Abstract
The incentive sensitization theory of addiction posits that repeated exposure to drugs of abuse, like cocaine, can lead to long-term adaptations in the neural circuits that support motivated behavior, providing an account of pathological drug seeking behavior. Although preclinical findings provide strong support for this theory, much remains unknown about the conditions that support incentive sensitization. The current study examined whether the mode of cocaine administration is an important factor governing that drug's long-term impact on behavior. Separate groups of rats were allowed either to self-administer intravenous cocaine or were given an equivalent number and distribution of unsignaled cocaine or saline infusions. During the subsequent test of incentive motivation (Pavlovian-to-instrumental transfer), we found that rats with a history of cocaine self-administration showed strong cue-evoked food seeking, in contrast to rats given unsignaled cocaine or saline. This finding indicates that the manner in which cocaine is administered can determine its lasting behavioral effects, suggesting that subjective experiences during drug use play a critical role in the addiction process. Our findings may therefore have important implications for the study and treatment of compulsive drug seeking.
Keywords: cocaine, self-administration, yoked, Pavlovian-to-instrumental transfer, incentive sensitization, reward
Introduction
One explanation for the development of addiction is that repeated drug exposure results in a persistent change in the way drugs and drug-related stimuli motivate behavior, allowing such stimuli to elicit intense “craving” and provoke compulsive drug-seeking (Robinson and Berridge, 1993). It is known that virtually all classes of abused drugs can stimulate and, with repeated exposure, sensitize dopamine neurotransmission in the mesotelencephalic system (Carboni et al., 1989; Di Chiara and Imperato, 1988; Pierce and Kalivas, 1997; Rowell et al., 1987; Wise, 1984), which is a critical substrate of cue-evoked incentive motivation (Ikemoto and Panksepp, 1999; Lex and Hauber, 2008; Ostlund and Maidment, 2012; Wassum et al., 2011). Furthermore, preclinical studies have demonstrated that animals pre-treated with abused drugs, including psychostimulants, such as amphetamine and cocaine, exhibit heightened levels of incentive motivation (Deroche et al., 1999; Harmer and Phillips, 1998; Mendrek et al., 1998). Such effects also appear to extend to behaviors motivated by natural rewards, including food (Harmer and Phillips, 1998; Mendez et al., 2009; Nocjar and Panksepp, 2002; Nordquist et al., 2007; Ranaldi et al., 2009; Taylor and Jentsch, 2001; Wyvell and Berridge, 2001). For instance, studies using the Pavlovian-to-instrumental transfer paradigm (PIT), a behavioral assay of Pavlovian incentive motivation (Balleine and Ostlund, 2007; Berridge and Robinson, 2003; Rescorla and Solomon, 1967), have shown that rats pre-treated with experimenter-administered amphetamine (Wyvell and Berridge, 2001) or cocaine (LeBlanc et al., in press) exhibit more vigorous instrumental food-seeking behavior in the presence of a separately trained food-paired cue than saline-treated rats.
While these studies demonstrate that experimenter-delivered drugs produce long-lasting neurochemical and behavioral alterations, voluntary drug taking in humans can be more directly modeled in rodents by allowing the subjects to actively self-administer drug. It has recently been shown that rats with a history of cocaine self-administration exhibit greater food-seeking behavior in response to food-paired cues than cocaine-naive rats (Saddoris et al., 2011). While this finding indicates that cocaine self-administration can result in potentiated cue-induced incentive motivation, it is not clear what role the mode of drug delivery played in this effect. Specifically, it remains unknown whether a subject's experience controlling their cocaine intake is an important factor in determining the degree of incentive sensitization supported by that drug or whether this effect can be more directly attributed to the purely pharmacological effects of the drug. Other lines of evidence suggest that the mode of drug delivery may play an important role. For instance, self-administered cocaine is more potent in stimulating dopamine release (Hemby et al., 1997; Kimmel et al., 2005; Lecca et al., 2007) and can support stronger and more persistent adaptations in the circuitry controlling dopamine signaling (Chen et al., 2008; Stefański et al., 2007) than passively administered intravenous cocaine. Given the role of the dopamine system in incentive motivation, one should therefore expect active and passive cocaine administration to support differential effects on motivated behavior. The current study investigates this question, examining whether the sensitizing impact of intravenous cocaine on Pavlovian incentive motivation depends on the mode of cocaine delivery.
