Abstract
N-hydroxylating monooxygenases (NMOs) are essential for pathogenesis in fungi and mycobacteria. NMOs catalyze the hydroxylation of lysine and ornithine in the biosynthesis of hydroxamate-containing siderophores. Inhibition of kynurenine monooxygenase (KMO), which catalyzes the conversion of l-kynurenine to 3-hydroxykynurenine, alleviates neurodegenerative disorders such as Huntington’s and Alzheimer’s diseases and brain infections caused by the parasite Trypanosoma brucei. These enzymes are examples of flavin-dependent monooxygenases, which are validated drug targets. Here, we describe the development and optimization of a fluorescence polarization assay to identify potential inhibitors of flavin monooxygenases. Fluorescently-labeled ADP molecules were synthesized and tested. An ADP-TAMRA chromophore bound to KMO with a Kd value of 0.60 ± 0.05 μM and to the NMOs from Aspergillus fumigatus and Mycobacterium smegmatis with Kd values of 2.1 ± 0.2 μM and 4.0 ± 0.2 μM, respectively. The assay was tested in competitive binding experiments with substrates and products of KMO and an NMO. Furthermore, we showed that this assay can be used to identify inhibitors of NMOs. A Z’-factor of 0.77 was calculated and we show that the assay exhibits good tolerance to temperature, incubation time, and DMSO concentration.
Keywords: Flavin-dependent monooxygenases, kynurenine monooxygenase, ornithine hydroxylase, lysine hydroxylase, siderophore, Huntington’s and Alzheimer’s diseases
INTRODUCTION
Flavin-dependent monooxygenases constitute a large family of enzymes that catalyze a variety of chemical reactions including epoxidation, hydroxylation, dehalogenation, aromatic hydrocarbon hydroxylation, and sulfur and nitrogen hydroxylation [1, 2]. The activities of these enzymes are of significant importance to human health and chemical applications. In mammals, the structurally and biochemically well-characterized flavin-monooxygenases (FMOs) are involved in the biodegradation of xenobiotics in the liver [3, 4]. The less characterized N-hydroxylating monooxygenases (NMOs) are involved in the biosynthesis of siderophores in fungi and bacteria. NMO catalyzes the NAD(P)H and oxygen-dependent hydroxylation of the side chain of lysine and ornithine to form the modified N6- or N5-hydroxy amino acids, respectively (Scheme 1) [5, 6]. Siderophores are essential for iron acquisition during infection by many human pathogens such as Aspergillus fumigatus and Mycobacterium tuberculosis [7, 8]. The ornithine hydroxylase from A. fumigatus, known as Af SidA, is essential for virulence [9, 10]. Mycobacterium tuberculosis contains an N6-lysine hydroxylase, known as MbtG, which has also been proposed as a potential drug target [7]. Recently, it was shown that inhibiting kynurenine monooxygenase (KMO) activity improves the pathology of Huntington’s and Alzheimer’s diseases and African trypanosomiasis [11-13]. KMO is also a flavin-dependent monooxygenase that catalyses the conversion of l-kynurenine to 3-hydroxykynurenine (Scheme 1) [13, 14].
Scheme 1.
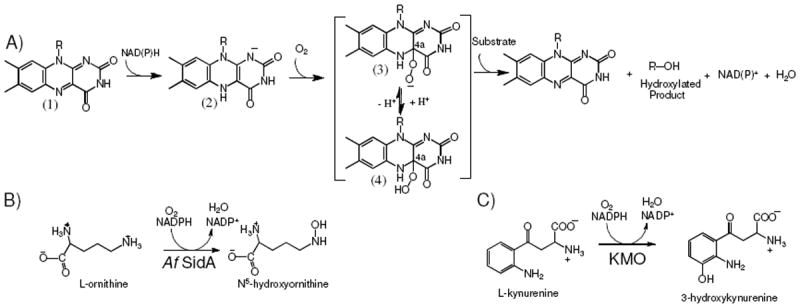
Flavin-dependent monooxygenases. (A) Reaction of oxidized flavin (1) with NAD(P)H to form the reduced flavin (2). Upon reaction with molecular oxygen, the C4a-peroxyflavin (3) is formed, which is stabilized by Baeyer-Villiger monooxygenases. Protonation of the C4a-peroxyflavin results in the formation of C4a-hydroperoxyflavin (4), which is stabilized by hydroxylases. (B) Reaction catalyzed by Af SidA. (C) Reaction catalyzed by KMO.
The important roles of these flavin monooxygenases in microbial pathogen growth, neurodegenerative diseases, and parasitic infections show that they are important drug targets. Here, we present the development and application of a fluorescence polarization binding assay to identify small molecule inhibitors of flavin monooxygenases. Since in all of these enzymes NADPH is a common substrate, we designed an ADP-based fluorescently-labeled ligand, which has affinity to several monooxygenases. It was shown that substrates and products displace the ADP-chromophore, indicating that the chromophore binds at the active site of both Af SidA and KMO. A screen of a small molecular library was performed and an inhibitor of Af SidA was identified. Furthermore, we show that this assay has a Z’ factor of 0.77 ± 0.01 and displays good temperature and dimethyl sulfoxide (DMSO) tolerance. More importantly, we show that this assay can be generally applied to other flavin monooxygenases, such as FMO and mycobacterium N-hydroxylating enzymes.
MATERIALS AND METHODS
Materials
All reagents were obtained from commercial sources and were used without further purification. All solvents were either reagent grade or high performance liquid chromatography (HPLC) grade. Anhydrous reactions were performed under argon. NMR spectral data were obtained using a Varian Inova spectrometer at 400 MHz. Chemical shifts were reported as δ-values relative to known solvent residue peaks. High-resolution mass spectra (HRMS) were obtained from the Mass Spec Incubator in the Department of Biochemistry at Virginia Tech. HPLC (Shimadzu LC 20A Prominence) was performed on a C18 reverse phase column (Phenomenex Luna C18 column, 250 × 21.20 mm, 5 micron, and Phenomenex Luna C18 (2) column, 250 × 4.6 mm, 5 micron) using water and acetonitrile as the elution solvents. All compounds were more than 95% pure as judged by HPLC and 1H NMR.
