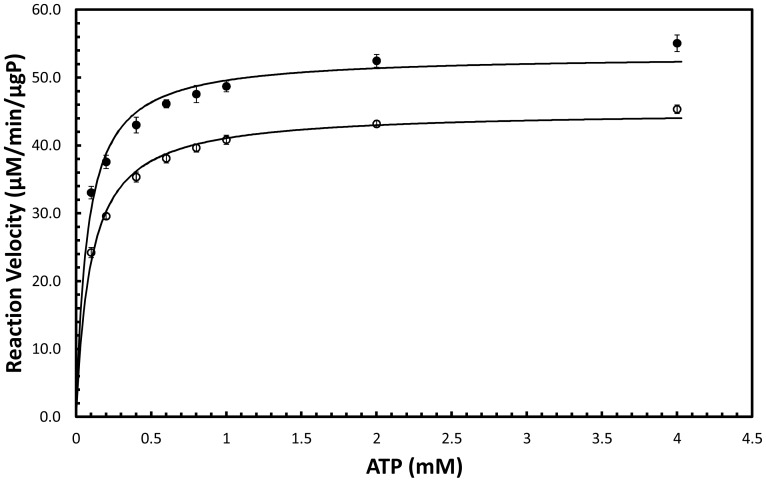
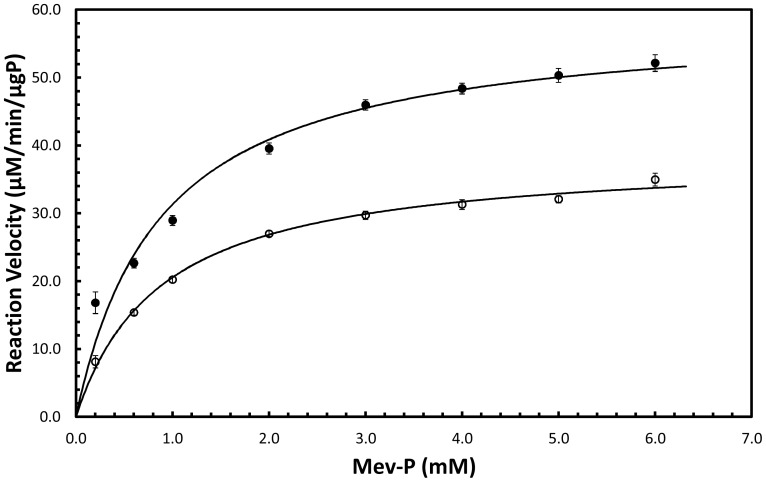
Kinetic constants were determined by nonlinear regression analysis using the solver function in Microsoft Excel. The KM for ATP, KM ATP, was determined to be 98.3 µM and 74.3 µM at 30°C and 37°C, respectively. The KM for mevalonate-5-phosphate, KM mev-p, was determined to be 885 µM and 880 µM at 30°C and 37°C, respectively (Figure 3). Vmax was determined to be 4.51 µmol/min/µg enzyme and 5.33 µmol/min/µg enzyme at 30°C and 37°C, respectively (Figure 4). In contrast, the KM ATP, KM mev-p, and Vmax for the Enterococcus faecalis PMK, which is Mn dependent, were reported to be 170 µM, 190 µM, and 3.9 µmol/min/mg enzyme [15]. The values for the Streptococcus pneumonia PMK were reported to be 74 µM, 4.2 µM, and 5.5 µmol/min/mg enzyme [19]. The values for pig liver PMK have been reported to be 43 µM, 12 µM, and 51 µmol/min/mg enzyme [16]. For the recombinant human PMK, the values were reported to be 107 µM, 34 µM, and 46 µmol/min/mg enzyme [20]. The high KM mev-p for the S. cerevisiae PMK makes it less ideal than enzymes with a low KM, as it would only reach its maximal rate at a high concentration of mevalonate-5-phosphate. Because of the Mn dependence of the E. faecalis PMK, it may not function fully if expressed in E. coli or other organisms. In contrast, the S. pneumonia, pig, and human PMKs have reasonable values for KM ATP and KM mev-p, making them better choices for a heterologous pathway. In terms of maximum rates, the mammalian enzymes are high than the microbial enzymes.
Figure 3. Initial reaction velocity as a function of ATP concentration.
Closed circles are data for incubation at 37°C and open circles are data for incubation at 30°C.
Figure 4. Initial reaction velocity as a function of mevalonate-5-phosphate concentration.
