Abstract
Cell-matrix adhesion is a fundamental biological process that governs survival, migration, and proliferation of living eukaryotic cells. Paxillin is an important central player in a network of adhesome proteins that form focal adhesion complexes. Phosphorylation of tyrosine and serine residues in paxillin is critical for the coordinated sequential recruitment of other adaptor and kinase proteins to adhesion complexes. Recently, the phosphorylation of serine178 in paxillin has been shown to be vital for epithelial cell adhesion and migration. In vivo and in vitro evidence have shown that transglutaminase (TG)-2 positively regulates this phosphorylation. Here, we propose three possible mechanisms that may explain these observations. First, TG-2 itself may be an adhesome member directly interacting with paxillin in a non-covalent way. Second, TG-2 may cross link a mitogen-activated protein kinase kinase kinase (MAP3K), which eventually activates c-Jun N-terminal kinase (JNK), and the latter phosphorylates paxillin. Lastly, TG-2 may have intrinsic kinase activity that phosphorylates paxillin. Future studies investigating these hypotheses on TG-2-paxillin relationships are necessary in order to address this fundamental process in cell matrix adhesion signaling.
Keywords: adhesion, signaling, paxillin, migration, transglutaminase, adhesome, review, cell culture
Role of paxillin in cell adhesion
Paxillin, a 68 kDa conserved cytoskeletal adhesome protein, is one of the earliest proteins to be detected in the nascent adhesions and is therefore clearly important in the assembly and disassembly of focal adhesions in eukaryotic cells.1,2 As a multi-domain adaptor protein crucial in the coordinated recruitment of a network of other adhesomes,3,4 paxillin is vital for cell-extracellular matrix adhesion, signal transduction, as well as cell migration.5 These biological processes are regulated by the subcellular localization of paxillin, as well as post-translational modification of paxillin. Mechanistically, these two events may involve protein–protein interactions.
Paxillin has multiple tyrosine, serine, and threonine phosphorylation sites, which are indispensable for normal cell adhesion, and is tightly regulated. These potential phosphorylation sites are targeted by various kinases activated by adhesion signaling and growth factors.6-9 The role of phosphorylation in adhesion complex signaling is relatively less understood compared with other conventional signaling paradigms and kinase cascades.
Paxillin comprises of several structural domains. The N-terminal of paxillin contains five leucine and aspartate-rich regions known as LD motifs (LD1–5). These 13 amino acids-long LD motifs with the consensus sequence of LDXLLXXL are highly conserved among species. At the C-terminal, there are four zinc-finger motifs known as LIM (Lin-11, Isl-1, Mec-3) domains (LIM1–4). Phosphorylation of different domains of paxillin has distinct functional effects, and has been associated with the formation of focal adhesions and actin stress fibers.10 In particular, the LD motifs control most of its signaling activities and the phosphorylation of these LD motifs is related to recruitment of downstream signaling molecules. For example, it has been reported that phosphorylation of paxillin LD4 domain at serine273 can regulate Rho GTPase signaling.11 On the other hand, phosphorylation on serine and threonine residues of LIM2 and 3 domains are essential for the localization of paxillin to the focal adhesions.9 Such molecular processes regulate maturation and turnover rate of focal adhesion complexes.8
What are the consequences of paxillin phosphorylation? Phosphorylation of paxillin leads to recruitment of signaling molecules, regulation of focal adhesion, and thus, cell migration. Although tyrosine phosphorylation of paxillin is more commonly studied,1 its serine residues are also heavily phosphorylated during cell adhesion and, thus, may be important. One key serine residue (Ser178) is located between the LD2 and LD3 motifs of paxillin, and is therefore not surprisingly important for signaling. It was previously demonstrated that mutation of Ser178 of paxillin to Ala178 (an amino-acid that cannot be phosphorylated) disrupted normal cytoskeletal remodeling, cell migration, and adhesion.12
Tranglutaminase-2 as a Regulator of Adhesion and Paxillin Phosphorylation
Paxillin can be phosphorylated by a variety of kinases.8 However, the upstream regulators involved in paxillin phosphorylation are not well characterized.