Protein expression and purification
Expression and purification of Af SidA was performed as previously described [5, 15]. Expression and purification of Mycobacterium smegmatis N6-lysine hydroxylase (MbsG) (~75% identical to the Mycobacterium tuberculosis enzyme, MbtG) was performed as previously described [5, 15]. The synthetic flavin monooxygenase gene from Methylophaga sp. strain SK1 was obtained from Genscript (Piscataway, NJ) and cloned into the pET55A vector, expressed, and purified following the procedures of Oppenheimer et al [16]. Kynurenine 3-monooxygenase from Pseudomonas fluorescens was a generous gift from Dr. Graham Moran, University of Wisconsin, Milwaukee [13].
Synthesis of ADP chromophores
AMP triethylammonium salt 1
Dowex 50WX8-200 (H+) resin (5 g) in Et3N (7 mL) and H2O (43 mL) was stirred at room temperature (rt) for 5 h. After filtration, the resin was washed with H2O and dried in vacuo to give Dowex 50WX8-200 (Et3NH+) resin. This resin (2 g) was added to a solution of adenosine monophosphate (AMP) (673 mg, 1.72 mmol) in H2O (10 mL), and the suspension was stirred at rt overnight before filtration and concentration to give triethylammonium salt 1 (800 mg, 99%). 1H NMR (400 MHz, D2O) δ 8.53 (s, 1H), 8.23 (s, 1H), 6.11 (d, J = 6.1 Hz, 1H), 4.77 – 4.74 (m, 1H), 4.48 (dd, J = 5.1, 3.4 Hz, 1H), 4.38-4.34 (m, 1H), 4.04 (dd, J = 4.7, 3.0 Hz, 2H), 3.18 (q, J = 7.3 Hz, 6H), 1.25 (t, J = 7.3 Hz, 9H) (Figure S1).
ADP-linker conjugate 3
Dimethylpyridine (114 μL, 0.9 mmol), Et3N (63 μL, 0.45 mmol) and trifluoroacetic anhydride (1mL, 1.4 M in acetonitrile) was added dropwise at 0 °C to a suspension of AMP triethylammonium salt 1 (100 mg, 0.22 mmol) in acetonitrile (3 mL). The resulting red brown solution was stirred for 15 min before being concentrated in vacuo and redissolved in acetonitrile (3 mL). After successive addition of molecular sieves (4 Å, 100 mg), Et3N (153 μL, 1.1 mmol), and methylimidazole (96 μL, 1.2 mmol) at 0 °C, a solution of phosphate 2 (70 mg, 0.18 mmol) in acetonitrile (1 mL) was added dropwise to the suspension at 0 °C, and the suspension was stirred at 0 °C for 1 h and at rt for 3 h. The suspension was then filtered and washed with H2O. The filtrate, was concentrated and purified by silica gel flash chromatography (CHCl3: MeOH: 1M NH4OAc = 5:4:1) to give ADP conjugate 3 (70 mg, 0.11 mmol, 50%). 1H NMR (400 MHz, D2O) δ 8.49 (s, 1H), 8.18 (s, 1H), 6.09 (d, J = 5.6 Hz, 1H), 4.73 (t, J = 5.4 Hz, 1H), 4.50 (t, J = 4.2 Hz, 1H), 4.36 (s, 1H), 4.20 (s, 2H), 3.80 (d, J = 6.3 Hz, 2H), 3.11 (t, J = 7.1 Hz, 2H), 1.45 – 1.37 (m, 2H), 1.36 – 1.27 (m, 2H), 1.14 – 1.02 (m, 4H). HRMS (MALDI-TOF): calcd. for C18H26F3N6O11P2 (M-H)-: 621.1087, found 621.1071 (Figure S2).
ADP conjugated amine 4
ADP-linker conjugate 3 (35 mg, 0.056 mmol) was dissolved in 3 M NH4OH (5 mL) and the resulting solution was stirred at rt for 2 h. After being concentrated in vacuo, the residue was re-dissolved in H2O and lyophilized to give amine 4 as a white powder (30 mg, 0.055 mmol, 98%). 1H NMR (400 MHz, D2O) δ 8.50 (s, 1H), 8.23 (s, 1H), 6.11 (d, J = 6.2 Hz, 1H), 4.72 – 4.69 (m, 1H), 4.54 – 4.49 (m, 1H), 4.39 – 4.34 (m, 1H), 4.21 – 4.17 (m, 2H), 3.84 – 3.78 (m, 2H), 2.89 (t, J = 7.5 Hz, 2H), 1.58 – 1.37 (m, 4H), 1.22-1.66 (m, 4H). HRMS (MALDI-TOF): calcd. for C16H27N6O10P2 (M-H)-: 525.1264, found 525.1225 (Figure S3).
Chromophore 1
A solution of rhodamine SE (1 mg, 2.0 μmol) in DMF (50 μL) was added to a solution of amine 4 (1.5 mg, 2.7 μmol) in NaHCO3 buffer (50 μL, 0.1 M, pH 8.3) at rt. After stirring at rt for 2 h, the solution was diluted with H2O (1.9 mL) and acetonitrile (0.1 mL), injected onto a reverse-phase HPLC column (Phenomenex Luna C18 (2) column, 250 × 4.6 mm, 5 micron) and purified at a flow rate of 1.0 mL/min with linear gradient elution (5% to 65% acetonitrile in H2O over 55 min, then 65% to 95% over 5 min) to afford chromophore 1 (1.2 mg, 69%). HRMS (MALDI-TOF): calcd. for C37H39N8O14P2 (M-H)-: 881.2061, found 881.1163 (Figure S4).