Closed circles are data for incubation at 37°C and open circles are data for incubation at 30°C.
Because the S. cerevisiae PMK has been used heterologously in E. coli for production of isoprenoids [4]–[8], [11], the temperature effect on PMK activity is important, particularly at E. coli's optimal growth temperature of 37°C. Despite expectations that PMK activity might diminish with increasing the temperature from the preferred 30°C growth temperature of S. cerevisiae to the 37°C preferred by E. coli, PMK activity was shown to slightly increase with the increase in temperature. This increased activity bodes well for the production of isoprenoid products, including advanced biofuels, via the mevalonate pathway if the low protein expression levels currently observed can be increased [12], [13]_ENREF_9. It should be noted that although we were able to achieve very high yields of PMK using pET-52b+ for the purpose of isolating and purifying the enzyme, increasing PMK expression in production strains by using high copy plasmids would be counterproductive to increasing overall biofuels production as doing so would divert an unnecessary amount of resources into the production of protein to the detriment of fuel titers.
One regulatory mechanism for controlling PMK activity we can rule out is feedback inhibition, as the presence of farnesyl pyrophosphate (FPP)—a known inhibitor of MK [18]—did not affect PMK activity at concentrations up to 10 µM FPP (data not shown). Of the publications reporting the kinetics of PMKs from various organisms, none have reported inhibition by prenyl phosphates. Furthermore, unlike S. cerevisiae mevalonate kinase [18], PMK did not demonstrate substrate inhibition. The lack of feedback and substrate inhibition in the S. cerevisiae PMK is an attractive feature for increasing production of a desired isoprenoid. Nevertheless, S. pneumonia PMK, which has a high Vmax and low KMs, is a much better enzyme and should be incorporated into future production strains. An additional advantage of the S. pneumonia PMK is that its crystal structure of the has been solved [21] and the kinetic mechanism of its catalysis has been described in detail [19].
With the addition of PMK from this study, the S. cerevisiae-derived mevalonate pathway enzymes that have been kinetically characterized include hydroxymethylglutaryl synthase [22], hydroxymethylglutaryl reductase [14], mevalonate kinase [18], phosphomevalonate decarboxylase [23], and farnesyl pyrophosphate synthase [24], leaving acetyl-CoA C-acetyltransferase and isopentenyl diphosphate isomerase uncharacterized. Although isopentenyl diphosphate isomerase has been isolated and studied [25], the difficulty associated with detecting the isomerization of a single bond is likely why the kinetic constants have yet to be determined. In combination with traditional genetic engineering techniques, such as varying promoter strength, and newly developed technologies for varying expression, such as RBS calculators [26], studying the kinetics of these remaining enzymes should allow isoprenoid production from engineered microbes to be optimized more rationally.
Materials and Methods
Codon Optimization of PMK
The original S. cerevisiae PMK sequence (accession number NM_001182727), which was downloaded from the BioCyc.org database, was codon optimized by DNA2.0 (Menlo Park, CA) for expression in E. coli. Codon optimization replaced codons rare for E. coli with more frequently used codons. The sequences of the original and codon-optimized versions of the genes are presented in Figure S1.
Expression Plasmid Construction
A chemically-competent strain of E. coli DH10B was transformed with pET-52b+ (Novagen, Germany) and then used to prepare the plasmid according to the instructions and materials in a Qiagen (Valencia, California) Spin Miniprep Kit. The codon-optimized PMK sequence was PCR amplified with primers that added a BsaI restriction site with an NcoI overhang on the 5′ end of the sequence and a SacI restriction site on the 3′ end of the sequence, then digested with the appropriate restriction enzymes (all enzymes from New England BioLabs, Ipswich, Massachusetts), and cloned into pET-52b+ to make expression plasmid pET-52b+_coPMK-His. Confirmation of expression plasmid construction was accomplished by sequencing the cloning region using T7 primers (sequencing and primers from Quintara Biosciences, Albany, California).
