Abstract
Summary
We studied the relationships among strength, muscle mass, and bone mineral density (BMD) with lifestyle change. Lifestyle therapy consisted of exercise, diet, and diet plus exercise. Diet was by caloric restriction to induce and maintain a weight loss of 10 % from baseline body weight. Exercise attenuated weight loss-induced muscle and bone losses. Exercise improved strength despite muscle loss in patients on diet and exercise. Changes in strength did not correlate with changes in BMD. However, changes in thigh muscle volume correlated with, and predicted changes in hip BMD.
Introduction
Losses of hip BMD and lean body mass are major complications of lifestyle therapy in frail, obese older adults; however, the contribution of mechanical strain loss from muscle loss is poorly defined. We determined the effect of changes in thigh muscle volume and muscle strength on BMD in frail, obese older adults undergoing lifestyle therapy aimed at intentional weight loss with or without exercise.
Methods
One hundred seven obese older adults were randomized to control, diet, exercise, and diet–exercise groups for 1 year. Thigh muscle volume was measured by magnetic resonance imaging, BMD by DXA, knee strength by dynamometry, total strength by one-repetition maximum (1-RM), and bone markers by immunoassay.
Results
Thigh muscle volume decreased in the diet group (−6.2±4.8 %) and increased in the exercise group (2.7±3.1 %), while it was not significantly different from the control in the diet–exercise group. Changes in hip BMD followed similar pattern as those in thigh muscle volume. Knee extension and flexion increased in the exercise group (23±20 %; 25±19 %) and diet–exercise group (20±19 %; 20.6±27 %) but were unchanged in the control and diet groups. Changes in thigh muscle volume correlated with changes in hip BMD (r=0.55, P=<0.001) and were an independent predictor of changes in hip BMD (β=0.12, P=0.03) in the multiple regression analyses after accounting for demographic factors and changes in weight and physical activity. There were no correlations between BMD changes and knee strength, 1-RM, and sclerostin changes.
Conclusions
Changes in thigh muscle volume predict hip BMD changes in obese older patients undergoing lifestyle therapy. The effect of exercise in attenuating thigh muscle loss when added to diet may in part account for the reduction in weight loss-induced bone loss in the diet–exercise group.
Keywords: Bone mineral, Density, Obesity, Lifestyle therapy, Thigh muscle
Introduction
We previously reported that diet-induced weight loss in the elderly obese is associated with bone loss at the hip and loss of lean body mass and thigh muscle volume [1]. However, despite this loss of lean body mass, elderly obese patients undergoing weight loss alone experienced an improvement in physical function which was attributed to loss of excess body weight leading to improved mobility. Conversely, the addition of exercise to weight loss resulted in an even greater improvement in physical function compared with weight loss alone suggesting an additional benefit from exercise. In fact, we found that exercise can attenuate the diet-induced weight loss associated reduction of hip bone mineral density (BMD), lean body mass, and thigh muscle volume [1, 2].
We also reported that the reduction in hip BMD from diet-induced weight loss was associated with an increase in sclerostin, an inhibitor of bone formation that increases in states of unloading [3]. This increase in sclerostin was inhibited by the addition of exercise, leading to attenuated bone loss in patients undergoing diet-induced weight loss and exercise compared with those undergoing diet-induced weight loss alone. Although we presumed that these changes in hip BMD with lifestyle intervention were in part mediated by changes in sclerostin as a result of changes in mechanical loading [3], the contribution from changes in mechanical strain on the hip due to changes in muscle strength and changes in volume of muscles in the thigh remains poorly defined. Thus, the objective of this study was to determine the effect of changes in thigh muscle volume and thigh muscle strength on hip BMD in frail, obese, older adults undergoing lifestyle intervention. We hypothesized that the negative effects of diet-induced weight loss and the protective effect of exercise on hip BMD would be predicted by changes in thigh muscle volume and thigh muscle strength. Given that the changes in BMD in our subjects were observed only at the hip, in this study we focused on the changes in strength of the knee flexion and knee extension which have been used in previous studies to assess strength of thigh muscles [4].
Subjects and methods
Subjects
The results described in this project were obtained from a secondary analysis of data from a previous study on the effect of lifestyle intervention on physical function in frail, obese, older adults [1]. This study was conducted at Washington University School of Medicine in accordance with the guidelines in the Declaration of Helsinki for the ethical treatment of human subjects. The protocol was approved by the Washington University Institutional Review Board. Participant recruitment was through advertisements. A written informed consent was obtained from each subject. Inclusion criteria/exclusion criteria were as reported previously [1]. Briefly, participants were ≥65 years of age, with BMI≥30 kg/m2, had sedentary lifestyle, stable body weight (±2 kg) over the past year and on stable medications for 6 months before enrollment. Those who were treated with bone-acting drugs (e.g., bisphosphonates, glucocorticoids, and sex-steroid compounds) during the previous year were excluded from participation. Reports describing the effects of weight loss and/or exercise training (ET) on measures of frailty, body composition, BMD, bone turnover markers, sclerostin levels, hip geometry parameters, specific physical functions, and quality of life on these subjects have been published [1–3]. The current manuscript reports the effects of weight loss and/or ET on muscle strength and thigh muscle volume and their relationship to the changes in hip BMD.