Chromophore 2
A solution of 5(6)-rhodamine-X SE (1 mg, 1.6 μmol) in DMF (50 μL) was added to a solution of amine 4 (1.0 mg, 1.8 μmol) in NaHCO3 buffer (50 μL, 0.1 M, pH 8.3) at rt. After stirring at rt for 2 h, the solution was diluted with H2O (1.9 mL) and acetonitrile (0.1 mL), injected onto a reverse-phase HPLC column (Phenomenex Luna C18 (2) column, 250 × 4.6 mm, 5 micron), and purified at a flow rate of 1.0 mL/min with linear gradient elution (5% to 65% acetonitrile in H2O over 55 min, then 65% to 95% over 5 min) to afford chromophore 2 (1.0 mg, 62%). HRMS (MALDI-TOF): calcd. for C43H50N9O15P2 (M-H)-: 994.2902, found 994.1824 (Figure S5).
Chromophore 3 and Chromophore 4
A solution of 5(6)-TAMRA-X SE (1 mg, 1.6 μmol) in DMF (50 μL) was added to a solution of amine 4 (1.0 mg, 1.8 μmol) in NaHCO3 buffer (50 μL, 0.1 M, pH 8.3) at rt. After stirring at rt for 5 h, the solution was diluted with H2O (1.9 mL) and acetonitrile (0.1 mL), injected onto a reverse-phase HPLC column (Phenomenex Luna C18 column, 250 × 21.50 mm, 5 micron), and purified at a flow rate of 5.0 mL/min with linear gradient elution (5% to 30% acetonitrile in H2O over 30 min, then 30% to 95% over 10 min) to afford 6-TAMRA chromophore 3 (0.5 mg, 30%) and 5-TAMRA chromophore 4 (0.6 mg, 37%).
Chromophore 3
1H NMR (400 MHz, D2O) δ 8.38 (s, 1H), 8.02 (dd, J = 8.1, 1.7 Hz, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.86 (s, 1H), 7.82 (d, J = 1.8 Hz, 1H), 7.15 (d, J = 9.6 Hz, 1H), 7.10 (d, J = 9.6 Hz, 1H), 6.89 (dd, J = 9.5, 2.3 Hz, 1H), 6.85 – 6.80 (m, 1H), 6.69 – 6.64 (m, 2H), 5.77 (d, J = 4.6 Hz, 1H), 4.45 (dt, J = 15.7, 4.9 Hz, 2H), 4.25 – 4.20 (m, 1H), 4.19 – 4.14 (m, 2H), 3.76 – 3.67 (m, 2H), 3.46 (t, J = 6.2 Hz, 2H), 3.25 (s, 6H), 3.23 (s, 6H), 2.81 (td, J = 6.3, 2.6 Hz, 2H), 2.15 (t, J = 7.0 Hz, 2H), 1.70 – 1.61 (m, 2H), 1.61 – 1.52 (m, 2H), 1.40 – 1.30 (m, 2H), 1.30 – 1.20 (m, 2H), 1.07 – 0.94 (m, 2H), 0.90 – 0.78 (m, 4H). HRMS (MALDI-TOF): calcd. for C47H58N9O15P2 (M-H)-: 1050.3528, found 1050.3514 (Figure S6).
Chromophore 4
1H NMR (400 MHz, D2O) δ 8.23 – 8.20 (m, 2H), 8.10 (dd, J = 8.1, 1.8 Hz, 1H), 8.02 (s, 1H), 7.45 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 9.4 Hz, 1H), 7.01 (d, J = 9.4 Hz, 1H), 6.91 (dd, J = 9.7, 2.5 Hz, 1H), 6.81 – 6.74 (m, 2H), 6.61 (d, J = 2.5 Hz, 1H), 5.74 (d, J = 4.9 Hz, 1H), 4.38 – 4.35 (m, 2H), 4.15 – 3.95 (m, 3H), 3.86 – 3.82 (m, 2H), 3.50 (t, J = 6.6 Hz, 2H), 3.26 (s, 6H), 3.20 (s, 6H), 3.07 (t, J = 7.0 Hz, 2H), 2.27 (t, J=6.8 Hz, 2H), 1.74 – 1.64 (m, 4H), 1.49 – 1.37 (m, 4H), 1.36 – 1.27 (m, 2H), 1.16 – 1.07 (m, 4H). HRMS (MALDI-TOF): calcd. for C47H58N9O15P2 (M-H)-: 1050.3528, found 1050.3481 (Figure S7).
Fluorescence polarization binding assay
Solutions containing serially diluted Af SidA, KMO, FMO, or MbsG and 30 nM of chromophore 4 in 0.05 M sodium phosphate buffer (pH 7.0) were incubated at rt for 5 minutes. Each experiment was done in triplicate in a 96-well black half-area flat-bottom plate (Corning, Corning, NY) at final volumes of 25 μL. The anisotropy values were measured (excitation at 544 nm and emission at 584 nm) on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). The Kd values were obtained by fitting the anisotropy data to Eq. 1, where m1 and m2 are the minimum and maximum anisotropy values, respectively, m3 is the Kd value, and Ct represents the total concentration of ADP chromophore.
| (1) |
Fluorescence polarization displacement assay
Solutions (25 μL) containing 2 μM of Af SidA or 0.6 μM KMO and 30 nM of chromophore 4 in 0.05 M sodium phosphate buffer (pH 7.0) were mixed with various concentrations of tested ligands (NADPH, NADP+, NAD+, ADP, lysine, ornithine, and kynurenine) and incubated at rt for 5 minutes. When NADPH was the ligand, the sample was tested immediately after mixing to minimize oxidation. Each assay was performed in triplicate. Anisotropy values were measured and the Kd values were obtained by fitting the data to Eq. 2, where m1 and m2 are the minimum and maximum anisotropy, respectively, m3 is the slope, and m4 is the Kd.
