Abstract
Cancer metastasis is the major cause of cancer-associated death. Accordingly, identification of the regulatory mechanisms that control whether or not tumor cells become “directed walkers” is a crucial issue of cancer research. The deregulation of cell migration during cancer progression determines the capacity of tumor cells to escape from the primary tumors and invade adjacent tissues to finally form metastases. The ability to switch from a predominantly oxidative metabolism to glycolysis and the production of lactate even when oxygen is plentiful is a key characteristic of cancer cells. This metabolic switch, known as the Warburg effect, was first described in 1920s, and affected not only tumor cell growth but also tumor cell migration. In this review, we will focus on the recent studies on how cancer cell metabolism affects tumor cell migration and invasion. Understanding the new aspects on molecular mechanisms and signaling pathways controlling tumor cell migration is critical for development of therapeutic strategies for cancer patients.
Keywords: cancer cell metabolism, cell migration, metastasis, glycolysis, glutamine
Introduction
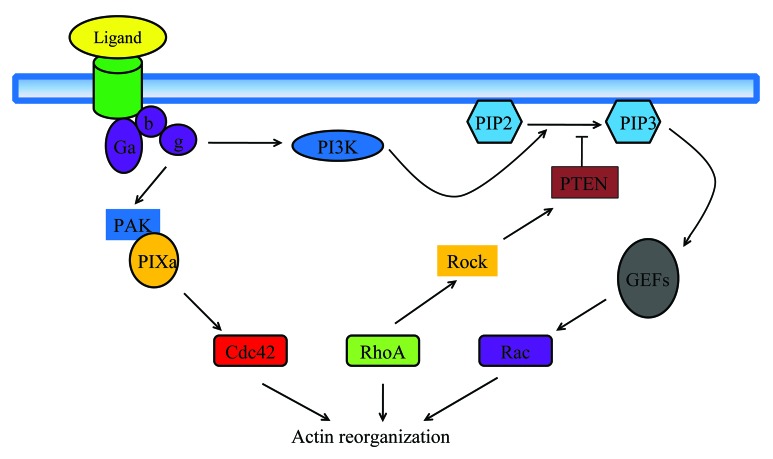
Tumor metastasis involves a series of interrelated events. Briefly, the initial steps involve vascularization of the primary tumor for aggressive growth through secretion of angiogenic factors, increased motility, and invasion of the tissue stroma through secretion of matrix metalloproteinases, and other changes in the tumor cells, such as the epithelial–mesenchymal-like transition (EMT-like). The invasive tumor cells penetrate blood vessels (intravasation) to enter the circulation or migrate through the lymphatic channels.1 The tumor cells also associate with bone marrow-derived cells, endothelial cells, stromal cells, and others, which provide a supportive microenvironment for the tumor cells.2 The circulating tumor cells extravagate into the parenchyma of a distal organ, where they undergo metastatic growth. Although tumor cell migration is a complicated procedure, the basic steps are similar to normal cell migration. For example, Rho GTPase-regulated cytoskeletal remodeling and PI-3K-defined leading edge are critical steps in both tumor cell migration and normal cell migration (Fig. 1).3
Figure 1. Model of chemoattractant signal transduction pathways in leukocyte polarization and migration. Binding of chemoattractant to G-protein coupled receptors releases the Ga heterodimer from the heterotrimeric Ga proteins. Dissociated Ga proteins stimulate PIP3 production via PI3K, lead to activation of PIP3-sensitive Rac-GEFs, and activation of the small GTPase Rac. Active Rac catalyzes the remodeling of the actin-cytoskeleton at the leading edge required for the formation of novel cell protrusions. G-proteins also stimulate Cdc42 activity, through complex formation with PAK and the Cdc42-GEF PIX. Active Cdc42 is required to localize RhoA at the back of the cell. RhoA activation at the trailing edge catalyzes the remodeling of the actomyosin-cytoskeleton required for uropod contraction. As an additional level of regulation, RhoA at the trailing edge activates its target Rock, which phosphorylates and activates PTEN; active PTEN at the back of the cell further strengthens the asymmetrical distribution of PIP3 at the leading edge, thus stabilizing the polarized shape and the orientation of the cell in the chemoattractant gradient.
The alternation of cancer cell metabolism was first observed by Otto Warburg in early 1921. He found that glucose carbons were mainly converted to lactate in proliferating ascites tumor cells, even with supply of abundant oxygen,4 a phenomenon known as the “Warburg effect.” He hypothesized that the metabolic alteration arose from the defects of mitochondria that lost their ability to effectively oxidize glucose carbon to CO2.5 Advances in cancer metabolism research over the last decade have enhanced our understanding of how aerobic glycolysis and other metabolic alterations observed in cancer cells support the anabolic requirements associated with cell growth and proliferation. High glycolytic rate allows cells to use the most abundant extracellular nutrient, glucose, to produce abundant ATP. Glucose degradation provides cells with intermediates needed for biosynthetic pathways, including ribose sugars for nucleotides; glycerol and citrate for lipids; nonessential amino acids; and, through the oxidative pentose phosphate pathway, NADPH. Therefore, the Warburg effect benefits both bioenergetics and biosynthesis necessary for cell growth and proliferation.6 A consequence of this changed metabolism is to increase acid production in tumor cells.7 This leads to normal cell death,8 and extracellular matrix degradation by proteolytic enzymes,9 these enhance cancer cell’s capability for migration and invasion.