One such candidate molecule may be the transglutaminase (TG)-2, a ubiquitous protein known to be important for skin fibroblast adhesion and migration.13 TG-2 is a member of the transglutaminase family that is best known for catalyzing post-translational modification of proteins via calcium-dependent cross-linking/transamidating activity. This catalytic action of TG-2 results in the formation of isopeptide bond that is resistant to mechanical and proteolytic degradation.14 TG-2 is a multifunctional protein (Fig. 1). Besides its cross-linking activity, TG-2 acts as a G-protein in signaling, a cell surface adhesion molecule via non-covalent binding of matrix molecules, and even as a protein kinase.14-17
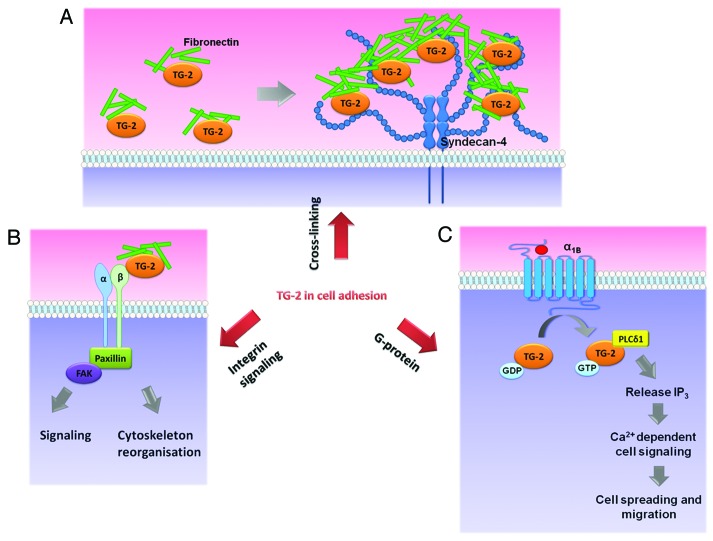
Figure 1. Diagram illustrating the multiple functions of transglutaminase 2 in cell matrix adhesion. (A) Syndecan-4 acts as a cell surface receptor for transglutaminase (TG)-2 in extracellular matrix. TG-2 is present in the extracellular matrix as non-covalent heteromers with fibronectin (green bars). The heparin sulfate chain of syndecan-4 concentrates TG-2-bound fibronectin in close proximity so that the TG-2 can enzymatically cross-link more neighboring fibronectin molecules, leading to the stiffening of the matrix. (B) Fibronectin has different domains (not shown) which can interact with either TG-2 or integrin. As an integrin co-receptor, TG-2 binds non-covalently to fibronectin, which recruits downstream adhesion complex proteins, leading to cytoskeleton reorganization and/or downstream signaling. (C) TG2 can function as a G protein. GDP bound TG-2 is inactive and its interaction with agonist (red sphere) stimulated α1B-adrenergic receptor (α1B) mediates the activation of phospholipase Cδ1 (PLCδ1), causing the release of inositol-1,4,5-trisphosphate (IP3), leading to calcium-dependent cell signaling which can affect cell spreading and migration.
The effect of TG-2 on cell adhesion may be mediated by a few mechanisms. Cell adhesion to surrounding substrate is greatly influenced by the rigidity of the matrix. In some cases, the enzymatic cross-linking function of TG-2 has been documented to contribute to the stability of extracellular matrix by cross-linking matrix molecules.18 In some reports, the adhesion signaling may not require the enzymatic transamidase function of TG-2.19,20 TG-2 may interact non-covalently with an array of matrix molecules such as integrin, growth factor receptors, and other cell surface or ECM proteins, such as fibronectin, to trigger adhesion signaling. Heparan sulfate proteoglycan (HSPG) syndecan-4, a transmembrane glycosylated protein, has also been reported as an important binding partner of TG-2.21,22 Syndecan-4 increases the concentration of the TG-2/fibronectin molecules in the matrix and, thereby, promotes the cross-linking of fibronectin.22,23 The increase in extracellular cross-linking will eventually raise the stiffness of the extracellular matrix. Since the fibronectin can also interact with the cell surface integrins, this process not only increases the supportive properties of the matrix, but may also result in integrin clustering through outside-in signaling.22
When TG-2 was reduced by short hairpin RNA in cultured human corneal epithelial (HCE-T) cells, the cytoskeleton of the cells, as evidenced by f-actin imaging, was disrupted. Using time-lapse imaging, these cells also showed a slower velocity of directional cell movement. This highlights the vital role of TG-2 in cellular adhesion and migration respectively.24 Previously extracellular TG-2 has been known to mediate integrin clustering leading to outside-in signaling, resulting in activation of downstream cell motility effectors, the rho proteins.19,20 The exact mechanism of how TG-2 achieves this remains to be defined.