| (2) |
Fluorescence polarization displacement assay in high throughput screening
We screened 160 small molecule compounds from “The Spectrum Collection” (MicroSource Discovery Systems, Inc., Gaylordsville, CT) using the fluorescence polarization displacement assay with Af SidA. Each sample contained 25 μL of 2 μM Af SidA, 30 nM chromophore 4, and 10 μM of test compound in 0.05 M sodium phosphate buffer, pH 7, at a DMSO concentration of 2% v/v. The mixtures were incubated for 5 min and read on a plate reader. The anisotropy values were normalized to a negative control (2 μM Af SidA and 30 nM chromophore 4 in 0.05 M sodium phosphate, pH 7.5) at 100% signal. A background control containing 30 nM and chromophore 4 without enzyme was also included. A positive control containing 2 μM Af SidA, 30 nM chromophore 4, and 50 μM NADP+, was present in every plate. One positive hit (sanguinarine sulfate) was identified, its affinity for Af SidA was determined, and the inhibitory effect evaluated in the ornithine hydroxylation assay. A negative hit, berberine, that has similar chemical structure to sanguinarine was selected to see if the binding was specific. The addition of up to 1 mM of this compound did not produce a decrease in anisotropy similar to sanguinarine (Figure 9S). Similarly, the negative hit, naproxen, did not bind to Af SidA (Figure 9S).
Activity assay and inhibition of Af SidA
The enzymatic activity of Af SidA was monitored by measuring the amount of N5-hydroxyornithine formed as previously described [15]. To determine the IC50 value for sanguinarine sulfate, the activity of Af SidA (2 μM) in 100 mM potassium phosphate, pH 7.5, in the presence of 200 μM NADPH and 1 mM ornithine was determined at various concentrations of the inhibitor (100 μM, 300 μM, 500 μM, 750 μM, and 1000 μM) (Figure 5B).
Figure 5.
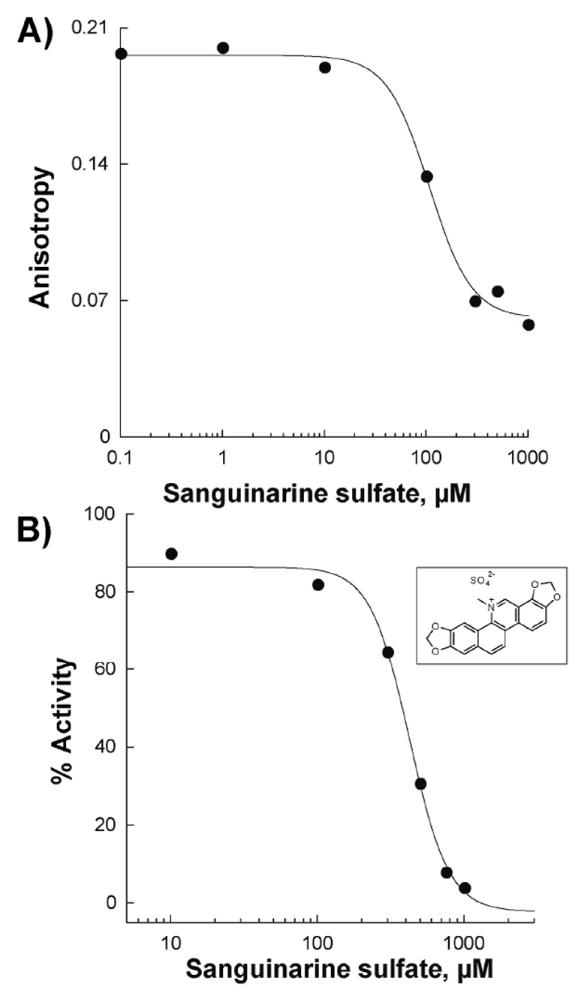
Determination of the Kd and IC50 values of sanguinarine to Af SidA. A) Anisotropy changes as a function of sanguinarine concentration were used to determin a Kd value of 110 ± 13 μM. B) Inhibition of the ornithine hydroxylation activity of Af SidA as a function of sanguinarine concentration. An IC50 value of 500 ± 90 μM was calculated.
Z′ factor vs. time
A 25 μL solution, containing 1 μM Af SidA and 30 nM chromophore 4 in 0.05 M sodium phosphate buffer (pH 7.0) was incubated in the presence of 30 μM ADP (positive control) or in the absence of ADP (negative control), and dispensed into 20 wells on a 96-well plate. The anisotropy value of each well was measured at the following time points: 10, 30, 60, 90, 120, 180, 240, 1080, and 1440 min. This experiment was repeated three times on three separate days. The Z′ factor was calculated using Eq. 3, where μ+ and σ+ represent the mean and standard error of the positive control, respectively, and μ− and σ− represent the mean and standard error of the negative control, respectively.
| (3) |
Z′ factor vs. temperature
A solution containing 1 μM Af SidA and 30 nM chromophore 4 in 0.05 M sodium phosphate buffer (pH 7.0) in the presence of 30 μM ADP (positive control) or in the absence of ADP (negative control) was dispensed into 20 wells on a 96-well black plate with a final volume of 25 μL. The plate was incubated at various temperatures (15, 20, 25, 30, and 35 °C) for 10 min. This experiment was repeated three times on three separate days. The Z′ factor was calculated using Eq. 3.
DMSO tolerance
A solution containing 1 μM Af SidA, 30 nM chromophore 4, and DMSO at various concentrations (1, 2, 3, 4, 5, 7, 9, 11, 15, and 20% v/v) in 0.05 M sodium phosphate buffer (pH 7.0) in the presence of 30 μM ADP (positive control) or in the absence of ADP (negative control) was dispensed into 20 wells on a 96-well black plate with a final volume of 25 μL. The plate was incubated at rt for 5 min. This experiment was repeated three times on three separate days. The Z′ factor was calculated using Eq. 3.
RESULTS
Chromophore design and synthesis
We used ADP as the backbone to synthesize a chromophore to target the NADPH binding site of flavin-monooxygenases. A linker containing a free amine was added such that the resulting compound 4 could be easily reacted with various amine reactive fluorophores (Scheme 2). Four chromophores, ADP-rhodamine (chromophore 1), ADP-rhodamine with an additional 5-carbon linker (chromophore 2), and ADP-TAMRA linked to the 6 position (chromophore 3) and 5 position (chromophore 4) were synthesized and isolated in 30-60% yield. The identity and purity of the compounds were determined by NMR and high resolution MS.