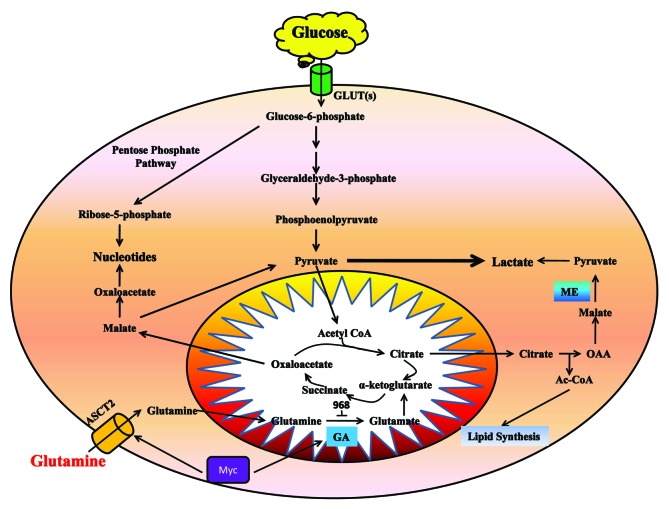
Decreased oxygen availability (hypoxia) in cancer cells is coordinated by the hypoxia-inducible factor 1 (HIF-1).10,11 HIF-1’s targets include genes encoding glucose transporters, glycolytic enzymes, and LDH-A.12,13 HIF-1 also can activate Myc,14 then Myc targets glutaminases for high activities in proliferating breast cancer cells.15 Experiments from carbon labeling metabolic studies demonstrated that glycolysis, glutaminolysis, the Kreb's cycle, the pentose phosphate pathway, and nucleotide biosynthesis are all coordinately enhanced in tumor cells (Fig. 2).16 Therefore, in this review, we will focus on the effects of glycolysis, glutamine metabolism, and pentose phosphate pathway on tumor cell migration and invasion.
Figure 2. In cancer cells, glycolysis is used to produce ATP and provides substrates to the pentose phosphate pathway for nucleotide synthesis. Glutamine metabolism mainly supply for metabolic intermediates for macromolecular synthesis.
How Does the Glycolysis Pathway Affect Tumor Cell Migration and Invasion?
The most cancer cells use glucose at high level and convert it to lactate instead of relying on mitochondrial oxidative phosphorylation to generate energy even with adequate oxygen, a phenomenon termed “Warburg effect.”4 Aerobic glycolysis is an inefficient way to generate ATP, but the inefficiency of the anaerobic pathway can be compensated by increased glucose flux.7 Switching to the aerobic glycolysis is a key characteristic of cancer metabolism and is not only critical for tumor cell growth but also essential for tumor cell migration.
Since the aerobic metabolism of glucose to lactate is substantially less efficient than oxidation to CO2 and H2O, tumor cells maintain ATP production by increasing glucose flux. A critical consequence of this altered metabolism is to increase lactate production in tumor cells.7 This leads to normal cell death via caspase-mediated activation of p53-dependent apoptotic pathway,8,17 whereas cancer cells are well equipped to export lactate by MCTs transporters resulting in the acidification of microenvironment.18 A low pH created by extracellular acidification provides a favorable microenvironment for the activation of proteases, including MMPs,19 urokinase-type plasminogen activator,20 and cathepsins B,21 D,22 and L,23 which induce extracellular matrix (ECM) degradation and facilitate tumor cells to metastasis.24 Goetze et al. found that sodium L-lactate but not D-lactate or changes in intracellular pH induced a time- and dose-dependent migration of human SQ20B squamous larynx carcinoma cells in a chemo-attractive experiment.25 Therefore, tumor cells become migratory and invasive because they disturb the environment so that it is optimal for their proliferation and toxic to the normal cells with which they compete for space and substrate.
Although no clinical diagnostic application has been developed to date, elevated levels of lactate have shown a correlation with poor patient prognosis and overall survival in different cancers.26,27 In addition, lactate is not only a metabolic intermediate but also acts as a signaling molecule.28 Lactate has been reported to activate hypoxia-inducible factor (HIF).29,30 The underlying pathway was shown to require lactate oxidation into pyruvate (LDH-1 reaction) in order to support a functional competition between pyruvate and 2-oxoglutarate (a by-product of the TCA cycle) for the control of HIF PHD activity. Pyruvate functionally competes with 2-oxoglutarate leading to PHD inactivation and, consequently, HIF-1 protein stabilization.30 HIF, as a transcription factor, drives the induction or repression of a myriad of genes controlling multiple cell functions such as angiogenesis, metabolism, invasion/metastasis, and apoptosis/survival. HIF activation by acidic microenvironment contributes to tumorigenesis and metastasis. Disruption of cell–cell and cell–extracellular matrix contacts promotes cell migration.31 A substantial number of proteins induced by HIF are involved in these processes, which includes vimentin, fibronectin, keratins 14, 18, 19, matrix metalloproteinase 2, cathepsin D.32 The loss of E-cadherin, a hallmark in invasion, is also linked to HIF activation and, thus, metastasis.33 Hypoxic environments select for tumor cells with stabilized HIF1 apha, which enhances invasion of tumor cells. An increase in environmental oxygen in combination with a mitochondrial-targeted catalase mimetic and a metabolism booster may be of interest to investigate as a treatment strategy for invasive cancer.34
Anoikis resistance, or the ability for cells to live detached from the extracellular matrix, is a property of epithelial cancers. A recent study focused on metabolic alterations in ovarian cancer cells with varying invasive capability under anoikis conditions found that pyruvate uptake was significantly higher for the highly invasive ovarian cancer cells compared with the less invasive ovarian cancer cells. These differences in metabolism would have an effect on cell migration, and pyruvate may be used by highly invasive ovarian cancer cells to migrate in attached conditions and, thus, may enhance metastatic potential.35
The enzymes in glycolysis also play important roles in tumor migration and invasion. Phosphoglucose isomerase (PGI, also known as glucose-6-phosphate isomerase or phosphohexose isomerase) is a housekeeping cytosolic enzyme that catalyzes the conversion of glucose-6-phosphate into fructose-6-phosphate in the second step of glycolysis.36 PGI is a secreted protein that behaves as a potent cytokine in extracellular environment. It has been demonstrated that PGI is an autocrine motility factor (AMF), and a tumor-secreted cytokine that stimulates cell migration in vitro and metastasis in vivo.37 PGI/AMF stimulates cell migration through binding to its seven-transmembrane receptor gp78 on the surface of target cells.38 PGI/AMF is critical for migration, invasion, metastasis of tumor cells, and contains anti-apoptotic effects on malignant tumor cells and its multiple roles in tumor progression are mediated by certain downstream pathways and effectors.39,40 A previous study showed that PGI/AMF induced interleukin (IL)-8 production and by which it induced tumor cell migration.41 IL-8 is a potent pro-inflammatory cytokine, which is expressed in various tumor cells, especially those with high metastatic indexes, such as melanoma cells42 and breast carcinoma cells.43 It was reported that PGI/AMF could increase IL-8 expression at both mRNA and protein levels in the early stage of melanoma cells and the migratory ability of melanoma cells could be inhibited by an anti-IL-8-neutralizing antibody. It was also reported that PGI/AMF directly stimulated tumor cell migration through RhoA and Rac1 pathways.44 However, the relationship of these pathways remains to be further defined.