Recently, our group has shown that phosphorylation of Ser178 paxillin is dependent on TG-2 status in cultured HCE-T cells using immunoblots with phosphoserine-specific antibody.24 This is a robust finding because the effect of TG-2 on paxillin phosphorylation does not require a specific extracellular ligand. The same results were obtained when the cells were grown on extracellular matrix proteins (fibronectin, laminin) coated surfaces.24 In other reports, the TG-2-dependent adhesion signaling required specific interaction between syndecan-4/TG-2 and fibronectin in the matrix.20
The relationship of TG-2 with paxillin phosphorylation was also demonstrated in vivo. Superficial mechanical wounds were constructed in the central cornea of homozygous TG-2-knockout and control mice, and observed at intervals until the epithelium was fully healed. In TG-2-knockout mice, reduced phospho-Ser178 paxillin was found at the leading edge of the migrating epithelial cells. This was associated with lack of strong adhesion between the leading edge of the migrating epithelium prior to closure and delayed wound closure.24 It is currently unknown whether the targeting of paxillin or phosphorylated form of paxillin to the leading edge of migrating cells is dependent on TG-2.
Transglutaminase-2 and Phosphorylation of Other Adhesomes
Apart from paxillin, examples of other important adhesomes include vinculin, talin, zyxin, and focal adhesion kinase (FAK). TG-2 was associated (Fig. 2) with phosphorylation24 of vinculin at Tyr822, and FAK at Tyr925 but not at Tyr576 (another residue known to be associated with adhesion signaling). In addition, TG-2 was linked to the activities of both the Rac1 and Cdc42 RhoGTPase proteins. Interestingly, it also participates in the phosphorylation of the cytoplasmic tail of β-3 integrin, previously known to be important in the signaling to Rac1.25
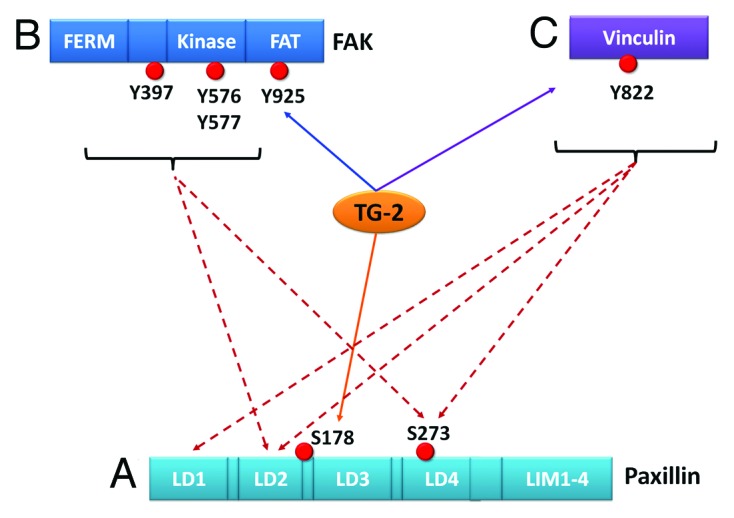
Figure 2. This is a schematic to show the possible relationships between TG-2 and adhesion proteins. (A) If a more direct association between TG-2 and a particular protein, for example, paxillin, can be demonstrated (orange solid line); but lack of association between TG-2 and other adhesion proteins (purple solid line), one can postulate that the signaling pathway is more likely to be TG2-paxillin. Although one cannot exclude simultaneous signaling between TG-2 and vinculin, etc., these effects may be more likely indirect via other intermediates. Conversely, it may also be possible to have a scenario where the TG-2 influences FAK (B), or vinculin (C) more directly than paxillin, suggesting alternative primary axes of signaling.