Scheme 2.
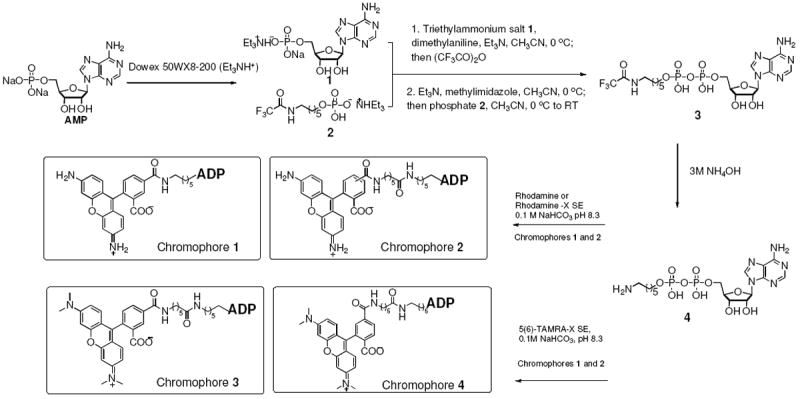
Synthesis of the fluorescently labeled ADP chromophores.
Chromophore binding to flavin-monooxygenases
Binding of each ADP-chromophore was tested with purified recombinant flavin-monooxygenases (Table 1). Chromophore 1 had weak affinity for Af SidA, FMO, and KMO. In contrast, chromophore 2 had low micromolar affinity for Af SidA, while the affinity for FMO and KMO was unaffected. To find a “universal” flavin-monooxygenase fluorescent ligand, we synthesized and tested ADP-TAMRA ligands (chromophores 3 and 4). TAMRA is a derivative of rhodamine that contains a positively charged dimethylamino group. It is photostable and can be excited at 544 nm with the emission measured at 584 nm. Longer excitation and emission wavelengths decrease the interference signals from the flavin cofactor and other molecules [17]. Binding of chromophore 3 was detected for all flavin-monooxygenases tested in this work. Chromophore 4 binds with higher affinity for all tested enzymes (Table 1 and Figure 1), thus, we chose this fluorescent ligand for further characterization. Interestingly, the binding affinity for MbsG remained relatively constant independent of what ligand was used (Table 1).
Table 1.
Affinity of ADP-chromophores to various flavin-dependent monooxygenases.
| Chromophore | Kd, μM
|
|||
|---|---|---|---|---|
| Af SidA | MbsG | FMO | KMO | |
| 1 | >10 | 1.8 ± 0.2 | >10 | >10 |
| 2 | 0.4 ± 0.1 | 2.0 ± 0.2 | >10 | >10 |
| 3 | 9.0 ± 5 | 5.7 ± 0.7 | 13 ± 2 | 10 ± 2 |
| 4 | 2.1 ± 0.2 | 4 ± 0.2 | 6.0 ±1 | 0.60 ± 0.05 |
Figure 1.
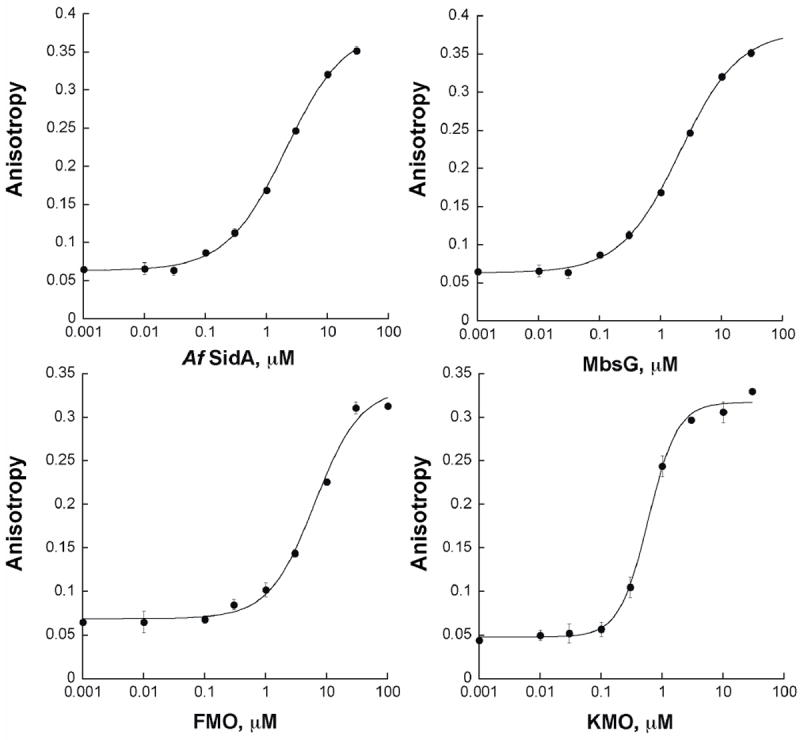
Binding curves of chromophore 4 (30 nM in 0.05 M sodium phosphate, pH 7.0) to various flavin-dependent monooxygenases.
Competitive displacement experiments
We further evaluated the binding of chromophore 4 to Af SidA and KMO. To determine if chromophore 4 indeed was binding at the active site of these enzymes, competition experiments with substrates and products were performed. Figure 2 shows a representative sample of the competition curves for NADP+, NAD+, ornithine, and lysine for Af SidA. The Kd values calculated from these experiments are summarized in Table 2. Competition by ADP was also tested and the binding affinity calculated (Table 2).
Figure 2.