Study design
The participants were randomized, with stratification by sex, for a 52-week study, to any of the following treatment groups: (1) control group, (2) diet-induced weight loss group (diet group), (3) ET group (exercise group), and (4) diet-induced weight loss and ET group (diet–exercise group). Details regarding the intervention strategies have been described previously [1]. Briefly, subjects in the control group were advised to continue their usual dietary or activity habits. The diet group was recommended a diet that would provide an energy deficit of 500 to 750 kcal/day from their daily energy requirement and asked to meet weekly as a group for adjustments of calorie intake and behavioral therapy with a dietitian. Their food diaries were reviewed and new goals were set based on diary reports. The objective was to achieve a ~10 % weight loss at 6 months and maintain this weight for an additional 6 months. The subjects in the exercise group were asked to maintain a weight-stable diet while participating in a multicomponent ET program supervised by a physical therapist. Each session was approximately 90min in duration: 15min of flexibility exercises, 30 min of aerobic exercises, 30 min of progressive resistance training, and 15 min of balance exercises. Subjects in the diet–exercise group participated in both weight management and ET programs described above. All subjects were provided supplements to ensure an intake of ~1,500 mg of calcium/day and ~1,000 IU vitamin D/day.
Outcome assessments
Muscle strength testing
Knee flexion and knee extension
Isokinetic knee extensor and flexor strength were evaluated using a Biodex System 3 dynamometer (Shirley, NY) as previously described [5]. During the testing, the participants were seated with their backs supported and hips positioned at 120° of flexion and secured to the seat of the dynamometer with thigh and pelvic straps. All tests were performed on the right leg. Testings were performed at an angular velocity of 60°/s. The best of the three maximal voluntary efforts for each of the knee flexion and extension was used as the measure of absolute strength and reported as peak torque at 60° in Newton meter (Nm) units. The biodex testing has the advantage that it minimizes the effects of neuromuscular learning on measurement outcomes, because the ET program did not include biodex exercise. These measurements were done at baseline, 6 and 12 months. The test–retest reliability of this method based on follow-up isokinetic testing done one week following the intial tests showed an intra-class correlation coefficient of 0.99.
One repetition maximum
The total one-repetition maximum (1-RM; the sum of the maximal weight a person can lift at one time) were done at baseline, 6 and 12 months as previously described [1]. The test–retest reliability of this method based on follow-up 1-RM determinations one week following the initial tests showed an intra-class correlation coefficient of 0.96.
Thigh Muscle Volume
Magnetic resonance imaging (MRI) of both thighs were obtained using a 1.5-T superconducting MRI instrument (Siemens, Iselin, NJ) and a T1-weighted pulse sequence as described previously [6]. Ten transverse images, 10 mm thick, were obtained just above the patella with no intersection gap between slices. Muscle volume and cross-sectional area were quantified in each slice by Analyze Direct software (version 5.0; Mayo Clinic, Rochester, MN), which distinguishes muscle from other tissues based on pixel brightness. Volume data for ten slices were summed to represent muscle volume in the 10-cm longitudinal region of interest. Muscle size data are reported as the sum of values from the right and left thighs. This test was done at baseline and 12 months. The coefficient of variation (CV) for repeated measures of total thigh muscle volume was <1.5 % as previously reported [5]).
Biochemical assays
Fasting venous blood samples were obtained at baseline, 6 and 12 months and samples were stored in −80 for future assays. Enzyme-linked immunosorbent assay was used to measure sclerostin (TECO Sclerostin; TECOmedical AG, Sissach, Switzerland), osteocalcin (Metra OC; Quidel, San Diego, CA, USA), and C-terminal telopeptide of type 1 collagen (CTX; Crosslaps: Nordic Bioscience Diagnostics Herlev, Denmark), while radioimmunoassay was used to measure Insulin-like growth factor-1 (IGF-1; Diagnostic Products Group), N-terminal propeptide of type 1 collagen (P1NP; Orion Diagnostica, Espoo, Finland), and serum 25-hydroxyvitamin D (25(OH)D; DiaSorin, Stilwater, MN, USA). Serum calcium was measured through Barnes–Jewish Hospital clinical laboratory using an automated colorimetric technique (Modular Analyzer, Roche). The CV for the above assays is as follows: sclerostin, <10 %; osteocalcin, 4.4 %; CTX, 2.1 %; IGF-1, <10 %; and 25(OH)D, <10 % as previously reported [2, 3]. The CV for PINP is 1.96 %.
Body weight, BMD, and body composition
Body weight was measured in the morning after the subjects had fasted for 12 h. BMD and body composition were measured using DXA (Delphi 4500-W; Hologic, Inc., Walthan, MA, USA) as previously reported [1, 2]. The CV for BMD at our center is 1.1 % for the lumbar spine and 1.2 % for the proximal femur [7]. The CV for fat free mass and fat mass in our laboratory is 1.5 % [8].