Once Ser178 is phosphorylated, a chain of events can take place, which facilitates paxillin to coordinate the recruitment of other adhesomes. Phosphorylation of paxillin at Ser178 by activated c-Jun N-terminal kinase (JNK) facilitates the binding of FAK26,27 to paxillin. The bound FAK then further facilitates phosphorylation of paxillin at Tyr 31 and 118, which, in turn, promotes binding of vinculin to paxillin.8,28 These changes suggest that phosphorylation of Ser178 paxillin may be upstream to phosphorylation of FAK and vinculin (Fig. 2). If TG-2 only affects phosphorylation of one adhesome initially, interaction with the most upstream Ser178 paxillin may be sufficient to interfere with post-translational changes of downstream FAK and vinculin.
Transglutaminase-2 Affects Adhesion in Diverse Cell Types
TG-2’s role in cell adhesion has already been demonstrated in more than one cell lineage.14,29 The effect of TG-2 on cell adhesion has been reported in NIH mouse 3T3 fibroblasts,19 human umbilical endothelial cells,29 human corneal epithelial cells,24 astrocytes,30 and a myelogenous leukemia cell line.31
Despite the possible multiplicity of extracellular and intrinsic factors that exist in different tissues, some aspects of the TG-2-paxillin relationship24 may be evolutionarily conserved. If so, this commentary addresses a fundamental issue, which will likely impact adhesion and cellular behavior in biology.
Hypothesis
Based on the initial assumption that TG-2 interacts most directly with paxillin (see above), three hypotheses (Fig. 3) can be proposed to explain the relationship between paxillin and TG-2 in this recent work.24 Since differential biochemical roles of TG-2 (cross-linking and non-cross-linking) have been implicated in fibroblast-mediated wound healing,32 each of these current hypotheses involves one distinct biochemical function of TG-2. These hypotheses are not necessarily mutually exclusive.
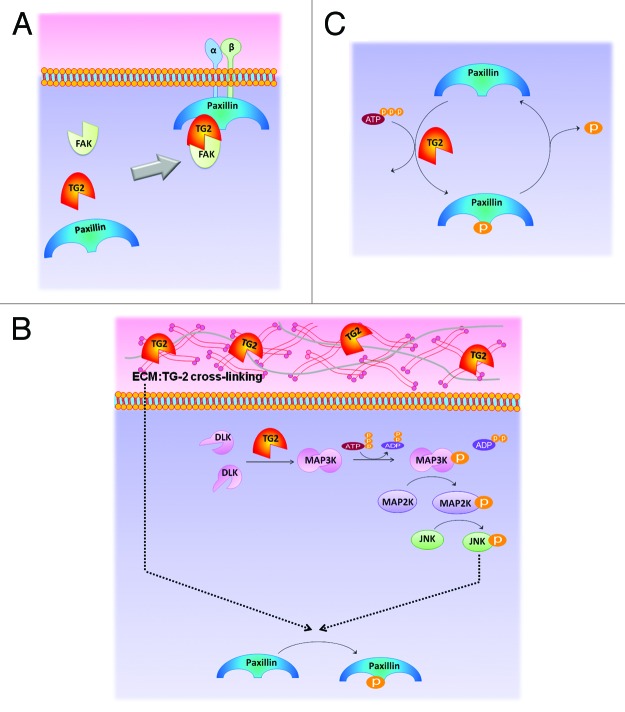
Figure 3. Diagrams illustrating the proposed hypotheses concerning transglutaminase (TG)2-paxillin Serine178 interactions. These mechanisms need not be mutually exclusive. (A) TG-2 acts as an adaptor protein, which brings various components of the adhesome into close proximity via non-covalent interactions, including paxillin to the cell surface integrins. (B) TG-2 cross-links different elements of the cell to activate phosphorylation of paxillin. First (left), upstream processes such as cross-linking of matrix and membrane proteins can subsequently signal to a kinase that phosphorylates paxillin. Alternatively, TG-2 can cross-link intracellular MAP3K and activates it. The subsequent kinase cascade in the cytoplasmic compartment results in the activation of c-Jun N-terminal kinase (JNK). JNK is targeted to focal adhesions and phosphorylates Ser178 of paxillin. (C) TG-2 may have intrinsic kinase activity, which is involved in paxillin phosphorylation.