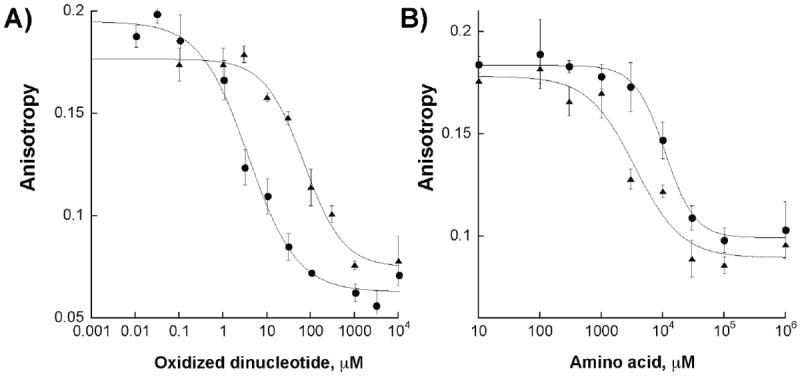
A) Competitive binding of NAD+ (triangles) and NADP+ (circles) to Af SidA. B) Competitive binding of ornithine (triangles) and lysine (circles) to Af SidA.
Table 2.
Kd values of substrates or inhibitor of Af SidA.
| Ligand | Af SidA | KMO |
|---|---|---|
| NADPH | 3.3 ± 0.1 μM | 152 ± 80 μM |
| NADP+ | 4± 1 μM | > 10 mM |
| NAD+ | 70 ± 20 μM | 670 ± 70 μM |
| ADP | 11 ± 1 μM | 50 ± 10 μM |
| Lysine | 11 ± 1 mM | n.d. |
| Ornithine | 4 ± 1 mM | n.d. |
| Kynurenine | n.d. | 180 ± 40 μM |
| Sanguinarine | 110 ± 10 μM | n.d. |
Determination of Z’ factor and fluorescence polarization assay optimization
The Z’ factor is a statistical parameter that estimates the reliability and robustness of the assay [18]. A value between 0.5-1 corresponds to a good assay response. We calculated the Z’ factor with chromophore 4 to be 0.77 ± 0.01 at 25 °C. The assay is resistant to temperature changes between 15-35 °C and to DMSO concentrations between 0-5% (Figure 3). In addition, we tested the stability and accuracy of the assay in the presence of 2% DMSO, which is the concentration used in the initial screening conditions. The results show that the fluorescence anisotropy values in all wells varies less than 5% and is stable for more than 4 hours after plating (Figure S8).
Figure 3.
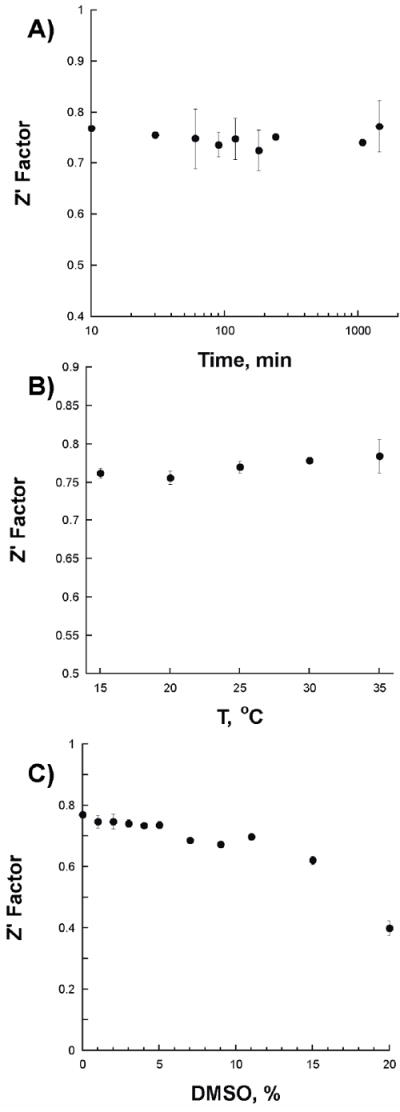
Changes in the Z’ factor value as a function of time (A), temperature (B), and dimethyl sulfoxide (DMSO) concentration (C).
Identification of inhibitors of Af SidA
We tested whether binding of small molecules to Af SidA could be identified using the assay developed here. We screened160 random compounds from “The Spectrum Collection” (MicroSource Discovery Systems, Inc., Gaylordsville, CT). This library is comprised of various known drugs, experimental bioactives, and pure natural products. Using 2 μM Af SidA and a fixed concentration of the compounds (10 μM), it was clear that several compounds did not decrease the anisotropy values, indicating that they did not compete for the binding site of the chromophore. One compound (sanguinarine sulfate) produced a decrease in the anisotropy value, but not as low as the positive control (containing 50 μM NADP+) (Figure 4). We determined a Kd value of 110 ± 13 μM for sanguinarine sulfate (ID number 00310035 from MicroSource Discovery Systems, Inc., Gaylordsville, CT). To determine if this compound was able to inhibit the activity of Af SidA, the IC50 value was determined by measuring the amount of hydroxylated ornithine produced in the ornithine hydroxylation assay. Sanguinarine sulfate was found to be a weak inhibitor of Af SidA with an IC50 value of 500 ± 90 μM (Figure 5).
Figure 4.
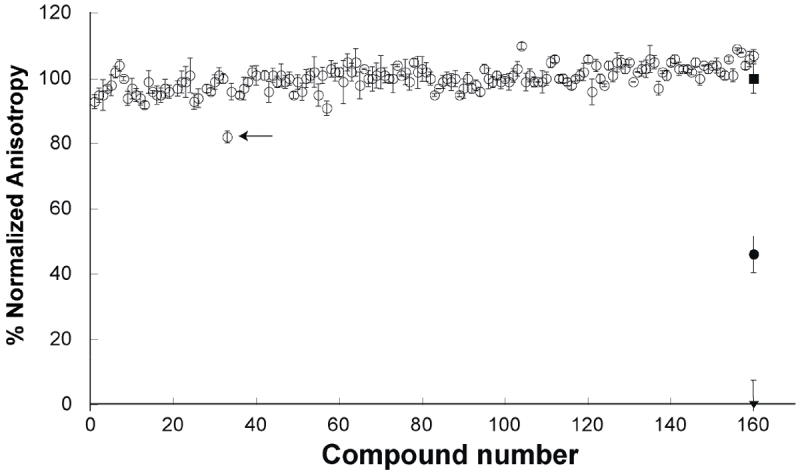
Identification of an Af SidA inhibitor. Binding of potential inhibitors to the active site of Af SidA was monitored by measuring the fluorescence anisotropy decrease as consequence of the displacement of chromophore 4. Open circles show the anisotropy value of the compounds tested. In the assay, we also included a negative control, which contains only Af SidA-chromophore complex (solid square), a sample with 50 μM NADP+ as the positive control (solid circle), and chromophore alone as the background (inverse solid triangle). The arrow points at the anisotropy value in well 32, which contains the compound sanguinarine. This molecule appears to bind Af SidA, as evident by a slight decrease in the anisotropy value. Assay conditions are described in the Materials and Methods section.