Materials and Methods
Subjects
Adult male Long Evans rats (mean weight: 322±5.9g) were used in this experiment. Rats were housed in a climate-controlled vivarium and were kept on a food restriction regimen (∼12g home chow per day) throughout training and testing to maintain them at approximately 85% of their free feeding bodyweight, but were provided ad libitum access to tap water in the home cage. All procedures were approved by the Animal Research Committee of University of California, Los Angeles, and were performed in accordance with National Research Council's Guide for the Care and Use of Laboratory Animals.
Apparatus and Training
Rats were trained in eight identical Med Associates (East Fairfield, VT) operant chambers housed within sound- and light-resistant shells. The chambers contained two retractable levers on one of the two aluminum end-walls that could be inserted into the chamber to the left and right side of a recessed food magazine, into which grain-based food pellets (45mg, Bioserv, Frenchtown, NJ) could be delivered. Magazine entries were detected by using a photobeam sensor that crossed the front of the food magazine. During self-administration training, the boxes were outfitted with a nosepoke manipulandum (Med Associates) on the opposite wall from the levers (which were retracted) and magazine. A 3-W, 24-V houselight was mounted on the top center of the opposite end wall and provided illumination during training sessions, with the exception of cocaine administration sessions. The chambers were also equipped with devices for generating the tone (3 kHz, 75dB) and clicker (2Hz, 75dB) stimuli.
Drugs
Cocaine hydrochloride, provided by the NIDA Drug Supply program, was dissolved in sterile saline (0.9% NaCl) and filtered-sterilized prior to administration.
Instrumental Training
Before instrumental training began, subjects received two magazine training sessions during each of which they received 20 food pellets on a fixed time 1-minute schedule. Magazine training was followed by 14 days of instrumental training, consisting of 30-minute sessions with continuous access to an active and inactive lever. Pressing on the active lever (either left or right) resulted in the delivery of grain pellets, while pressing on the inactive lever was monitored but was without consequence. The schedule of reinforcement used for the active lever was modified over days. On the first day rats were continuously reinforced, but were then shifted to a random interval (RI) schedule for the rest of training, with one day at RI-5s, one day at RI-15s, one day at RI-30s, followed by 10 days at RI-45s.
Pavlovian Training
Rats were then given Pavlovian training, which consisted of 14 daily 30-minute sessions. For the first 11 sessions, the presentation of one of the two auditory stimuli (CS+; either the tone or clicker; 30-s duration) was followed immediately by the delivery of 3 pellets. Each session consisted of 10 CS+ presentations, delivered on a variable time 150s schedule. The last 3 sessions were identical to previous sessions except for the addition of two non-reinforced presentations of the alternative auditory stimulus (CS−), which were made at the middle and end of the sessions (i.e., after the 5th and 10th CS+ presentation). Magazine entries were recorded to monitor acquisition of conditioned approach behavior.
Catheter surgery
Cather surgery was performed as described previously (Leblanc et al., 2012). Briefly, rats were deeply anesthetized with isoflurane, and a silicon catheter was placed into the right or left jugular vein. Rats were given 5 days to recover from surgery and catheters were maintained with twice daily heparin injections (0.1 ml of 10 units/ml) for the duration of the experiment. Any catheter of questionable patency was tested by evaluating the sedative effectiveness of 0.2ml of 1% propofol. Any subject not sedated was excluded. One subject died during surgery (yoked saline group).