The first hypothesis is that TG-2 acts as a scaffolding or adaptor molecule, which targets paxillin non-covalently to the focal adhesion. Via further binding to other adhesome proteins, TG-2 facilitates recruitment of these adhesome members, which may or may not be kinases. Due to the close proximity between the newly recruited molecules and paxillin, kinases present in the complex may have easier access to phosphorylate paxillin at Ser178. If this hypothesis is true, TG-2 fulfills the definition of an adhesome and should be regarded as a bona fide adhesome member.3
There have been a few studies which evaluates TG-2 as an integrin co-receptor.22,33-35 These suggest that TG-2 may serve as a “scaffolding protein” in the extracellular compartment. Although there is no direct evidence that TG-2 can act as an intracellular adaptor protein, there were at least three findings that support this possibility.
First, TG-2 mediates the activation of protein kinase Cα, leading to its binding with β1 integrins.22 Second, when TG-2 transcripts are targeted with antisense oligonucleotides, there is reduced detection of PKCα in the membrane fraction.32 Lastly, there is evidence that paxillin can bind PKC.36 Taken together, there is a possibility that TG-2 can interact with paxillin through direct or indirect translocation of kinases to the membrane.
A second hypothesis is that TG-2’s cross-linking function may be involved in paxillin phosphorylation. This can occur through upstream processes, such as crosslinking of matrix or cell membrane proteins like syndecan-4 as described above. Alternatively, TG-2 may be able to cross-link an intracellular kinase such as c-Jun N-terminal kinase (JNK). In experiments with human corneal epithelial cells, the JNK can be co-immunoprecipitated with paxillin in the absence of the JNK inhibitor (SP600125) but in the presence of this inhibitor, the JNK no longer interacts with paxillin.12
By serving as a transamidase, TG-2 oligomerises and activates dual leucine zipper kinase or DLK (MAP3K12), which then activated JNK. The activated JNK (pJNK) is therefore the immediate kinase phosphorylating paxillin. This mechanism of TG-2 on JNK has been reported in the context of cell death signaling.37,38 Consistent with this observation, silencing of TG-2 has been associated with reduced pJNK in malignancies.37,39,40
Moreover, it has been reported that JNK can localize to paxillin-containing focal adhesions in corneal epithelial cells.6,12,41 Various studies have shown that pJNK can be a kinase for Ser178 of paxillin.7,42 Our in-vitro kinase screening assay (data unpublished) with a panel of 229 kinases shows that JNK is a relatively powerful kinase for recombinant human paxillin substrate.
Lastly, TG-2 itself may be the kinase that phosphorylates paxillin. Although the kinase function of TG-2 is not well described, there are reports that described TG-2 acts as a serine/threonine kinase.15-17
One study has found TG-2 serving as a kinase that phosphorylates insulin-like growth factor binding protein (IGFBP)-3. TG-2 purified from guinea pig liver as well as recombinant human TG-2 were able to phosphorylate IGFBP-3. In contrast to the transamidase function, the intrinsic kinase activity of TG-2 could be inhibited by calcium in a concentration-dependent way.17 Another study found TG-2 to phosphorylate the transcription factor p53. Since the phosphorylation occurs at Ser15 and Ser20 of p53, which are important for interactions with its main inhibitor Mdm2, this kinase activity is important functionally.16 It is also interesting that the TG-2 can phosphorylate histone proteins H1 and H3 in chromatin preparations. For example, TG-2 phosphorylates the histone H1 at Ser10 of H3, and since this enhances acetylation, it may have further effects on the epigenetics and control of gene expression.15
Other Intricacies in the Paxillin Regulation of Cell Adhesion
Apart from the full-length paxillin called paxillin-α protein mentioned above, other members of the paxillin family also exist, e.g., Hic-5, a natural paxillin antagonist. Hic-5′s LD-domains compete with paxillin for its binding partners. Recently, a shorter form of paxillin called paxillin-δ, which lacks the LD1 domain, has been reported. It strongly localizes to focal adhesions, suppresses the tyrosine phosphorylation of full-length paxillin, and competitively inhibits integrin signaling. Tyrosine signaling downstream of the LD1 domain of paxillin-α is inhibited by paxillin-δ. Since the original Ser178 residue of paxillin-α is intact in paxillin-δ, it remains to be shown if the phospho-Ser178-dependent adhesion function is suppressed when both paxillin isoforms are present.43
Conclusion
Further research should focus on whether TG-2 binds to paxillin or another adhesion protein. If TG-2 binds paxillin directly, it is relevant to determine the specific part of TG-2 that binds paxillin as well as the specific domain of paxillin that is involved in this interaction. In addition, it may be important to investigate if conformational changes of paxillin occur due to this interaction.