DISCUSSION
Flavin-dependent monooxygenases catalyze a number of chemical reactions. This chemical versatility originates from different active sites that accommodate a variety of substrates and from the modulation of the chemical nature of the oxygenated flavin intermediate [1, 19]. In all flavin-monooxygenases, the flavin cofactor must be reduced to react with molecular oxygen and form the C4a-flavin adduct (Scheme 1). If the protein catalyzes the nucleophilic addition of molecular oxygen, such as in the case of Baeyer-Villiger monooxygenases, the C4a-peroxyflavin is stabilized. If hydroxylation is the desired chemical outcome, the C4a-hydroperoxyflavin is stabilized. The hydroxylation products of several monooxygenases have been shown to be important for virulence in many human pathogens. For example, to proliferate and establish infection under iron-limiting conditions, such as those found in mammals, microbial pathogens must obtain iron from the host [1]. One mechanism of iron acquisition is via the use of iron chelators known as siderophores [20]. It has been shown that hydroxamate-containing siderophores in Mycobacterium tuberculosis are essential for virulence. In A. fumigatus, the deletion of the Af SidA gene results in a mutant strain that does not synthesize the hydroxamate-containing siderophore ferricrocin and is unable to establish infection in mice, which links the activity of a single protein to the virulence of this pathogenic fungus [9]. Flavin-monooxygenases have also been identified as potential drug targets against parasitic and neurodegenerative diseases. It has recently been shown that targeted inhibition of kynurenine monooxygenase results in an increase in kyneuric acid, a neuroprotective compound in the brain. High levels of kyneuric acid alleviate neurodegeneration in mouse models of Alzheimer’s and Huntington’s diseases [11]. Similarly, targeted inhibition of KMO has been shown to diminish the pathology of African trypanosomiasis, or sleeping sickness [12]. Thus, identification of inhibitors of flavin-monooxygenases can result in lead compounds that can be developed into potential drugs for treating tuberculosis, aspergillosis, sleeping sickness, and Alzheimer’s and Huntington’s diseases.
The need for inhibitors of these enzymes and the potential health impact prompted us to develop an assay that can be used to perform high-throughput screening of chemical libraries. This assay must identify small molecule compounds that bind to the active site of these proteins, be sensitive, and use small amounts of protein and other materials to reduce cost. Since the only common substrate in all of these enzymes is NAD(P)H, we used this molecule as the building block for the design of a fluorescent ligand. In the reaction of all of the enzymes tested here, the nicotinamide ring of NAD(P)H must bind close to the flavin in order to transfer a hydride from the C4-position of NAD(P)H to the flavin N5-position [21]. We reasoned that it could be possible to use the ADP portion of this substrate to function as a carrier molecule that will recognize the NAD(P)H binding site. To increase the affinity of the ligand, we selected a three aromatic ring chromophore that might interact with the flavin via aromatic stacking interactions. A linker was added to ADP to enhance flexibility and to allow the chromophore to reach and interact with the flavin. In our initial experiments, we used the chromophore rhodamine, which resulted in binding to only MbsG (Table 1). Addition of an extra 5-carbon linker (Compound 2) resulted in similar binding to MbsG and a significant increase in binding to Af SidA; however, very low affinity for FMO and KMO was observed. Therefore, we added the chromophore TAMRA, which we showed has high affinity to other flavin proteins [17]. In addition, low background fluorescence from the bound flavin and other aromatic compounds is obtained with TAMRA because it absorbs at 545 nm and emits at 584 nm [17]. Commercially available TAMRA is sold as a mixture of the aminohexanoyl spacer bound at the 5 and 6 position of benzoic acid. We isolated each isomer and determined that the ADP-5-TAMRA (chromophore 4) bound with high affinity to all of the enzymes (Table 1). Further characterization of the binding of chromophore 4 was performed with Af SidA and KMO. Since ADP was used as the building block of the chromophore, addition of NADP+, NAD+, and ADP should displace the chromophore from its binding site, resulting in a decrease in anisotropy. A decrease in anisotropy was observed when the concentrations of these ligands were increased, permitting the calculation of the Kd values (Table 2 and Figure 2). The Kd values for NADPH, NADP+, and NAD+ of Af SidA are consistent with values previously determined using other methods [5, 22]. KMO has very low affinity to NADP+ and no reports of NAD+ binding have been published; however, our Kd value for NADPH is consistent with the value reported in the literature (Table 2) [14]. These results suggest that chromophore 4 is binding in the NADPH binding site, as expected. We also tested whether chromophore 4 could be displaced from the active site of Af SidA and KMO by molecules that do not resemble NADPH. With Af SidA, we determined the binding of ornithine and the substrate analog, lysine [5]. Kynureine was tested with KMO. Binding was determined by measuring the change in anisotropy as a function of increasing ligand concentrations (Table 2 and Figure 2). Clearly, chromophore 4 can be displaced by both NADPH-like compounds and other small molecules that bind to the active site of these enzymes. The assay was further characterized and it was with shown to have a Z’ value of 0.77 ± 0.01 and to display good temperature and DMSO tolerance, indicating that the assay is suitable for high-throughput screening. The ability of this assay to report on the binding of potential inhibitors of Af SidA was further tested by screening 160 low molecular weight compounds (Figure 4) [17]. The screen clearly shows that 159 compounds did not bind to Af SidA, since the anisotropy values were similar