PMK-His Expression and Purification
Ideal conditions for PMK expression were screened on NuPAGE 10% Bis-Tris SDS-PAGE gels and the supplies indicated in the accompanying protocol (Invitrogen, Grand Island, New York) from 5-mL cultures that spanned a range of media types, growth temperatures, inducer concentrations, and growth times. Protein expression was ultimately accomplished by growing a 2-L culture in Terrific Broth (Invitrogen) to OD600 = 0.6 at 37°C, inducing with 100 µM IPTG (Sigma Aldrich, St. Louis, Missouri), then growing at 18°C for approximately two days (until stationary phase was reached). Cells were pelleted in 250-mL portions, flash frozen in liquid nitrogen after medium removal, and then stored at −80°C prior to further processing. On ice, cells from one 250-mL portion were suspended in 25 mL of a lysis buffer (10 mM Imidizole, 300 mM NaCl, 50 mM NaH2PO4, pH = 8.0; Sigma Aldrich), sonicated for 10 minutes in a water bath to break up residual clumps, then homogenized with two passes through an EmulsiFlex®-C3 (Avestin, Canada). Cell debris was removed by centrifugation at 12,000 X g for 30 minutes. Cleared lysate was bound to 2-mL of Ni-NTA resin (Qiagen) at 4°C by rocking gently for 30 minutes. The resin was then bedded in a column, washed with 20 column volumes (CV) of buffer containing 20 mM imidizole, then the protein was eluted with 10 CV of buffer containing 500 mM imidizole. Buffer exchange into 20 mM Tris, 50 mM NaCl, pH = 7.0 was accomplished on an AKTA (GE Healthcare Life Sciences, Pittsburgh, Pennsylvania) using a GE Healthcare HiPrep 26/10 Desalting Column (17-5087-01). Protein was then concentrated using VivaSpin 20 3,000-MWCO filters (Sartorius, Bohemia, New York). Protein concentration was determined using a Nanodrop (Thermo Scientific, West Palm Beach, Florida). The protein was then diluted so that glycerol (Sigma) was 50% v/v and stored at −20°C.
Activity Assay
All chemicals and supporting enzymes were purchased from Sigma-Aldrich. Reaction progress was monitored spectrophotometrically at 339 nm for NADH consumption on a 96-well plate in a Spectramax M2 (Molecular Devices, Sunnyvale, California). 100-µL enzymatic assay mixtures contained 200 mM Tris (pH = 7.2), 100 mM KCl, 10 mM MgCl2, 0.81 mM NADH, 1.5 mM phosphoenolpyruvate, 0.682U pyruvate kinase, 0.990 U lactate dehydrogenase, 0.1 µg PMK, 0.1–8.0 mM ATP, and 0.2–10.0 mM mevalonate-5-phosphate. Stock concentrations of NADH and pH neutralized ATP were confirmed through their extinction coefficients (ATPε259 nm = 15.4 mM−1 cm−1, NADHε339 nm = 6.22 mM−1 cm−1). All conditions were repeated twelve times for statistical analysis, from which KM (µM) and reaction velocities (µM mev-PP formed*minute−1 * µg PMK−1) were calculated. When studying pH effect and divalent cation dependence, ATP and mevalonate-5-phosphate were held constant and data were normalized to the maximum observed reaction velocities. To ensure PMK was the rate-limiting enzyme, when necessary the following standard controls and results were verified: doubling the PMK added doubled the observed rate, doubling the supporting enzymes added did not affect the observed rate, and doubling the phosphoenolpyruvate concentration did not affect the observed rate.
Supporting Information
Sequences of the original PMK and the codon-optimized version of PMK.
(DOCX)
Funding Statement
This work was funded by the Joint BioEnergy Institute (JBEI), which is funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, under contract number DE-AC02-05CH11231. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kuzuyama T (2002) Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Bioscience Biotechnology and Biochemistry 66: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 2. Wilding EI, Brown JR, Bryant AP, Chalker AF, Holmes DJ, et al. (2000) Identification, evolution, and essentiality of the mevalonate pathway for isopentenyl diphosphate biosynthesis in gram-positive cocci. Journal of Bacteriology 182: 4319–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuzuyama T, Seto H (2012) Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis. Proceedings of the Japan Academy Series B-Physical and Biological Sciences 88: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nature Biotechnology 21: 796–802. [DOI] [PubMed] [Google Scholar]
- 5. Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, et al. (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440: 940–943. [DOI] [PubMed] [Google Scholar]
- 6. Chang MCY, Eachus RA, Trieu W, Ro D-K, Keasling JD (2007) Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nature Chemical Biology 3: 274–277. [DOI] [PubMed] [Google Scholar]
- 7. Bokinsky G, Peralta-Yahya PP, George A, Holmes BM, Steen EJ, et al. (2011) Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 108: 19949–19954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, et al. (2011) Identification and microbial production of a terpene-based advanced biofuel. Nature Communications 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang FZ, Rodriguez S, Keasling JD (2011) Metabolic engineering of microbial pathways for advanced biofuels production. Current Opinion in Biotechnology 22: 775–783. [DOI] [PubMed] [Google Scholar]
- 10. Lee SK, Chou H, Ham TS, Lee TS, Keasling JD (2008) Metabolic engineering of microorganisms for biofuels production: from bugs to synthetic biology to fuels. Current Opinion in Biotechnology 19: 556–563. [DOI] [PubMed] [Google Scholar]
- 11.Chou HH, Keasling JD (2011) Host cells and methods for producing 3-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, and 3-methyl-butan-1-ol. The Regents of the University of California.