Statistical analyses
The primary outcomes for this report were the 12-month changes in muscle strength (as assessed by knee flexion and extension) and thigh muscle volume in relation to changes in hip BMD. Secondary outcomes included changes in sclerostin, IGF-1, and biochemical markers of bone turnover. Intention-to-treat analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA), with inclusion of all participants who provided any data after baseline. Baseline characteristics were compared using analyses of variance (ANOVA) or Fisher's exact tests. Longitudinal changes between groups were tested with mixed-model repeated-measures ANOVA, adjusting for baseline values and sex. Within the framework of the mixed model, when the p value for an interaction was significant, the specific contrasts were used to test the null hypothesis that changes between two specific time points in one group were equal to the corresponding changes in another group. Analyses testing for within-group changes were also performed using mixed-model repeated-measures ANOVA. Pearson's correlation was used to examine relationships among changes in selected variables and changes in hip BMD, followed by stepwise multiple linear regression analysis to identify which variables were independent contributors to the changes in hip BMD. Data are presented in the tables and text as mean±SD and in the figures as mean±SE. All statistical tests were two-tailed, and P<0.05 was considered statistically significant. Our study was powered to detect a difference of 18 % in the percentage change in muscle strength and 5% in the percentage change in thigh muscle volume between study arms with 80 % power and α=0.05.
Results
The results of screening, enrollment, randomization, follow-up and compliance have been reported [1]. Briefly, of the 107 volunteers who were randomized, 93 (87 %) completed the study. Fourteen participants discontinued the intervention due to personal or medical reasons but were included in the intention-to-treat analyses. The median attendance at exercise sessions was 89 % (interquartile 86 to 93) at 6 months and 87 % (interquartile 84 to 92) at 12 months among participants in the exercise group and was 88 % (interquartile 76 to 90) at 6 months and 79 % (interquartile 75 to 88) at 12 months among those in the diet–exercise group. As reported, the diet group lost an average of 9.7±5.4 kg (10 %) of body weight, whereas those in the diet–exercise group lost 8.6±3.8 kg (9 %) of body weight. There was no weight loss in the exercise group (−1.8±2.7 kg or a loss of 1 %) and the control group (−0.9±1.5 kg or a loss <1 %) [1].
There were no significant differences in baseline characteristics, including age, sex, race, weight, BMI, hip BMD, and Tscore and serum concentrations of sclerostin, IGF-1, and bone markers (Table 1) among the treatment groups [1, 2]. In addition, the four groups did not significantly differ at baseline in thigh muscle volume, and strength of knee extension and flexion (Table 1).
Table 1.
Baseline characteristics of study participants
| Control (n=27) | Diet (n=26) | Exercise (n=26) | Diet-exercise (n=28) | P value | |
|---|---|---|---|---|---|
| Age (year) | 69±4 | 70±4 | 70±4 | 70±4 | 0.85 |
| Female sex (no. (%)) | 18 (67) | 17 (65) | 16 (61) | 16 (57) | 0.89 |
| White race (no. (%)) | 22 (81) | 23 (88) | 21 (81) | 25 (89) | 0.78 |