to the negative control. However, one compound decreased the anisotropy value by 20%, suggesting that this compound binds to the active site of Af SidA, albeit with low affinity. A Kd value of 110 ± 13 μM was calculated for this compound, which was identified as sanguinarine. We then determined if this compound could inhibit the activity of Af SidA in the presence of NADPH and ornithine. An IC50 value of 500 ± 90 μM was calculated and 100 % inhibition was observed (Figure 4). These data clearly show that small molecule compounds that inhibit Af SidA can be identified using this assay, even those with low affinity. Sanguinarine has been previously described as an inhibitor of cancer cell proliferation by a depolymerizing effect on cellular microtubules [23]. This compound is also reported as a potent inhibitor of mitogen-activated protein kinase phosphatase-1 (MKP-1) [24]. Thus, it appears that it has some affinity to ATP binding enzymes. The identification of compound as ligand of Af SidA is consistent with the fact that this enzyme also binds nucleotides. We also show that this assay can be generally used for the identification of other flavin-dependent monooxygenases since chromophore 4 can effectively bind to MbsG and KMO. Thus, inhibitors that can be used to treat diseases such as tuberculosis, Alzheimer’s, and Huntington’s can be obtained using the assay presented here.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the NIH: RO1 AI082542 (R. Tarleton, PI), and by a grant from the National Science Foundation: MCB-1021384 (P. Sobrado, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.van Berkel WJ, Kamerbeek NM, Fraaije MW. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J Biotechnology. 2006;124:670–689. doi: 10.1016/j.jbiotec.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Palfey BA, McDonald CA. Control of catalysis in flavin-dependent monooxygenases. Arch Biochem Biophy. 2010;493:26–36. doi: 10.1016/j.abb.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Poulsen LL, Ziegler DM. The liver microsomal FAD-containing monooxygenase. Spectral characterization and kinetic studies. J Biol Chem. 1979;254:6449–6455. [PubMed] [Google Scholar]
- 4.Ziegler DM. An overview of the mechanism, substrate specificities, and structure of FMOs. Drug Metab Rev. 2002;34:503–511. doi: 10.1081/dmr-120005650. [DOI] [PubMed] [Google Scholar]
- 5.Chocklett SW, Sobrado P. Aspergillus fumigatus SidA is a highly specific ornithine hydroxylase with bound flavin cofactor. Biochemistry. 2010;49:6777–6783. doi: 10.1021/bi100291n. [DOI] [PubMed] [Google Scholar]
- 6.Meneely KM, Lamb AL. Biochemical characterization of a flavin adenine dinucleotide-dependent monooxygenase, ornithine hydroxylase from Pseudomonas aeruginosa, suggests a novel reaction mechanism. Biochemistry. 2007;46:11930–11937. doi: 10.1021/bi700932q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Micro. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 8.Eisendle M, Oberegger H, Zadra I, Haas H. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC) Mol Micro. 2003;49:359–375. doi: 10.1046/j.1365-2958.2003.03586.x. [DOI] [PubMed] [Google Scholar]
- 9.Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infec Immun. 2005;73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krithika R, Marathe U, Saxena P, Ansari MZ, Mohanty D, Gokhale RS. A genetic locus required for iron acquisition in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:2069–2074. doi: 10.1073/pnas.0507924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodgers J, Stone TW, Barrett MP, Bradley B, Kennedy PG. Kynurenine pathway inhibition reduces central nervous system inflammation in a model of human African trypanosomiasis. Brain. 2009;132:1259–1267. doi: 10.1093/brain/awp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crozier KR, Moran GR. Heterologous expression and purification of kynurenine-3-monooxygenase from Pseudomonas fluorescens strain 17400. Protein Expr Purif. 2007;51:324–333. doi: 10.1016/j.pep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Crozier-Reabe KR, Phillips RS, Moran GR. Kynurenine 3-monooxygenase from Pseudomonas fluorescens: substrate-like inhibitors both stimulate flavin reduction and stabilize the flavin-peroxo intermediate yet result in the production of hydrogen peroxide. Biochemistry. 2008;47:12420–12433. doi: 10.1021/bi8010434. [DOI] [PubMed] [Google Scholar]
- 15.Robinson R, Sobrado P. Substrate binding modulates the activity of Mycobacterium smegmatis G (MbsG), a flavin-dependent monooxygenase involved in the biosynthesis of hydroxamate-containing siderophores. Biochemistry. 2011;50:8489–8496. doi: 10.1021/bi200933h. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheimer M, Pierce BS, Crawford JA, Ray K, Helm RF, Sobrado P. Recombinant expression, purification, and characterization of ThmD, the oxidoreductase component of tetrahydrofuran monooxygenase. Arch Biochem Biophy. 2010;496:123–131. doi: 10.1016/j.abb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Qi J, Oppenheimer M, Sobrado P. Fluorescence polarization binding assay for Aspergillus fumigatus virulence factor UDP-galactopyranose mutase. Enz Res. 2011;2011:11. doi: 10.4061/2011/513905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 19.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269:22459–22462. [PubMed] [Google Scholar]
- 20.Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 21.Joosten V, van Berkel WJ. Flavoenzymes. Curr Opin Chem Biol. 2007;11:195–202. doi: 10.1016/j.cbpa.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Mayfield JA, Frederick RE, Streit BR, Wencewicz TA, Ballou DP, DuBois JL. Comprehensive spectroscopic, steady state, and transient kinetic studies of a representative siderophore-associated flavin monooxygenase. J Biol Chem. 2010;285:30375–30388. doi: 10.1074/jbc.M110.157578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopus M, Panda D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J. 2006;273:2139–2150. doi: 10.1111/j.1742-4658.2006.05227.x. [DOI] [PubMed] [Google Scholar]
- 24.Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