It is possible that TG-2-paxillin relationships may vary in the presence of different cell substrates or tissue origins. This requires clarification in further studies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Png E, Tong L. Transglutaminase-2 in cell adhesion: All roads lead to paxillin? Cell Adhesion & Migration 2013; 7:In press
Financial Support
This research is supported by the Singapore National Research Foundation under its clinician scientist award NMRC/CSA/045/2012 administered by the Singapore Ministry of Health’s National Medical Research Council and the Singapore Ministry of Health’s National Medical Research Council under its individual research grant NMRC/1206/2009 and center grant NMRC/CG/SERI/2010.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/26344
References
- 1.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–23. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–81. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 3.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/S0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Yan DP, Ge BX. JNK regulates cell migration through promotion of tyrosine phosphorylation of paxillin. Cell Signal. 2008;20:2002–12. doi: 10.1016/j.cellsig.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Chang AY, Lin-Feng MH, Tsou WI, Chiang IH, Lai MZ. Paxillin phosphorylation by JNK and p38 is required for NFAT activation. Eur J Immunol. 2012;42:2165–75. doi: 10.1002/eji.201142192. [DOI] [PubMed] [Google Scholar]
- 8.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–90. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–45. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura K, Teranishi S, Yamauchi J, Nishida T. Role of JNK-dependent serine phosphorylation of paxillin in migration of corneal epithelial cells during wound closure. Invest Ophthalmol Vis Sci. 2008;49:125–32. doi: 10.1167/iovs.07-0725. [DOI] [PubMed] [Google Scholar]
- 13.Yiu TW. Transglutaminase and cell adhesion and migraion. Sydney: University of New South Wales, Australia; 2012. [Google Scholar]
- 14.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–56. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 15.Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ. Phosphorylation of histones by tissue transglutaminase. J Biol Chem. 2006;281:5532–8. doi: 10.1074/jbc.M506864200. [DOI] [PubMed] [Google Scholar]
- 16.Mishra S, Murphy LJ. The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem Biophys Res Commun. 2006;339:726–30. doi: 10.1016/j.bbrc.2005.11.071. [DOI] [PubMed] [Google Scholar]
- 17.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem. 2004;279:23863–8. doi: 10.1074/jbc.M311919200. [DOI] [PubMed] [Google Scholar]
- 18.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–70. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 19.Janiak A, Zemskov EA, Belkin AM. Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell. 2006;17:1606–19. doi: 10.1091/mbc.E05-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Collighan RJ, Gross SR, Danen EH, Orend G, Telci D, Griffin M. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 α5β1 integrin co-signaling. J Biol Chem. 2010;285:40212–29. doi: 10.1074/jbc.M110.123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem. 2009;284:18411–23. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telci D, Wang Z, Li X, Verderio EA, Humphries MJ, Baccarini M, Basaga H, Griffin M. Fibronectin-tissue transglutaminase matrix rescues RGD-impaired cell adhesion through syndecan-4 and beta1 integrin co-signaling. J Biol Chem. 2008;283:20937–47. doi: 10.1074/jbc.M801763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verderio E, Scarpellini A. Significance of the syndecan-4-transglutaminase-2 interaction. ScientificWorldJournal. 2010;10:1073–7. doi: 10.1100/tsw.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong L, Png E, Aihua H, Yong SS, Yeo HL, Riau A, Mendoz E, Chaurasia SS, Lim CT, Yiu TW, et al. Molecular mechanism of transglutaminase-2 in corneal epithelial migration and adhesion. Biochim Biophys Acta. 2013;1833:1304–15. doi: 10.1016/j.bbamcr.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Gayer CP, Chaturvedi LS, Wang S, Alston B, Flanigan TL, Basson MD. Delineating the signals by which repetitive deformation stimulates intestinal epithelial migration across fibronectin. Am J Physiol Gastrointest Liver Physiol. 