| Weight (kg) | 101 ± 16 | 104±15 | 99±17 | 99±17 | 0.66 |
| BMI (kg/m2 ) | 37.3±4.7 | 37.2±4.5 | 36.9±5.4 | 37.2±5.4 | 0.93 |
| BMD at total hip (g/cm2 ) | 0.962±0.132 | 1.021±0.139 | 0.958±0.151 | 1.014±0.151 | 0.25 |
| T-score at total hip | −0.18±0.91 | 0.34±97 | −0.25±1.1 | 0.18±1.1 | 0.07 |
| Sclerostin (ng/mL) | 1.51±0.42 | 1.50±0.33 | 1.41±0.23 | 1.57±0.39 | 0.54 |
| IGF-1 (ng/mL) | 102.8±31.9 | 102.9±31.2 | 88.0±26.9 | 91.2±24.5 | 0.06 |
| CTX (ng/mL) | 0.407±0.197 | 0.309±0.123 | 0.350±0.143 | 0.320±0.116 | 0.09 |
| Osteocalcin (ng/L) | 12.4±4.4 | 11.6±3.6 | 13.2±4.4 | 12.2. ±4.4 | 0.64 |
| PINP (µg/L) | 52.2±26.3 | 41.5± 12.2 | 45.2±14.1 | 43.1 ± 13.1 | 0.13 |
| Thigh muscle volume (cm3) | 1138±290 | 1271±280 | 1188±234 | 1261±253 | 0.27 |
| Knee extension (Nm) | 70.0±26.0 | 70.8±24.4 | 72.5±28.7 | 71.4±24.0 | 0.99 |
| Knee flexion (Nm) | 44.7±17.4 | 50.3±18.0 | 46.4±16.5 | 49.1±13.8 | 0.78 |
Values are means±SD
BMD bone mineral density, BMI body mass index, IGF-1 insulin-like growth factor 1, CTX C-terminal telopeptide of type 1 collagen, PINP N-terminal propeptide of type 1 collagen, Nm Newton meter
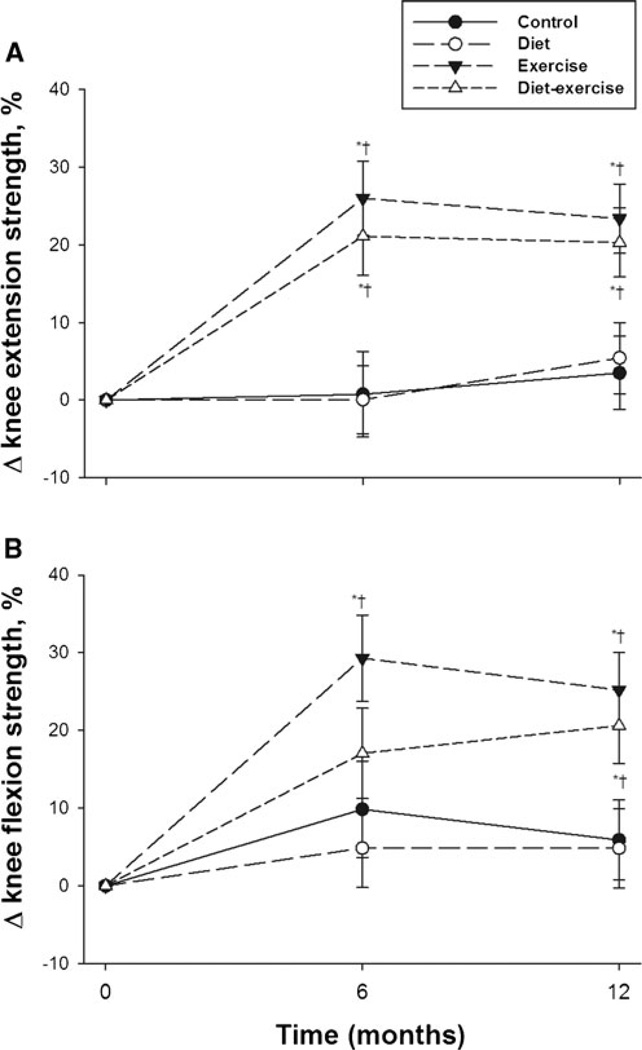
Our results showed that patients in the exercise and diet– exercise groups had a significant increase in the strength of knee extension at 6 (26.0±28.0 and 21.1±20.2 %, respectively) and 12 months (23.4±20.2 and 20.3±19.2 %, respectively) (Fig. 1a). The strength of knee flexion also significantly increased at 6 and 12 months among patients randomized to exercise (29.3±15.3 % and 25.2±19.1 %, respectively) and at 12 months (20.6±27.5 %) among those randomized to diet– exercise (Fig. 1b). Thus, at the end of 12 months, the strength of knee extension and flexion was comparable in both exercise and diet–exercise groups. There were no changes in both knee extension and knee flexion among subjects randomized to control and diet groups.
Fig. 1.
Mean percent changes in strength of knee extension (a) and knee flexion (b) during the 1-year intervention. Values are mean±standard error. *P<0.05 compared with baseline; †P<0.05 compared with control and diet
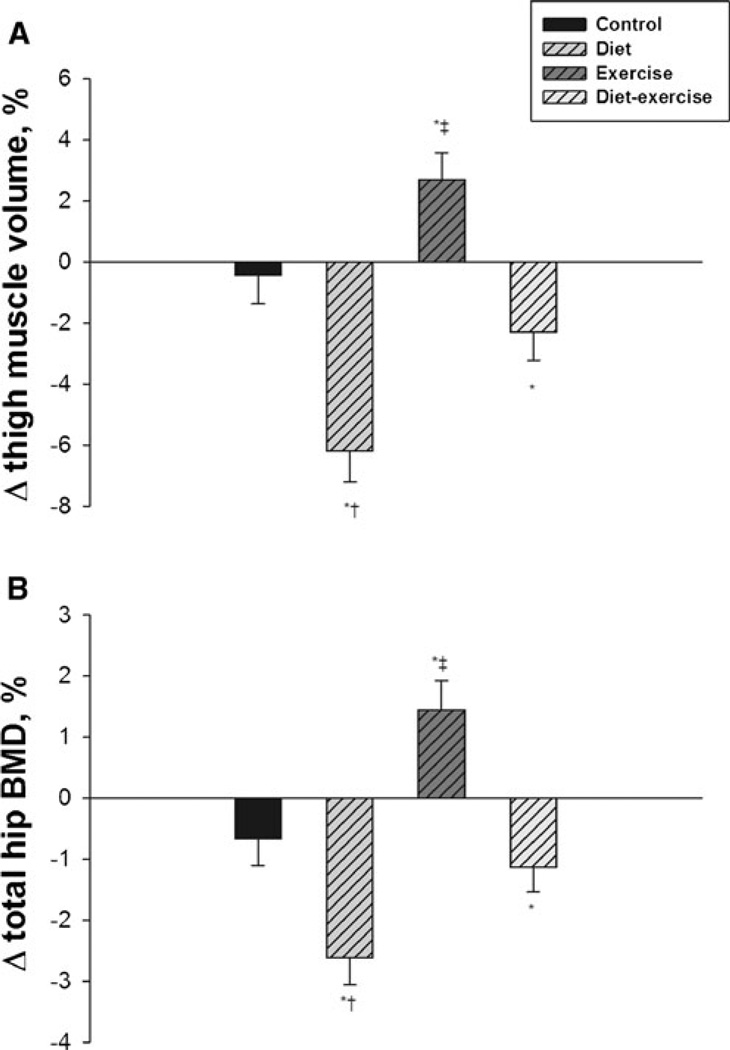
As reported, there was a significant decrease in thigh muscle volume among patients randomized to diet (−6.2±4.8 %) and a significant increase in patients randomized to exercise (2.7±3.1 %) (Fig. 2a) [1]. Patients in the diet– exercise group had a modest decrease (−2.3±5.1 %) in thigh muscle volume which was not significantly different from patients in the control group (−0.4±4.3 %). Analysis of changes in total hip BMD showed that, similar to changes in thigh muscle volume, patients in the diet group experienced a significant decrease in BMD (−2.6±2.1 % ) while patients in the exercise group had a significant increase in BMD (1.5±1.6 %) (Fig. 2b). Meanwhile, patients in the diet–exercise group had a modest decrease in BMD (−1.1±2.7 %) which was not different from patients in the control group (−0.7±1.8 %). A similar trend was observed for BMD changes at the femoral neck although the change was only significant for those in the diet group; the diet group lost an average of −2.3±2.8 %. The exercise group had an average change in BMD of 1.0±3.2 %, the diet–exercise group of −0.9±2.8 %, and the control group of 0.05±2.9 %, all of which were not significantly different from baseline.