Cocaine sensitization
Subjects were divided into three groups: a master group (n = 8) that self-administered cocaine, a yoked cocaine group (n = 8), in which rats were noncontingently administered cocaine using intervals set by their counterparts in the master group, and a yoked saline group (n = 7), in which rats received saline based on the intervals of their master counterparts. Thus, each master rat set the cocaine delivery times for one rat and the saline delivery times for another. To facilitate discrimination between training phases, no house light illumination was provided during this phase of the experiment. The chambers were also distinguished by adding a punched stainless steel floor plate, side-wall panels with black-and-white vertical lines (1″ wide), and an odor cue (0.1ml of 10% almond extract, placed on a paper towel positioned below floor plate). An LED light positioned within the nose poke hole was used to signal cocaine availability for the master group; this light was never illuminated for rats in the yoked groups. For the master group, each nosepoke resulted in the delivery of 0.23mg of cocaine over 4.35s followed by a 20-s time out during which the nosepoke light was extinguished. All groups received 14 once- daily sessions. Sessions lasted for 2 h or until 30 outcomes had been earned by a master group rat. Although locomotor sensitization was not assessed in the current study, it should be noted that similar cocaine-administration protocols have been shown to support locomotor sensitization (Hooks et al., 1994; Knackstedt and Kalivas, 2007; Phillips and Di Ciano, 1996; Sutton et al., 2000). One rat (yoked cocaine group) was excluded from the rest of the study due to a lack of catheter patency. One set of rats (a master rat and his yoked cocaine and yoked saline counterparts) was eliminated from the study because the master rat failed to meet criterion for adequate self-administration (at least 50 cocaine infusions over the 14-d period). After sensitization, rats were withdrawn for 10 d during which they remained in their homecages. One rat (master group) died before testing.
Pavlovian-to-instrumental transfer testing
The cocaine administration cues were removed from the chamber for the remainder of training and testing. Rats (n=6 for all groups) were retrained for 3 d on the instrumental response on a RI-45s schedule. On the following day, rats received a 30-min extinction session in which both levers were available but produced no rewards, which was done to suppress lever press rates to facilitate detection of the PIT effect. A PIT test was conducted on the following day. All lever presses were recorded during this session but no rewards were delivered. The two auditory cues (CS+ and CS−) were noncontingently presented 4 times each in strict alternation (tone, click) using a 2.5-min ITI to assess their ability to influence lever press performance. We subtracted the average number of presses performed on each lever during the 30-sec CS period from the average number of presses occurring 30-sec before cue onset (Pre-CS) to generate difference scores (CS – Pre-CS), which reflect the cue-related changes in lever press performance.
Results
All rats acquired the instrumental response for food reward, with no significant difference in lever pressing behavior between groups on the last two days (Masters: 30.24 ± 4.63, yoked cocaine: 24.30 ± 3.05, yoked saline: 25.35 ± 4.06). A one-way between-subjects ANOVA found no effect of group (F (2,17) = 0.64, p > 0.05). All subjects also learned to discriminate between the CS+ and CS− by the last two days of Pavlovian conditioning. Because our analysis of baseline (pre-CS) magazine entries found no main effect of CS (F (1,15) = 0.015, p > 0.05) or group (F (1,15) = 1.84, p > 0.05), and found no evidence of a CS by group interaction (F (1,15) = 0.31, p > 0.05), we used a difference score (see Methods) to quantify conditioned approach behavior. As shown in Table 1, on the final 2 days of training rats in all groups showed higher rates of magazine entry during the CS+ compared to the CS−. A mixed group × CS (CS+, CS−) ANOVA found a significant main effect of CS (F (1,15) = 13.72, p = 0.002), but no CS by group interaction (F (1,15) = 0.231, p > 0.05) and no main effect of group (F (1,15) = 0.077, p > 0.05), demonstrating that all groups learned the Pavlovian conditioned approach response.
Table 1.
Pavlovian training data – magazine entries. Values are averages ± SEM.