Increasing evidence suggested that the conversion of epithelial cells to more mesenchymal-like cells facilitated cell migration, invasion, and metastasis. Molecular analysis showed that PGI/AMF suppressed epithelial marker expression and enhanced mesenchymal marker expression.45,46 The acquisition of migratory and invasive properties by epithelial cells may be associated with the gain of mesenchymal characteristics and the loss of epithelial features.47 PGI/AMF induce epithelial-to-mesenchymal transition (EMT) by decreasing the E-cadherin expression48 through NFκB pathway, which is activated by RhoA and Rac1 pathways.49,50 It has been reported that PGI/AMF-induced EMT was regulated by miR-200s in breast cancer cells.51 MiR-200s negatively regulated expression of ZEB1/ZEB2, a mesenchymal marker and target gene of NFκB.52 MiR-200s can alter the relative expression of epithelia and mesenchymal markers, and decrease aggressiveness and migration of tumor cells (Fig. 3).
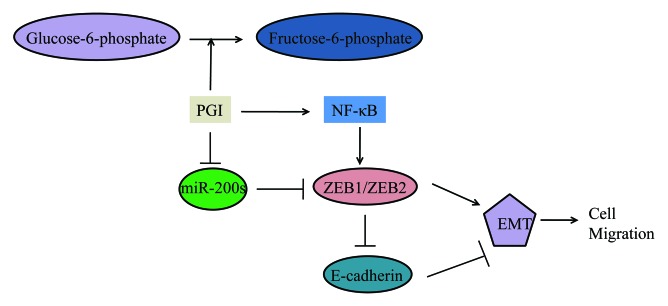
Figure 3. Schematic representation of regulation of EMT and migration of breast cancer cells by PGI/AMF. The miR200s seem to play a key role in the induction of EMT by PGI/AMF. The complex relationship between miR200s, NFκB, ZEB1/ZEB2, and E-catering, and their regulation by PGI/AMF might be crucial to the acquisition of EMT and aggressive behavior of breast cancer cells.
Fructose-1,6-bisphosphatase (FBP1), a gluconeogenesis enzyme, which catalyzes the splitting of fructose-1,6-bisphosphate (F-1,6-BP) into fructose 6-phosphate, also plays an important role in EMT. Study shows that Loss of FBP1 in basal-like breast cancer induces glycolysis and results in increased glucose uptake, and macromolecule biosynthesis. This metabolic reprogramming is intertwined with the development of basal-like breast cancer, because loss of FBP1 is required for EMT induction and enhanced cancer invasiveness.53
Pyruvate kinase (PK) mediates the final rate-limiting step of glycolysis by catalyzing the dephosphorylation of phosphoenolpyruvate (PEP) to pyruvate.54 Studies have found that cancer cells exclusively express PKM2,55 but there might be different expressing patterns and roles of PKM2 in different tumors. As PKM2 expression is strongly correlated with gastric cancer differentiation, it may play different roles in differently differentiated gastric cancer cell types. In differentiated gastric cancer cells, knockdown of PKM2 can decrease the expression of E-cadherin and, thus, activate downstream signaling pathway of EGFR, such as PLC-γ1 and ERK1/2, and promote cell migration and invasion. While in undifferentiated gastric cancer cells that lack E-cadherin, PKM2 can enhance EGFR downstream signaling activation and promote cell migration and invasion.56 In colorectal cancer, the PKM2 expression is increased and increased PKM2 expression was associated with later stage and lymph metastasis of the tumors. Knocking-down of PKM2 suppressed the proliferation and migration of colon cancer RKO cells.57
Lactate dehydrogenase (LDH) is a key metabolic enzyme catalyzing the transition of pyruvate to lactate. There are two types of subunits of LDH, designated M (muscle-type; LDHA gene product) and H (heart-type; LDHB gene product). Normal cells contain five different LDH isozymes with different substrate reactivities as a result of the five different combinations of the two different subunits: LDH1 (H4); LDH2 (MH3); LDH3 (M2H2); LDH4 (M3H); LDH5 (M4). The expression levels of LDHA and LDHB determine the cell's isozyme pattern.58 LDH5 effectively catalyzes the conversion of pyruvate to lactate, and an isozyme shift to LDH5 has been linked with metastatic cancer.59 This shift is mediated by increased LDHA expression via HIF-1α.60 LDHA induction via HIF-1α is critical for maintaining glycolysis in cancer cells and increasing its invasive activity. In glioma cells, lactate metabolism regulates TGF-β2-mediated migration.61 Transforming growth factor-β2 (TGF-β2) is an important regulator for invasion of high-grade gliomas.62 TGF-β2 plays an important role in glioma cell motility and migration via several mechanisms that involve certain extracellular matrix (ECM) proteins such as versican and ECM-degrading enzymes such as MMPs.63-66 LDH-A and lactate can regulate TGF-β2 expression in glioblastoma cells and increase MMP-2 expression, resulting in enhanced glioma cell migration. Conversely, downregulation of LDH-A can decrease TGF-β2 protein levels and result in reduced glioma cell migration.61
Another study showed that suppressed LDHB expression plays a critical role in hepatoma cell invasiveness by inducing claudin-1 (Cln-1), a tight junction protein. The increased lactate production was due to LDH isozyme shifts to LDH5 by LDHB downexpression rather than LDHA induction. The ectopic expression of LDHB attenuated the invasiveness of both SNU 354 and 449 cells, whereas LDHB knockdown significantly augmented the invasiveness of Chang cells with Cln-1 induction.67
Beside the glycolysis enzymes we discussed above, other glycolytic enzymes also play a potential role in the process of tumor cell migration. Hexokinase 2 (HK2) and 6-phosphofructo-2-kinase (PFKFB) have been reported to be transcriptional targets of HIF-1.68,69 Based on these findings, drugs have been developed to inhibit glycolysis pathways and small molecule inhibitors of HIF are being actively sought. Other strategies like manipulation of the extracellular and/or intracellular pH of tumors may also have considerable potential in cancer therapy.