2009;296:G876–85. doi: 10.1152/ajpgi.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura K, Teranishi S, Yamauchi J, Nishida T. Role of JNK-dependent serine phosphorylation of paxillin in migration of corneal epithelial cells during wound closure. Invest Ophthalmol Vis Sci. 2008;49:125–32. doi: 10.1167/iovs.07-0725. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Yan D-P, Ge B-X. JNK regulates cell migration through promotion of tyrosine phosphorylation of paxillin. Cell Signal. 2008;20:2002–12. doi: 10.1016/j.cellsig.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–45. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RA, Nicholas B, Mian S, Davies PJ, Griffin M. Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerisation of fibronectin. J Cell Sci. 1997;110:2461–72. doi: 10.1242/jcs.110.19.2461. [DOI] [PubMed] [Google Scholar]
- 30.van Strien ME, Brevé JJ, Fratantoni S, Schreurs MW, Bol JG, Jongenelen CA, Drukarch B, van Dam AM. Astrocyte-derived tissue transglutaminase interacts with fibronectin: a role in astrocyte adhesion and migration? PLoS One. 2011;6:e25037. doi: 10.1371/journal.pone.0025037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silano M, Vincentini O, Luciani A, Felli C, Caserta S, Esposito S, Villella VR, Pettoello-Mantovani M, Guido S, Maiuri L. Early tissue transglutaminase-mediated response underlies K562(S)-cell gliadin-dependent agglutination. Pediatr Res. 2012;71:532–8. doi: 10.1038/pr.2012.4. [DOI] [PubMed] [Google Scholar]
- 32.Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci. 2004;117:3389–403. doi: 10.1242/jcs.01188. [DOI] [PubMed] [Google Scholar]
- 33.Ruggiero L, Sarang Z, Szondy Z, Finnemann SC. αvβ5 integrin-dependent diurnal phagocytosis of shed photoreceptor outer segments by RPE cells is independent of the integrin coreceptor transglutaminase-2. Adv Exp Med Biol. 2012;723:731–7. doi: 10.1007/978-1-4614-0631-0_93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SH, Lin CY, Lee LT, Chang GD, Lee PP, Hung CC, Kao WT, Tsai PH, Schally AV, Hwang JJ, et al. Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Res. 2010;30:4177–86. [PubMed] [Google Scholar]
- 35.Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem. 2009;284:16693–703. doi: 10.1074/jbc.M109.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romanova LY, Holmes G, Bahte SK, Kovalchuk AL, Nelson PJ, Ward Y, Gueler F, Mushinski JF. Phosphorylation of paxillin at threonine 538 by PKCdelta regulates LFA1-mediated adhesion of lymphoid cells. J Cell Sci. 2010;123:1567–77. doi: 10.1242/jcs.060996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park MK, Lee HJ, Shin J, Noh M, Kim SY, Lee CH. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim Biophys Acta. 2011;1811:1021–9. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Robitaille K, Daviau A, Lachance G, Couture JP, Blouin R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 2008;15:1522–31. doi: 10.1038/cdd.2008.77. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Xu X, Bai L, Chen W, Lin Y. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. J Biol Chem. 2011;286:21164–72. doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park MK, You HJ, Lee HJ, Kang JH, Oh SH, Kim SY, Lee CH. Transglutaminase-2 induces N-cadherin expression in TGF-β1-induced epithelial mesenchymal transition via c-Jun-N-terminal kinase activation by protein phosphatase 2A down-regulation. Eur J Cancer. 2013;49:1692–705. doi: 10.1016/j.ejca.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 41.Teranishi S, Kimura K, Nishida T. Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro. Invest Ophthalmol Vis Sci. 2009;50:5646–52. doi: 10.1167/iovs.08-2534. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto Y, Torii T, Yamamori N, Eguchi T, Nagao M, Nakamura K, Tanoue A, Yamauchi J. Paxillin is the target of c-Jun N-terminal kinase in Schwann cells and regulates migration. Cell Signal. 2012;24:2061–9. doi: 10.1016/j.cellsig.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial-mesenchymal transformation: a putative role for paxillin delta. J Cell Sci. 2005;118:4849–63. doi: 10.1242/jcs.02615. [DOI] [PubMed] [Google Scholar]