| CS+ | CS− | |
|---|---|---|
| Masters | 9.63 ± 2.45 | 2.67 ± 1.94 |
| Yoked cocaine | 10.60 ± 3.68 | 3.17 ± 2.17 |
| Yoked saline | 8.25 ± 1.87 | 3.5 ± 1.73 |
All but one rat in the master group learned to self-administer cocaine by nosepoking. Group average nosepoke rates (pokes per minute) at the end of training (last 2 days) reveal that the groups displayed very different levels of nosepoke behavior, with rats in the master group responding at a high rate (19.25 ± 2.64), rats in the yoked cocaine group responding at a low rate (5.08 ± 3.57), and rats in the yoked saline group showing no nosepoke behavior (0 ± 0). Due to the total lack of nosepokes for rats in the yoked saline group, the data from this group violated assumptions of normality and equal distribution of variance and was excluded from the statistical analysis. Multiple rats in the yoked cocaine group also performed no nosepokes, resulting in a non-normal distribution of scores in this group. We therefore used a nonparametric test (Mann-Whitney-U), which revealed a significant difference in nosepokes between yoked cocaine and master groups (U = 3.5, p < 0.05). As expected, we also observed an increase in the mean number of cocaine infusions for rats in the master (and yoked cocaine) group over days (First day: 1.38 ± 0.37, Last day: 18.67 ± 2.50). A paired-samples t-test revealed that intake significantly increased from the first day to the last day of self-administration (t (5) = −6.43, p <0.001).
The rats were then administered a PIT test to evaluate group differences in cue-evoked food seeking. Training and testing procedures were based on a previous study (Wyvell and Berridge, 2001) that established parameters that support minimal PIT performance in normal subjects, facilitating the detection of drug-induced enhancements in this effect. The results of this test are presented as a difference score in Figure 1, with baseline (pre-CS) press rates reported in Table 2. Consistent with the incentive sensitization hypothesis, we found that masters showed a selective increase in their rate of pressing during the CS+, whereas the performance of saline-treated rats was generally unaffected by either cue. However, this facilitation of PIT performance in the master group did not appear to be due to the absolute pharmacological properties of cocaine, since the yoked cocaine group also showed no evidence of the PIT effect. This interpretation was supported by a mixed group × CS ANOVA, which found no overall effect of CS (F (1, 15) = 0.448, p > 0.05) or group (F (2,15) = 2.82, p = 0.09), but did detect a significant CS by group interaction (F (2,15) = 5.16, p < 0.05), in line with the hypothesis that the food-paired cue was differentially effective in eliciting food-seeking behavior across the various cocaine-exposure conditions. To further examine this interaction we conducted planned pairwise comparisons of the CS effect (the difference between CS+ and CS−scores) between the three groups. Whereas the master group significantly differed from the yoked saline group (t (15) = 2.65, p <0.05) and the yoked cocaine group (t (15) = 2.89, p = 0.01), no difference was detected between the yoked cocaine and yoked saline groups (t (15) = −0.24, p > 0.05). Further analysis (paired, 2-tailed t-tests) found that the CS+ was significantly more effective in eliciting lever pressing than the CS− in the master group (t (5) = 3.78, p = 0.01) but not in the yoked saline (t (5) = 1.28, p > 0.05) or yoked cocaine (t (5) = 1.00, p > 0.05) group.
Figure 1.
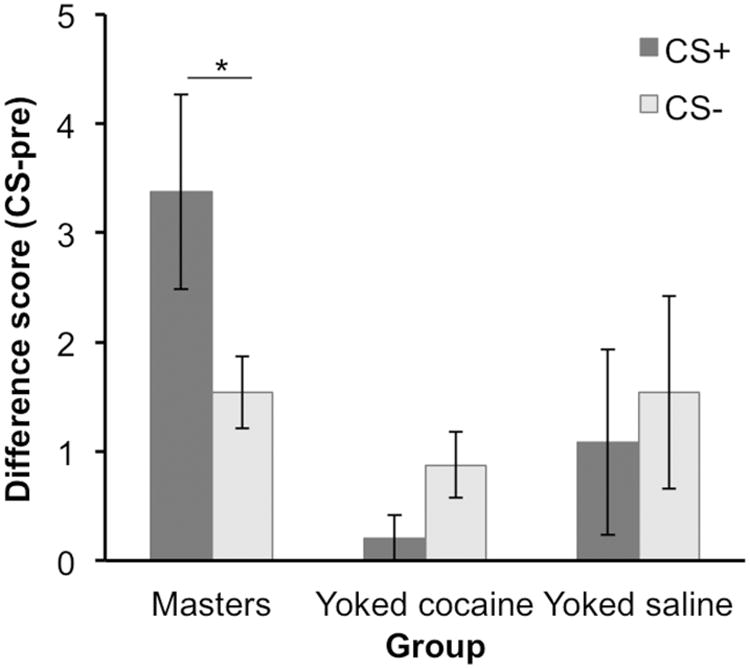
PIT test results, displayed as a difference score (baseline subtracted: CS − pre CS). Averages ± SEM. * = p < 0.05.