How Does Glutamine Metabolism Affect Tumor Cell Migration and Invasion?
Along with increased aerobic glycolysis, enhanced metabolism of glutamine is now recognized as a key feature of the metabolic profile of cancer cells. As the most abundant amino acid in plasma, glutamine is consumed and utilized by most tumors at much higher rates than other amino acids.70 Once transported into cells, glutamine could be used as an amino acid for protein synthesis or as a nitrogen donor for nucleic acid synthesis. In actively growing cells, glucose is secreted as a lactate, which will cause a dramatic decrease of intermediates in the tricarboxylic acid (TCA) cycle. Glutamine can replenish the TCA cycle by a process termed glutamine-dependent anaplerosis,71 in which glutamine is transported into mitochondria and catabolized to glutamate by the mitochondrial enzyme glutaminase. Glutamate is then catabolized by glutamate dehydrogenase to α-ketoglutarate to feed the TCA cycle.
Recent studies suggested that glutamine metabolism contributed to cancer cell migration. Transformed fibroblasts and the highly invasive MDA-MB231 and SKBR3 breast cancer cells showed significantly higher glutaminase activity, compared with non-transformed cells and normal human mammary epithelial cells (HMECs), indicating the importance of glutamine metabolism. In screening for inhibitors of Rho GTPase-mediated cell transformation, a small molecule inhibitor 968 was found to be a potent inhibitor of Rho GTPases-mediated cell transformation. Further experiments identified glutaminase as the direct target of 968. In cell invasion assays, the migratory activity of the transformed fibroblasts and cancer cells was severely compromised when they were treated with 968, suggesting the contribution of glutamine metabolism to cancer cell migration.72 In prostate cancer cell line PC3, the c-Myc oncogenic transcription factor represses miR-23a and miR-23b, resulting in greater expression of their target protein, mitochondrial glutaminase (GLS). This leads to upregulation of glutamine catabolism. Knocking-down c-Myc by siRNA was also associated with reduction of GLS expression. Importantly, PC3 cell proliferation is markedly attenuated by siGLS but not by control siRNA, indicating that GLS is necessary for cell proliferation.73 Moreover, glutamine restriction inhibits attachment, spreading, and migration of melanoma cell lines via inhibition of specific integrin expression and modulation of actin cytoskeleton remodeling.74 In addition, glutamine catabolism, leading to glutamate formation, plays specific role in neoplastic phenotype. It was reported that high extracellular concentration of glutamate favors glioma cell migration.75 Glutamate was also observed to increase the invasion and migration of pancreatic cancer cells via AMPA receptor activation and kRas-MAPK signaling.76 On the other hand, glutamate antagonists decreased motility and invasive activities of adenocarcinoma and breast and lung carcinoma cells.77
Glutamine taken up through SLC1A5 glutamine transporter was rapidly exported in exchange for essential amino acids,78 which can activate the mammalian target of rapamycin complex 1 (mTORC1) activity.79 Recent data have shown that mTOR also plays a critical role in the regulation of tumor cell migration and metastasis.80 It has been reported that rapamycin inhibited cell migration, indicating the role of mTORC1 in cell motility.81 X-387, a novel active-site inhibitor of mTOR, displayed superior activity than rapamycin in inhibiting cell migration of A549 cells.82 The high utilization of glutamine may contribute to cancer cell migration partly by activating the mTORC1 activity.
Glutamine plays a role in lipogenesis by providing both acetyl-CoA and NADPH. The direct contribution of glutamine to de novo lipogenesis is particularly apparent under conditions of hypoxia or mitochondrial dysfunction, in which cells were shown to depend almost exclusively on the reductive metabolism of α-ketoglutarate to synthesize acetyl-CoA.83,84 Glutamine metabolism may promote cancer cell migration partly by supporting lipogenesis, which, in turn, regulates the activation of AKT.85 Phosphoinositide 3-kinase/Akt pathway is an extensively studied pathway, which has been involved in migratory and invasive behavior of many cancer cell lines.86,87
Glutamine metabolism uses several steps of the TCA cycle to produce α-ketoglutarate, succinate, fumarate, and oxaloacetate.88 Mutations in the genes encoding the TCA cycle enzymes succinate dehydrogenase (SDH) and fumarate hydratase (FH) render the enzymes inactive, leading to the accumulation of succinate and fumarate in mitochondria.89 This prevents the degradation of HIF-1α and HIF-2α, and promotes cell migration.90,91 Silencing HIF-1α has been reported to have a significant inhibition on migration of gliomas and glioblastoma U87 cells.92
Glutamine is hydrolyzed by different isoforms of glutaminases in different tissues/cells: liver-type glutaminase (LGA) and kidney-type glutaminase (KGA).93 Normally, the expression of KGA in cancer cells promotes their growth and migration. However, stable transfection of T98G cells with a vector carrying human LGA sequence resulted in increased LGA protein activity, and the transfected cells showed a 45% reduction of cell migration compared with non-transfected cells.94 LGA was also identified as a novel target of p53 and plays an important role in energy metabolism and antioxidant function.95 Taken together, glutamine plays an important role in contributing to the core metabolism of proliferating cells by supporting energy production and biosynthesis. Glutamine availability and metabolism can also modulate activity of signal transduction pathways and then regulates cancer cell growth and migration (Fig. 2).96
Cancer cells metabolic reprogramming includes a shift in energy production from oxdative phopsphrylation to less efficient glycolysis even in the presence of oxygen (Warburg effect) and use of glutamine for increased biosynthetic needs. This necessitates greatly increased glucose and glutamine uptake, both of which enter the hexosamine biosynthetic pathway (HBP). The HBP end product UDP-N-actylglucosamine (UDP-GlcNAc) is used in enzymatic post-translational modification of many cytosolic and nuclear proteins by O-linked β-N-acetylglucosamin (O-GlcNAc). A number of these targeted proteins are implicated in cancer.97 The increased HBP flux and hyper-O-GlcNAcylation were observed in human pancreatic ductal adenocarcinoma (PDAC). Reducing hyper-O-GlcNAcylation had no effect on non-transformed pancreatic epithelial cell growth, but inhibited PDAC cell proliferation, anchorage-independent growth, orthotopic tumor growth, and triggered apoptosis.98 Therefore, targeting HBP should be a potential therapeutic strategy in the treatment of cancer.