Fig. 2.
Mean percent changes in thigh muscle volume (a) and total hip BMD (b) during the 1-year intervention. Values are mean±standard error. *P<0.05 compared with baseline; †P<0.05 compared with control and exercise; ‡P<0.05 compared with control and diet
Serum CTX, OC, and P1NP significantly increased in the diet group (31±53 %, 24±32 %, and 9±29 %, respectively), decreased in the exercise group (−13±31 %, −15±28 % and −15±25 %, respectively), and did not change in the dietexercise and control groups [2]. Moreover, while there were no significant changes in IGF-1 at the end of 12 months, serum sclerostin increased in the diet group (10.5±1.9 % ) but this increase in sclerostin was prevented by the addition of exercise (0.5±9.3 %) [3]. Serum 25 (OH) vitamin D levels increased in the control (from 19.2±8.2 to 22.4±8.3 ng/mL), diet (from 22.0±5.7 to 24.5 ng/mL), exercise (from 21.4±8.4 to 26.3 ng/mL), and diet–exercise (from 20.9 to 25.6 ng/mL) groups [2]. Average serum calcium levels which were normal at baseline among the treatment groups did not significantly change during the study (data not shown).
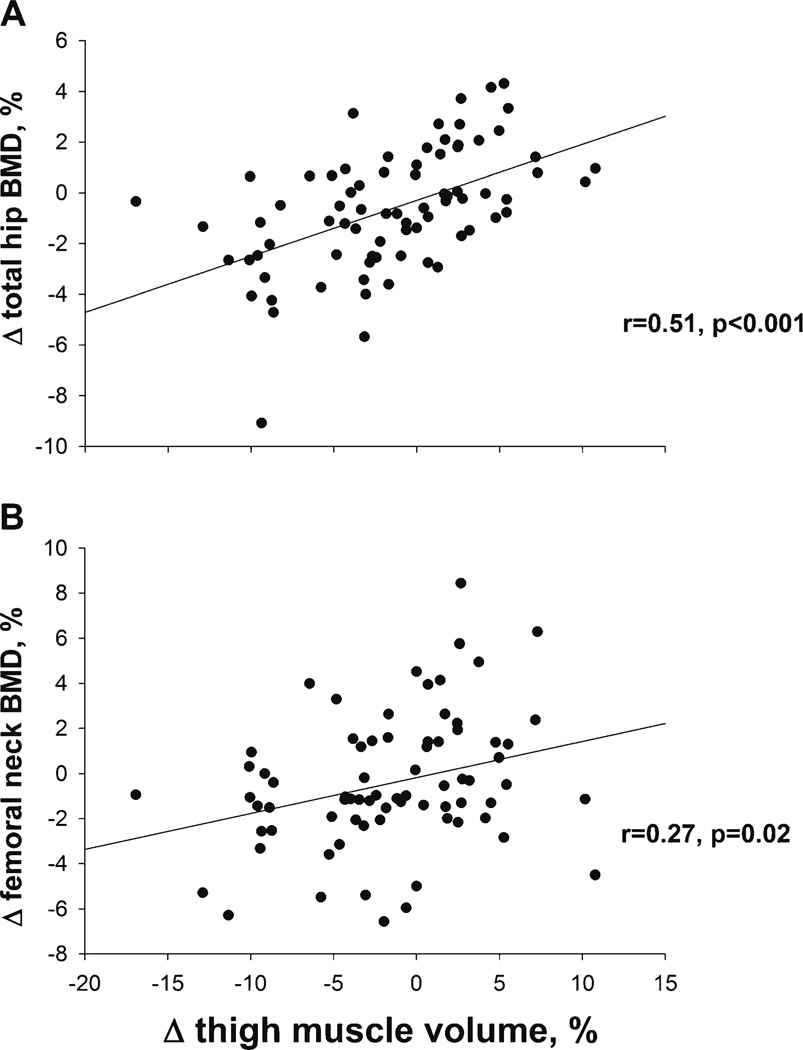
Pearson correlation analysis showed a positive correlation between changes in total hip BMD and thigh muscle volume (Fig. 3a), r=0.51, P<0.001; and between changes in femoral neck BMD and thigh muscle volume (Fig. 3b), r=0.27, P=0.02. There were no correlations between changes in total hip BMD and changes in strength of knee extension and flexion, and changes in sclerostin (r=−0.01, −0.07, and −0.12, respectively, all P>.05). As reported previously, changes in total hip BMD correlated with changes in lean body mass (r=0.46, P=<0.001), total 1-RM (r=0.23, P=0.04), and markers of bone turnover including CTX (r=−0.32, P=0.004), PINP (r=−0.30, P=0.005), and osteocalcin (r=−0.37, P=0.001) [2]. Multiple stepwise regression analysis using variables that correlated with changes in total hip BMD by Pearson correlation (changes in thigh muscle volume, lean body mass, osteocalcin, P1NP, CTX, and 1-RM strength) showed that changes in thigh muscle volume were an independent predictor of changes in total hip BMD (Table 2). The effect of changes in total 1-RM strength, an independent predictor of total hip BMD in our prior report [2], was lost with the inclusion of changes in thigh muscle volume into the model. The final model which also included change in lean body mass and change in osteocalcin, explained 39.9 % of the variance in total hip BMD changes. In adjusting the model for age, gender, and change in weight, thigh muscle volume remained a significant predictor of total hip BMD (adjusted P values were, 0.04, 0.049, and 0.03, respectively). Likewise, in adjusting the model for level of physical activity using measures such as change in total 1-RM strength as a marker of improved physical fitness as well as treatment group assignment, thigh muscle volume also remained a significant predictor of total hip BMD (adjusted P values were 0.04 and 0.03, respectively).
Fig. 3.
Relationships between changes in total hip BMD and changes in thigh muscle volume (a) and between changes in femoral neck BMD and changes in thigh muscle volume (b)
Table 2.