Table 2.
Pre-CS baseline lever presses. Values are averages ± SEM.
| Pre-CS+ | Pre-CS− | |
|---|---|---|
| Masters | 1.42 ± 0.42 | 1.04 ± 0.48 |
| Yoked cocaine | 1.58 ± 0.52 | 1.67 ± 0.50 |
| Yoked saline | 2.79 ± 0.45 | 2.67 ± 0.45 |
Discussion
We found that self-administered intravenous cocaine exposure enhances Pavlovian incentive motivation and facilitates expression of cue-evoked reward seeking. We extend a previous finding (Saddoris et al., 2011) by showing that this long-lasting consequence of self-administered cocaine cannot be fully understood by the simple pharmacological properties of that drug, in that rats given passive (noncontingent) intravenous cocaine injections using a yoking procedure showed no evidence of incentive sensitization. Instead, it appears that the subjective experience of taking cocaine (e.g., being able to predict and/or regulate drug intake) is a critical variable in this process. These differential behavioral effects of self-administered and yoked cocaine intake coincide with previous findings showing that self-administered cocaine is more effective than yoked cocaine in eliciting dopamine release (Hemby et al., 1997; Kimmel et al., 2005; Lecca et al., 2007) and in supporting long-lasting adaptations in the neural circuitry controlling dopamine release (Chen et al., 2008; Stefanski et al., 1999). Given the role of the dopaminergic system in incentive motivation (Robinson and Berridge, 2008), this increased ability of response-contingent cocaine to provoke changes in dopaminergic circuitry could account for the observed increase in cue-evoked reward-seeking reported here.
This finding indicates that that mode of delivery is a critical factor in determining the impact of a drug exposure regimen on incentive motivation. It should be noted, however, that some forced (involuntary) drug administration regimens can be effective in sensitizing the incentive motivational system (Mendez et al., 2009; Nocjar and Panksepp, 2002; Nordquist et al., 2007; Ranaldi et al., 2009; Taylor and Jentsch, 2001; Wyvell and Berridge, 2001). For instance we have recently shown that rats given repeated intraperitoneal cocaine injections (6d; 15 mg/kg per injection) exhibit a potentiation of the PIT effect (LeBlanc et al., in press). Thus both experimenter-delivered and self-administered cocaine can alter food-motivated behavior, indicating that the presence of a response-cocaine contingency is not a strict requirement for the development of cocaine-induced incentive sensitization. Interestingly, Chen et al (2008) showed that both experimenter-administered intraperitoneal cocaine injections and self- administered intravenous injections induce long-term potentiation of excitatory (glutamatergic) signaling in dopamine neurons in the ventral tegmental area, whereas unsignaled intravenous cocaine injections do not. Such changes would be expected to make the dopamine system more responsive to excitatory input and may therefore be the mechanism through which these cocaine-exposure regimens enhance the expression of the dopamine-dependent PIT effect.
Why yoked cocaine injections do not result in incentive sensitization (or, for that matter, long-term potentiation in ventral tegmental dopamine cells) is not entirely clear. One possibility is that it is the ability to anticipate and presumably prepare for cocaine intake that determines its impact on the incentive motivational system. With both experimenter- and self-administered cocaine, receipt of the drug is signaled, either by injection cues (e.g., special handling and needle insertion) or by execution of the instrumental action. No such signals were provided to rats in the yoked cocaine group. Consistent with this account, previous studies have shown that cues signaling drug delivery can influence the drug's acute behavioral and neurochemical effects (Cepeda-Benito and Tiffany, 1995; Duvauchelle et al., 2000; Hinson and Poulos, 1981; Hinson and Siegel, 1982), and are known to play an important role in the development of drug sensitization (Browman et al., 1998a, b; Robinson et al., 1998). It should be noted, however, that while associative learning and drug anticipation may very well contribute to the induction of incentive sensitization, it is also possible that the opportunity to actively self-regulate cocaine intake was the determining factor between the two cocaine exposure groups in the current study. Although the studies mentioned above have shown that forced drug exposure regimens can also induce incentive sensitization, the various procedural differences for drug administration between these studies and the current experiment (e.g., intraperitoneal injections of a single, high dose drug bolus each day vs. multiple relatively low dose intravenous injections distributed over hours each day) make direct comparisons problematic.