How Does Pentose Phosphate Pathway Affect Tumor Cell Migration and Invasion?
The pentose phosphate pathway (PPP) is involved in the degradation of glucose in which glucose is catalyzed by different enzymes through oxidative and non-oxidative ways, leading to production of lactate and more nucleotides.99 Because the PPP provides two substrates—ribose5-phosphate and NADPH—necessary for dividing cells and buffering the ROS damage, it is not surprising that changes in PPP activity usually occur during cancer development and progression. An upregulation of the PPP is generally associated with invasive and metastasizing tumors.100 Overexpression of the oxidative branch enzyme-G6PD was found in the central nervous system metastases of breast cancers.101 An increased activation of the non-oxidative branch seems functional to provide increased energetic needs of a highly invasive renal cancer. In light of these results, some studies have proposed that the activation of the non-oxidative branch of the PPP can be a hallmark of metastatic tumors.99
The non-oxidative branch of pentose phosphate pathway is catalyzed by transketolases (TKT). TKT is a ubiquitous thiamin diphosphate and Me2+-dependent enzyme that catalyzes the reversible transfer of two-carbon ketol units between ketose and aldose phosphates in the non-oxidative part of the pentose phosphate pathway (PPP). TKT, along with transaldolase (TAL), which transfers three-carbon units, a reversible connection between glycolysis, and the PPP.102 A mutated transketolase transcript (TKTL1) is upregulated in human malignancies, and the overexpression of TKTL1 has been reported in different cancers.103-105 TKTL1 is responsible for around 60% or 70% of transketolase activity in human hepatoma and colon-cancer cells. It has been demonstrated that knockdown of TKTL1 by RNAi in human HCT116 colon carcinoma cells resulted in reducing cancer cell migration along with a significantly low glucose consumption and lactate production.106,107 As one of the five lactate dehydrogenase (LDH) isoenzymes, LDH5 plays an important role in catalyzing pyruvate into lactate. Kayser et al. found that overexpression of TKTL1 led to overexpression of LDH5, thereby enhanced the production of pyruvate and lactate.108 High lactate concentration could induce the necrosis and apoptosis of normal tissues and release of cathepsin B and other proteolytic enzymes, which results in the degradation of extracellular matrix and initiates cancer cell migration.19-24 It is reported that metastasis of tumors is promoted by lactate-induced secretion of hyaluronan that creates a milieu favorable for migration.109 Intriguingly, lactate itself has been found to induce the migration of cancer cells.110 The data from patients also suggest that TKTL1 plays a critical role in cancer migration. Langbein and his co-workers (2006) found that strong TKTL1 activity was observed in invasive tumors, whereas no or weak activity of TKTL1 was detected in non-invasive colon carcinomas. They also found that increased TKTL1 protein expression was observed in tumor tissue of all patients with metastasized kidney cancer, whereas no expression or weak expression in non-progressing tumors.99 Finally, a recent study showed that overexpression of TKTL1 induced the expression of HIF-1a in vivo and in vitro. Conversely, gene silence of TKTL1 by siRNA significantly decreased the HIF-1a level.111 HIF-1, a transcriptional factor, regulates the transcription of hundreds of genes that encode proteins involved in every aspect of cancer biology, including cell proliferation, division, migration, invasion, and metastasis (Fig. 4).31 Taken together, the enzymes that function in pentose phosphate pathway may affect tumor cell migration and tumor invasion through multiple mechanisms.
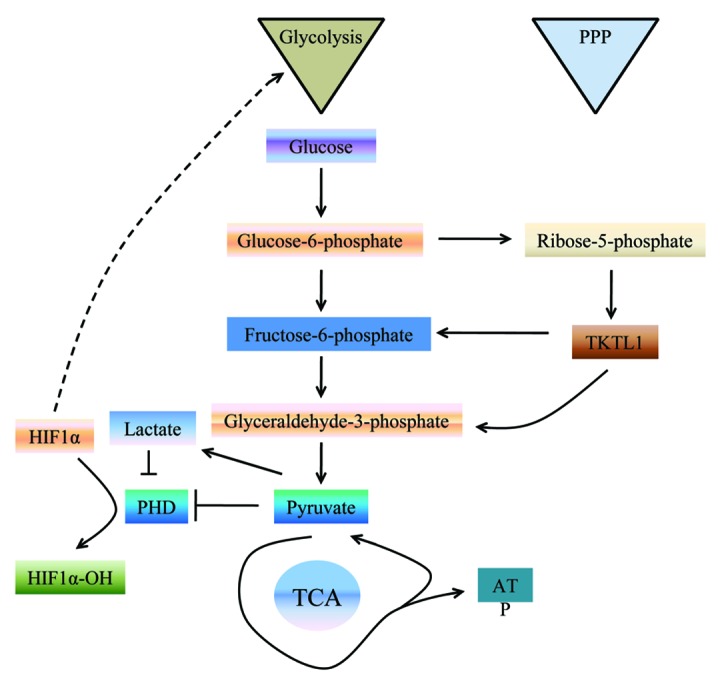
Figure 4. The PPP is directly connected to glycolysis, as fructose-6-phosphate and glyceraldehyde-3-phosphate are the intermediates in both pathways. We hypothesized that TKTL1 could increase the production of fructose-6-phosphate and glyceraldehydes-3-phosphate, increasing aerobic glycolysis.