Final model in the stepwise multiple regression analysis identifying predictors of changes in total hip BMD
| R2 | Regression coefficient |
95% Confidence interval |
P value | |
|---|---|---|---|---|
| Δ lean body mass | 39.9 | 0.17 | 0.025 to 0.323 | 0.02 |
| Δ osteocalcin | −0.02 | −0.037 to −0.003 | 0.02 | |
| Δ thigh muscle volume | 0.12 | 0.012 to 0.220 | 0.03 |
Variables entered into the model: Δ thigh muscle volume, Δ lean body mass, Δ body weight, Δ total 1-RM, Δ osteocalcin, Δ P1NP, Δ CTX, Δ level of physical activity, Δ sclerostin, age, and gender
Other relevant correlation studies revealed a negative correlation between changes in thigh muscle volume and changes in sclerostin (r=−0.23, P<0.05), and a borderline correlation between changes in thigh muscle volume and changes in knee flexion strength (r=0.23, P=0.06).
Discussion
Our results demonstrated that despite some degree of muscle loss, patients in the diet–exercise group also experienced a significant improvement in the strength of knee extension and flexion at the end of the study comparable to that of the exercise group. In addition, although there was a reduction in muscle volume among patients in the diet group, their strength remained stable at the end of the 12-month intervention period similar to controls. Our analysis also showed parallel changes in thigh muscle volume and hip BMD; patients randomized to diet had significant loss of thigh muscle volume and total hip BMD while patients in the exercise group had significant increase in thigh muscle volume and total hip BMD [1, 2]. Although subjects in the diet–exercise group were experiencing some degree of loss of thigh muscle volume and total hip BMD, losses in both were modest and not significantly different from the control group. More importantly, changes in thigh muscle volume positively correlated with changes in total hip BMD, and were an independent predictor of changes in the total hip BMD.
Falls account for the majority of hip fractures in the elderly [9]. Several nonskeletal factors have been recognized to influence BMD and the risk of fractures. Some of the most relevant nonskeletal factors associated with fall risk in the elderly include reduced balance, mobility, and lower limb strength [10–14]. The positive impact of regular exercise on muscle strength, gait, and balance in elderly has been demonstrated in several studies [15, 16]. However, little information is available on the effects of regular ET on the BMD in frail, elderly subjects and whether the positive effects of exercise on the muscles have any effect on BMD. This information is highly useful in the care of the obese subset who could be at high risk for falls by having the worst combination of being frail, old, and fat [17, 18]. Results from our group showed that weight loss leads to improvement in physical function in frail, elderly, obese patients but at the expense of loss of lean body mass and bone mass [1]. Consistent with reports from previous investigators [19, 20], we found that these adverse effects are however, mitigated by the addition of regular ET [2] and resulted in even greater improvement in physical function [1] underscoring the benefits of exercise to both bone metabolism and muscle function even in the frail, elderly, obese subjects.