The current results shed new light on the way drugs of abuse impact motivated behavior and may have important implications for our understanding of addiction. For instance, they suggest that subjective factors (e.g., ability to prepare for and/or regulate drug intake) play a role in determining how influential cues will be in motivating behavior after prolonged drug use, which could predict whether experimental drug use will develop into a pathological behavior. Such information may be useful for predicting vulnerability to drug abuse and for developing novel approaches to prevent and treat drug addiction.
Acknowledgments
This research was supported by Grant DA029035 to SBO, Grants DA009359 and DA005010 to NTM, and training fellowship T32-DA024635 to KHL.
Footnotes
Author contribution: KHL, SBO, and NTM conceived of and designed the experiment. KHL conducted the experiments. KHL and SBO analyzed the data. KHL, SBO, and NTM wrote the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats. J Pharmacol Exp Ther. 1998a;287:1007–1014. [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. The influence of environment on the induction of sensitization to the psychomotor activating effects of intravenous cocaine in rats is dose-dependent. Psychopharmacology (Berl) 1998b;137:90–98. doi: 10.1007/s002130050597. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Tiffany ST. Role of drug-administration cues in the associative control of morphine tolerance in the rat. Psychopharmacology (Berl) 1995;122:312–316. doi: 10.1007/BF02246554. [DOI] [PubMed] [Google Scholar]
- Chen B, Bowers M, Martin M, Hopf F, Guillory A, Carelli R, Chou J, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza P. Cocaine self-administration increases the incentive motivational properties of the drug in rats. European Journal of Neuroscience. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res. 2000;862:49–58. doi: 10.1016/s0006-8993(00)02091-6. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Siegel S. Nonpharmacological bases of drug tolerance and dependence. J Psychosom Res. 1982;26:495–503. doi: 10.1016/0022-3999(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Hooks M, Duffy P, Striplin C, Kalivas P. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl) 1994;15:265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kimmel H, Ginsburg B, Howell L. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56:129–134. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. J Pharmacol Exp Ther. United States: 2007. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization; pp. 1103–1109. [DOI] [PubMed] [Google Scholar]
- LeBlanc K, Maidment N, Ostlund S. Repeated Cocaine Exposure Facilitates the Expression of Incentive Motivation and Induces Habitual Control in Rats. PLoS One. doi: 10.1371/journal.pone.0061355. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc KH, Ostlund SB, Maidment NT. Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci. 2012;126:681–689. doi: 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res. 2009;201:74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Di Ciano P. Behavioral sensitization is induced by intravenous self- administration of cocaine by rats. Psychopharmacology (Berl) 1996;124:279–281. doi: 10.1007/BF02246669. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Egan J, Kest K, Fein M, Delamater AR. Repeated heroin in rats produces locomotor sensitization and enhances appetitive Pavlovian and instrumental learning involving food reward. Pharmacol Biochem Behav. 2009;91:351–357. doi: 10.1016/j.pbb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Robinson T, Berridge K. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive- sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Rowell P, Carr L, Garner A. Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49:1449–1454. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Stamatakis A, Carelli RM. Neural correlates of Pavlovian-to- instrumental transfer in the nucleus accumbens shell are selectively potentiated following cocaine self-administration. Eur J Neurosci. 2011;33:2274–2287. doi: 10.1111/j.1460-9568.2011.07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee S, Cadet J, Goldberg S. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stefański R, Ziółkowska B, Kuśmider M, Mierzejewski P, Wyszogrodzka E, Kołomańska P, Dziedzicka-Wasylewska M, Przewłocki R, Kostowski W. Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Neuropsychopharmacology. United States: 2000. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats; pp. 626–641. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr. 1984;50:15–33. [PubMed] [Google Scholar]
- Wyvell C, Berridge K. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