Conclusion and Future Directions
In this review, we provide an overview of recent experimental studies that investigate the effects of cancer cell metabolism on tumor cell migration and invasion. These experimental studies have provided great insight into how the enzymes that control cancer metabolisms affect tumor cell migration and invasion. The ability to switch from a predominantly oxidative metabolism to glycolysis and the production of lactate even when oxygen is plentiful is a key characteristic of cancer cells. This metabolic switch, known as the Warburg effect, was first described in the 1920s, and not only affected tumor cell growth but also affected tumor cell migration. In general, there are several pathways including glycolysis, glutamine metabolism, and pentose phosphate pathway that are involved in cancer cell metabolism. There is a concomitantly increase of glucose metabolism in tumor cells, leading to generation of ATP, NADPH, lactate, and nucleic acids. Emerging studies suggest that not only the key enzymes that control cancer metabolism but also the metabolic products from cancer cells significantly affect tumor cell migration and invasion. However, the detailed molecular mechanisms on how cancer metabolism regulates tumor cell migration and cancer metastasis are not clear.
While anaerobic glycolysis promotes energy production under hypoxia, aerobic glycolysis, the Warburg effect, is favorable not only for cancer cell growth, but also tumor migration and invasion. These metabolic switches in cancer cells lead to the changes of HIF1 activation, lactate release, and redox production, which all link the development of invasive phenotype of cancers. A recent mathematical model provided a hypothesis of acid-mediated tumor invasion.24 In this model, increased acid production due to altered glucose metabolism serves as a key intermediate by producing H+ flow alone concentration gradients into adjacent normal tissue. This chronic exposure of peritumoral normal tissue to an acidic microenvironment induces normal cell death and extracellular matrix degradation through the release of cathepsin B and other proteolytic enzymes, which permits cancer cells to invade the damaged adjacent normal tissue despite the acid gradient. However, this model is still needed to be proven by more in vivo experimental evidence. In addition, the underlying mechanisms of lactic acidosis and metastasis, lactate shuttle, the influence of lactate on redox homeostasis, lactate signaling, and lactate-induced angiogenesis in the cancer context are still needed to be further investigated in the future.
In summary, we are here summarizing the recent advances in the effects of cancer cell metabolism on its invasive phenotype. Targeting the cancer cell metabolism may provide novel strategies for inhibiting tumor cell migration and cancer metastasis and make cancer treatable.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Ceshi Chen (Kunming Institute of Zoology, Chinese Academy of Science) for discussion and reading of the manuscript. This work was supported by a starting fund from Nanchang University.
Glossary
Abbreviations:
- PGI
phosphoglucose isomerase
- AMF
autocrine motility factor
- IL
interleukin
- EMT
epithelial-to-mesenchymal transition
- LDH
lactate dehydrogenase
- TGF
transforming growth factor
- MMPs
matrix metalloproteinases
- ECM
extracellular matrix
- HK
hexokinase
- PFKFB
6-phosphofructo-2-kinase
- PK
pyruvate kinase
- HIF
hypoxia-inducible factor
- TCA
tricarboxylic acid
- HMECs
human mammary epithelial cells
- SDH
succinate dehydrogenase
- FH
fumarate hydratase
- LGA
liver-type glutaminase
- KGA
kidney-type glutaminase
- PPP
pentose phosphate pathway
- TKT
transketolases MCT, monocarboxylate transporter
- PHD
HIF-α-prolylhydroxylase
- FBP1
Fructose-1,6-bisphosphatase
- F-1,6-BP
fructose-1,6-bisphosphate
- PEP
phosphoenolpyruvate
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/26345
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–40. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 6.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 2003;63:3847–54. [PubMed] [Google Scholar]
- 8.Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene. 1999;18:3199–204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- 9.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–30. [PubMed] [Google Scholar]
- 10.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelson H, Fredlund E, Ovenberger M, Landberg G, Påhlman S. Hypoxia-induced dedifferentiation of tumor cells--a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005;16:554–63. doi: 10.1016/j.semcdb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.O’Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem. 1996;241:403–10. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63. [PubMed] [Google Scholar]
- 14.Huang LE. Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ. 2008;15:672–7. doi: 10.1038/sj.cdd.4402302. [DOI] [PubMed] [Google Scholar]
- 15.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–83. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan TW, Kucia M, Jankowski K, Higashi RM, Ratajczak J, Ratajczak MZ, Lane AN. Rhabdomyosarcoma cells show an energy producing anabolic metabolic phenotype compared with primary myocytes. Mol Cancer. 2008;7:79. doi: 10.1186/1476-4598-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751–6. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 18.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y, Ozawa S, Tsukuda M, Kubota E, Miyazaki K, St-Pierre Y, Hata R. Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007;274:3171–83. doi: 10.1111/j.1742-4658.2007.05848.x. [DOI] [PubMed] [Google Scholar]
- 20.Kindzelskii AL, Amhad I, Keller D, Zhou MJ, Haugland RP, Garni-Wagner BA, Gyetko MR, Todd RF, 3rd, Petty HR. Pericellular proteolysis by leukocytes and tumor cells on substrates: focal activation and the role of urokinase-type plasminogen activator. Histochem Cell Biol. 2004;121:299–310. doi: 10.1007/s00418-004-0639-3. [DOI] [PubMed] [Google Scholar]
- 21.Szpaderska AM, Frankfater A. An intracellular form of cathepsin B contributes to invasiveness in cancer. Cancer Res. 2001;61:3493–500. [PubMed] [Google Scholar]
- 22.Tedone T, Correale M, Barbarossa G, Casavola V, Paradiso A, Reshkin SJ. Release of the aspartyl protease cathepsin D is associated with and facilitates human breast cancer cell invasion. FASEB J. 1997;11:785–92. doi: 10.1096/fasebj.11.10.9271363. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–75. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 24.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–23. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 25.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–63. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 26.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21. [PubMed] [Google Scholar]