- 12. Singh P, Batth TS, Juminaga D, Dahl RH, Keasling JD, et al. (2012) Application of targeted proteomics to metabolically engineered Escherichia coli. Proteomics 12: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 13. Redding-Johanson AM, Batth TS, Chan R, Krupa R, Szmidt HL, et al. (2011) Targeted proteomics for metabolic pathway optimization: Application to terpene production. Metabolic Engineering 13: 194–203. [DOI] [PubMed] [Google Scholar]
- 14. Ma SM, Garcia DE, Redding-Johanson AM, Friedland GD, Chan R, et al. (2011) Optimization of a heterologous mevalonate pathway through the use of variant HMG-CoA reductases. Metabolic Engineering 13: 588–597. [DOI] [PubMed] [Google Scholar]
- 15. Doun SS, Burgner JW, Briggs SD, Rodwell VW (2005) Enterococcus faecalis phosphomevalonate kinase. Protein Science 14: 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eyzaguirre J, Valdebenito D, Cardemil E (2006) Pig liver phosphomevalonate kinase: Kinetic mechanism. Archives of Biochemistry and Biophysics 454: 189–196. [DOI] [PubMed] [Google Scholar]
- 17. Bloch K, Chaykin S, Phillips AH, Dewaard A (1959) MEVALONIC ACID PYROPHOSPHATE AND ISOPENTENYLPYROPHOSPHATE. Journal of Biological Chemistry 234: 2595–2604. [PubMed] [Google Scholar]
- 18. Primak YA, Du M, Miller MC, Wells DH, Nielsen AT, et al. (2011) Characterization of a Feedback-Resistant Mevalonate Kinase from the Archaeon Methanosarcina mazei. Applied and Environmental Microbiology 77: 7772–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilloff D, Dabovic K, Romanowski MJ, Bonanno JB, Doherty M, et al. (2003) The kinetic mechanism of phosphomevalonate kinase. Journal of Biological Chemistry 278: 4510–4515. [DOI] [PubMed] [Google Scholar]
- 20. Herdendorf TJ, Miziorko HM (2006) Phosphomevalonate kinase: functional investigation of the recombinant human enzyme. Biochemistry 45: 3235–3242. [DOI] [PubMed] [Google Scholar]
- 21. Andreassi J, Vetting M, Bilder P, Roderick S, Leyh T (2009) Structure of the ternary complex of phosphomevalonate kinase: the enzyme and its family. Biochemistry 48: 6461–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleto.B (1972) KINETIC MECHANISM OF 3-HYDROXY-3-METHYLGLUTARYL-COENZYME-A SYNTHASE FROM BAKERS-YEAST. Biochemical Journal 126 : 35––&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krepkiy D, Miziorko HM (2004) Identification of active site residues in mevalonate diphosphate decarboxylase. Abstracts of Papers of the American Chemical Society 228: U187–U187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song LS, Poulter CD (1994) YEAST FARNESYL-DIPHOSPHATE SYNTHASE - SITE-DIRECTED MUTAGENESIS OF RESIDUES IN HIGHLY CONSERVED PRENYLTRANSFERASE DOMAIN-I AND DOMAIN-II. Proceedings of the National Academy of Sciences of the United States of America 91: 3044–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson MS, Muehlbacher M, Street IP, Proffitt J, Poulter CD (1989) ISOPENTENYL DIPHOSPHATE - DIMETHYLALLYL DIPHOSPHATE ISOMERASE - AN IMPROVED PURIFICATION OF THE ENZYME AND ISOLATION OF THE GENE FROM SACCHAROMYCES CEREVISIAE. Journal of Biological Chemistry 264: 19169–19175. [PubMed] [Google Scholar]
- 26.Salis HM (2011) THE RIBOSOME BINDING SITE CALCULATOR. In: Voigt C, editor. Synthetic Biology, Pt B: Computer Aided Design and DNA Assembly. pp. 19–42.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of the original PMK and the codon-optimized version of PMK.
(DOCX)