Of note, in addition to the to the corresponding changes in thigh muscle volume and hip BMD across the different treatment groups, our analysis also revealed a correlation between changes in thigh muscle volume and changes in hip BMD. Subjects who lost thigh muscle volume also had a reduction in BMD and vice-versa. Taken together, these findings may suggest that the changes occurring in muscle and bone are related or a result of an interaction between the two organs. In agreement with this concept are data from association studies showing that patients with low muscle mass also have low bone mass and vice-versa [21–23]. Furthermore, patients with history of vertebral fractures have been reported to have lower lean body mass than nonfractured controls [24]. Of particular interest, are reports showing that fractures that are covered by intact muscles are more likely to heal rapidly than those with damaged muscles [25, 26]. Despite speculations that muscles secrete certain osteogenic factors, this apparent relationship between muscle and bone metabolism is presently incompletely understood. Several factors produced by muscle tissues including IGF 1, IGFBP5, basic fibroblast growth factor-2, osteonectin, transforming growth factor-β1, matrix metalloproteinase 2, leukemia inhibitory factor, and osteoglycin, have been found to stimulate osteogenesis [27, 28]. Notwithstanding the significant differences in BMD and thigh muscle volume changes in this cohort, we did not find significant differences in IGF-1 among the different treatment groups [2]. However, our group found increased expression of mechanogrowth factor, an isoform of IGF-1, in exercising muscles [29]. Whether this is released into the circulation or in a paracrine fashion affects the neighboring bone remains uncertain. Conversely, PGE2 secreted by osteocytes has been found to induce myogenesis [30]. Alternatively, both myoblast and osteoblast come from the same mesenchymal stem cells, and events that drive these progenitor cells to differentiate results in activation of both myoblastic and osteoblastic signaling [31, 32]. Indeed, Wnt3A a component of the Wnt signaling system, which is crucial for bone development also enhances myogenesis and muscle fiber size [33]. Nevertheless, whether one factor or a combination of factors mediates the cross-talk and the coordinated regulationship between muscle and bone is unclear at this time.
Conversely, we previously reported increased levels of sclerostin, an inhibitor of bone formation, in patients undergoing weight loss [3]. Sclerostin is a protein almost exclusively produced by osteocytes, the mechanosensors of bones, which goes up in states of unloading resulting in bone loss [34, 35]. It is possible that sclerostin also mediates the enhanced or reduced mechanical stimulation on the skeleton from increase or loss of muscle mass leading to increase or decrease in BMD, respectively. A recent report indicated a correlation between hand grip with wrist BMD, and bone quality as assessed by ultrasonogragphy [36]. We previously showed that subjects in the diet group with high sclerostin levels had deterioration in the parameters of hip geometry indicating poor bone quality while subjects in diet–exercise group with sclerostin levels not different from the control appeared to have preservation in these parameters [3]. Taken together, it is possible that the mechanical stain on the muscles from regular exercise was able to neutralize the effect of unloading on muscle loss and increase in sclerostin levels resulting in diminished bone loss and maintenance of bone quality.
Our results also suggest that muscle volume and muscle strength may not be equivalent, at least in our subjects. Despite some degree of muscle loss in the diet–exercise group, there was an improvement in the strength of knee flexion and extension. More importantly, the patients in the diet group who had muscle loss had no deterioration in muscle strength, and their strength at the end of the study was similar to the control group who did not experience muscle loss. This is not surprising as obesity is associated with increase in inter and intramuscular fat, and the measured muscle mass may not reflect pure muscle by itself [37]. Fat in the muscle tissue may impair muscle quality not only from a reduction in the quantity of muscle fibers but also from increased production of inflammatory cytokines [38, 39]. Although we have not quantified the degree of fatty infiltration in the muscles of our subjects, it is likely that this may explain the relatively poor muscle strength in the obese control patients who had no change in muscle volume. Conversely, it is possible that the improvement in muscle strength experienced by the dietexercise group and the relatively preserved muscle strength in the diet group are perhaps a result of the reduction in fatty infiltration and cytokine production leading to improvement in muscle quality despite loss in muscle volume in both groups. This may also partially explain the improvement in physical function in the diet group even with a significant reduction in lean body mass as we previously reported [1]. Nevertheless, whether the improvement in strength attenuated the bone loss in the diet-exercise group is uncertain as there was no direct correlation between changes in strength of knee flexion and extension and changes in hip BMD in this study.
Conclusions
Our results indicate that ET is beneficial in improving muscle strength and in maintaining thigh muscle volume and hip BMD in obese older adults undergoing lifestyle intervention which includes weight loss. Changes in both thigh muscle volume and hip BMD appear to parallel and correlate with each other possibly suggesting a common or a related mechanism regulating these changes. Furthermore, thigh muscle volume does not necessarily correlate with strength perhaps because muscle strength is more of a reflection of muscle quality than volume. Despite some degree of muscle volume loss in the diet–exercise group, ET improves muscle strength implying improved muscle quality. Our data indicate that ET is advantageous for improving both muscle and bone even in frail, obese, and older adults.
Acknowledgments
The authors thank the participants for their cooperation and the staff of the Intensive Research Unit of the Institute of Clinical and Translational Sciences for their skilled assistance in the performance of this study. This study was supported by grants RO1-AG025501, R01-AG031176, P30-DK56341 (Clinical Nutrition Research Unit), and UL1-RR024992 (Clinical and Translational Science) and resources at the New Mexico VA Health Care System.
Footnotes
Conflicts of Interest The authors have no conflicts of interests.