- 27.Yokota H, Guo J, Matoba M, Higashi K, Tonami H, Nagao Y. Lactate, choline, and creatine levels measured by vitro 1H-MRS as prognostic parameters in patients with non-small-cell lung cancer. J Magn Reson Imaging. 2007;25:992–9. doi: 10.1002/jmri.20902. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–12. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–39. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 31.Brahimi-Horn C, Pouysségur J. The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull Cancer. 2006;93:E73–80. [PubMed] [Google Scholar]
- 32.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 33.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 34.Enns L, Ladiges W. Mitochondrial redox signaling and cancer invasiveness. J Bioenerg Biomembr. 2012;44:635–8. doi: 10.1007/s10863-012-9467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caneba CA, Bellance N, Yang L, Pabst L, Nagrath D. Pyruvate uptake is increased in highly invasive ovarian cancer cells under anoikis conditions for anaplerosis, mitochondrial function, and migration. Am J Physiol Endocrinol Metab. 2012;303:E1036–52. doi: 10.1152/ajpendo.00151.2012. [DOI] [PubMed] [Google Scholar]
- 36.Achari A, Marshall SE, Muirhead H, Palmieri RH, Noltmann EA. Glucose-6-phosphate isomerase. Philos Trans R Soc Lond B Biol Sci. 1981;293:145–57. doi: 10.1098/rstb.1981.0068. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960–3. [PubMed] [Google Scholar]
- 38.Shimizu K, Tani M, Watanabe H, Nagamachi Y, Niinaka Y, Shiroishi T, Ohwada S, Raz A, Yokota J. The autocrine motility factor receptor gene encodes a novel type of seven transmembrane protein. FEBS Lett. 1999;456:295–300. doi: 10.1016/S0014-5793(99)00966-7. [DOI] [PubMed] [Google Scholar]
- 39.Haga A, Funasaka T, Niinaka Y, Raz A, Nagase H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expression. Int J Cancer. 2003;107:707–14. doi: 10.1002/ijc.11449. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10:7775–84. doi: 10.1158/1078-0432.CCR-04-1015. [DOI] [PubMed] [Google Scholar]
- 41.Araki K, Shimura T, Yajima T, Tsutsumi S, Suzuki H, Okada K, Kobayashi T, Raz A, Kuwano H. Phosphoglucose isomerase/autocrine motility factor promotes melanoma cell migration through ERK activation dependent on autocrine production of interleukin-8. J Biol Chem. 2009;284:32305–11. doi: 10.1074/jbc.M109.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norgauer J, Metzner B, Schraufstätter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996;156:1132–7. [PubMed] [Google Scholar]
- 43.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, Vignon F, Lazennec G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256–65. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsutsumi S, Gupta SK, Hogan V, Collard JG, Raz A. Activation of small GTPase Rho is required for autocrine motility factor signaling. Cancer Res. 2002;62:4484–90. [PubMed] [Google Scholar]
- 45.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 46.Wang JM, Taraboletti G, Matsushima K, Van Damme J, Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990;169:165–70. doi: 10.1016/0006-291X(90)91449-3. [DOI] [PubMed] [Google Scholar]
- 47.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 48.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 49.Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–75. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 50.Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–9. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–24. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 53.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969–80. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–8. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Wang LY, Liu YP, Chen LG, Chen YL, Tan L, Liu JJ, Jazag A, Ren JL, Guleng B. Pyruvate kinase M2 plays a dual role on regulation of the EGF/EGFR signaling via E-cadherin-dependent manner in gastric cancer cells. PLoS One. 2013;8:e67542. doi: 10.1371/journal.pone.0067542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou CF, Li XB, Sun H, Zhang B, Han YS, Jiang Y, Zhuang QL, Fang J, Wu GH. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life. 2012;64:775–82. doi: 10.1002/iub.1066. [DOI] [PubMed] [Google Scholar]
- 58.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 59.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 60.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18:1319–30. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 61.Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, Kastenberger M, Kreutz M, Nickl-Jockschat T, Bogdahn U, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11:368–80. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wick W, Naumann U, Weller M. Transforming growth factor-beta: a molecular target for the future therapy of glioblastoma. Curr Pharm Des. 2006;12:341–9. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- 63.Deryugina EI, Bourdon MA, Luo GX, Reisfeld RA, Strongin A. Matrix metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci. 1997;110:2473–82. doi: 10.1242/jcs.110.19.2473. [DOI] [PubMed] [Google Scholar]
- 64.Arslan F, Bosserhoff AK, Nickl-Jockschat T, Doerfelt A, Bogdahn U, Hau P. The role of versican isoforms V0/V1 in glioma migration mediated by transforming growth factor-beta2. Br J Cancer. 2007;96:1560–8. doi: 10.1038/sj.bjc.6603766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–23. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 66.Platten M, Wick W, Wild-Bode C, Aulwurm S, Dichgans J, Weller M. Transforming growth factors beta(1) (TGF-beta(1)) and TGF-beta(2) promote glioma cell migration via Up-regulation of alpha(V)beta(3) integrin expression. Biochem Biophys Res Commun. 2000;268:607–11. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 67.Kim JH, Kim EL, Lee YK, Park CB, Kim BW, Wang HJ, Yoon CH, Lee SJ, Yoon G. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects. Exp Cell Res. 2011;317:1108–18. doi: 10.1016/j.yexcr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr. 2007;39:223–9. doi: 10.1007/s10863-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 69.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Foundation symposium proceedings 2007:35-53. [DOI] [PubMed] [Google Scholar]
- 70.Eagle H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med. 1955;102:37–48. doi: 10.1084/jem.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu YM, Zhang H, Ding M, Li YQ, Fu X, Yu ZX, Meadows GG. Specific amino acid restriction inhibits attachment and spreading of human melanoma via modulation of the integrin/focal adhesion kinase pathway and actin cytoskeleton remodeling. Clin Exp Metastasis. 2004;21:587–98. doi: 10.1007/s10585-004-5515-y. [DOI] [PubMed] [Google Scholar]
- 75.Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–8. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 76.Herner A, Sauliunaite D, Michalski CW, Erkan M, De Oliveira T, Abiatari I, Kong B, Esposito I, Friess H, Kleeff J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer. 2011;129:2349–59. doi: 10.1002/ijc.25898. [DOI] [PubMed] [Google Scholar]
- 77.Rzeski W, Ikonomidou C, Turski L. Glutamate antagonists limit tumor growth. Biochem Pharmacol. 2002;64:1195–200. doi: 10.1016/S0006-2952(02)01218-2. [DOI] [PubMed] [Google Scholar]
- 78.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuchs BC, Finger RE, Onan MC, Bode BP. ASCT2 silencing regulates mammalian target-of-rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol Cell Physiol. 2007;293:C55–63. doi: 10.1152/ajpcell.00330.2006. [DOI] [PubMed] [Google Scholar]
- 80.Zhou H, Huang S. mTOR signaling in cancer cell motility and tumor metastasis. Crit Rev Eukaryot Gene Expr. 2010;20:1–16. doi: 10.1615/CritRevEukarGeneExpr.v20.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wedel S, Hudak L, Seibel JM, Makarević J, Juengel E, Tsaur I, Wiesner C, Haferkamp A, Blaheta RA. Impact of combined HDAC and mTOR inhibition on adhesion, migration and invasion of prostate cancer cells. Clin Exp Metastasis. 2011;28:479–91. doi: 10.1007/s10585-011-9386-8. [DOI] [PubMed] [Google Scholar]
- 82.Chen SM, Liu JL, Wang X, Liang C, Ding J, Meng LH. Inhibition of tumor cell growth, proliferation and migration by X-387, a novel active-site inhibitor of mTOR. Biochem Pharmacol. 2012;83:1183–94. doi: 10.1016/j.bcp.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 83.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–8. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–6. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, Testa JR. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–82. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 86.Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M, et al. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31:2389–400. doi: 10.1038/onc.2011.419. [DOI] [PubMed] [Google Scholar]
- 87.Walker L, Millena AC, Strong N, Khan SA. Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis. 2013;30:13–23. doi: 10.1007/s10585-012-9494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forbes NS, Meadows AL, Clark DS, Blanch HW. Estradiol stimulates the biosynthetic pathways of breast cancer cells: detection by metabolic flux analysis. Metab Eng. 2006;8:639–52. doi: 10.1016/j.ymben.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 89.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–82. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 90.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 91.Sudarshan S, Shanmugasundaram K, Naylor SL, Lin S, Livi CB, O’Neill CF, Parekh DJ, Yeh IT, Sun LZ, Block K. Reduced expression of fumarate hydratase in clear cell renal cancer mediates HIF-2α accumulation and promotes migration and invasion. PLoS One. 2011;6:e21037. doi: 10.1371/journal.pone.0021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen SH, Kwan AL, Chen YY, Wang ZX. Effect of silencing HIF-1α on proliferation, invasion and migration of glioblastoma U87 cells. Neurol Sci. 2013;34:365–71. doi: 10.1007/s10072-012-1010-4. [DOI] [PubMed] [Google Scholar]
- 93.Szeliga M, Obara-Michlewska M. Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem Int. 2009;55:71–5. doi: 10.1016/j.neuint.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Szeliga M, Obara-Michlewska M, Matyja E, Łazarczyk M, Lobo C, Hilgier W, Alonso FJ, Márquez J, Albrecht J. Transfection with liver-type glutaminase cDNA alters gene expression and reduces survival, migration and proliferation of T98G glioma cells. Glia. 2009;57:1014–23. doi: 10.1002/glia.20825. [DOI] [PubMed] [Google Scholar]
- 95.Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107:7455–60. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. 2012;23:362–9. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 97.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–30. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langbein S, Frederiks WM, zur Hausen A, Popa J, Lehmann J, Weiss C, Alken P, Coy JF. Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer. 2008;122:2422–8. doi: 10.1002/ijc.23403. [DOI] [PubMed] [Google Scholar]
- 100.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–36. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 101.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR, 3rd, Felding-Habermann B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–86. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 102.Schneider S, Lüdtke S, Schröder-Tittmann K, Wechsler C, Meyer D, Tittmann K. A δ38 deletion variant of human transketolase as a model of transketolase-like protein 1 exhibits no enzymatic activity. PLoS One. 2012;7:e48321. doi: 10.1371/journal.pone.0048321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94:578–85. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Földi M, Stickeler E, Bau L, Kretz O, Watermann D, Gitsch G, Kayser G, Zur Hausen A, Coy JF. Transketolase protein TKTL1 overexpression: A potential biomarker and therapeutic target in breast cancer. Oncol Rep. 2007;17:841–5. [PubMed] [Google Scholar]
- 105.Schultz H, Kähler D, Branscheid D, Vollmer E, Zabel P, Goldmann T. TKTL1 is overexpressed in a large portion of non-small cell lung cancer specimens. Diagn Pathol. 2008;3:35. doi: 10.1186/1746-1596-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu X, Zur Hausen A, Coy JF, Löchelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer. 2009;124:1330–7. doi: 10.1002/ijc.24078. [DOI] [PubMed] [Google Scholar]
- 107.Zhang S, Yang JH, Guo CK, Cai PC. Gene silencing of TKTL1 by RNAi inhibits cell proliferation in human hepatoma cells. Cancer Lett. 2007;253:108–14. doi: 10.1016/j.canlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 108.Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K, Stickeler E, Werner M, Passlick B, zur Hausen A. Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol. 2010;5:22. doi: 10.1186/1746-1596-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 110.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–5. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 111.Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, Zhao M, Rudek MA, Ha PK, Califano JA. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res. 2010;16:857–66. doi: 10.1158/1078-0432.CCR-09-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]