Contributor Information
R. Armamento-Villareal, New Mexico VA Health Care System, Albuquerque, NM, USA University of New Mexico School of Medicine, Albuquerque, NM, USA.
L. Aguirre, New Mexico VA Health Care System, Albuquerque, NM, USA
N. Napoli, Campus Biomedico, Rome, Italy Washington University School of Medicine, St. Louis, MO, USA.
K. Shah, University of Rochester School of Medicine, Rochester, NY, USA
T. Hilton, Ithaca College, Rochester, NY, USA
D. R. Sinacore, Washington University School of Medicine, St. Louis, MO, USA
C. Qualls, University of New Mexico School of Medicine, Albuquerque, NM, USA
D. T. Villareal, New Mexico VA Health Care System, Albuquerque, NM, USA, Dennis.Villareal@va.gov University of New Mexico School of Medicine, Albuquerque, NM, USA; Washington University School of Medicine, St. Louis, MO, USA.
Reference
- 1.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshikawa Y, Iida T, Muramatsu M, Ii N, Nakajima Y, Chumank K, et al. Thigh muscularity and strength in teenage soccer players. Int J Sports Med. 2013;34(5):415–423. doi: 10.1055/s-0032-1323780. [DOI] [PubMed] [Google Scholar]
- 5.Villareal DT, Holloszy JO. DHEA enhances effects of weight training onmusclemass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 6.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napoli N, Villareal DT, Mumm S, Halstead L, Sheikh S, Cagaanan M, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20:232–239. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 9.Youm T, Koval KJ, Kummer FJ, Zuckerman JD. Do all hip fractures result from a fall? Am J Orthop (Belle Mead NJ) 1999;28:190–194. [PubMed] [Google Scholar]
- 10.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 11.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 12.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 14.Marsh AP, Rejeski WJ, Espeland MA, Miller ME, Church TS, Fielding RA, et al. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P) J Gerontol A Biol Sci Med Sci. 2011;66:1376–1383. doi: 10.1093/gerona/glr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 16.Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J. Effect of exercise on extraskeletal risk factors for hip fractures in elderly women with low BMD: a population-based randomized controlled trial. J Bone Miner Res. 2006;21:772–779. doi: 10.1359/jbmr.060116. [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 18.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 19.Ryan AS, Nicklas BJ, Dennis KE. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol. 1998;84:1305–1310. doi: 10.1152/jappl.1998.84.4.1305. [DOI] [PubMed] [Google Scholar]
- 20.Salamone LM, Cauley JA, Black DM, Simkin-Silverman L, Lang W, Gregg E, et al. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. AmJ Clin Nutr. 1999;70:97–103. doi: 10.1093/ajcn/70.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Lebrasseur NK, Achenbach SJ, Melton LJ, III, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and seruminsulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27:2159–2169. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CH, Yang KC, Chang HH, Yen JF, Tsai KS, Huang KC. Sarcopenia is Related to Increased Risk for Low Bone Mineral Density. J Clin Densitom. 2013;16(1):98–103. doi: 10.1016/j.jocd.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Verschueren S, Gielen E, O'Neill TW, Pye SR, Adams JE, Ward KA, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 24.Capozza RF, Cure-Cure C, Cointry GR, Meta M, Cure P, Rittweger J, et al. Association between low lean body mass and osteoporotic fractures after menopause. Menopause. 2008;15:905–913. doi: 10.1097/gme.0b013e318164ee85. [DOI] [PubMed] [Google Scholar]
- 25.Richards RR, McKee MD, Paitich CB, Anderson GI, Bertoia JT. A comparison of the effects of skin coverage and muscle flap coverage on the early strength of union at the site of osteotomy after devascularization of a segment of canine tibia. J Bone Joint Surg Am. 1991;73:1323–1330. [PubMed] [Google Scholar]
- 26.Stein H, Perren SM, Cordey J, Kenwright J, Mosheiff R, Francis MJ. The muscle bed—a crucial factor for fracture healing: a physiological concept. Orthopedics. 2002;25:1379–1383. doi: 10.3928/0147-7447-20021201-16. [DOI] [PubMed] [Google Scholar]
- 27.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–47. doi: 10.1097/JES.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287:11616–11628. doi: 10.1074/jbc.M111.292193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–229. doi: 10.2174/1872208311206030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Suarez S, Mo C, Lara N, Jaehn K, Johnson M, Bonewald L, et al. The B-catenin activating factor, Wnt3a, stimulates skeletal myogenesis. J Bone Miner Res. 2013 in press. [Google Scholar]
- 34.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 35.Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, et al. Mechanobiology of the skeleton. Sci Signal. 2009;2:t3. doi: 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cipriani C, Romagnoli E, Carnevale V, Raso I, Scarpiello A, Angelozzi M, et al. Muscle strength and bone in healthy women: effect of age and gonadal status. Hormones (Athens ) 2012;11:325–332. doi: 10.14310/horm.2002.1361. [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 38.Jo E, Lee SR, Park BS, Kim JS. Potential mechanisms underlying the role of chronic inflammation in Age-related Muscle Wasting. Aging Clin Exp Res. 2012;5:412–422. doi: 10.3275/8464. [DOI] [PubMed] [Google Scholar]
- 39.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]