Abstract
In developing therapeutic alternatives to liver transplantation, we have used the strategy of applying a small intestinal segment as a scaffold for hepatocyte transplantation and also as a portocaval shunt (PCS) system to address both liver dysfunction and portal hypertension. The aim of this study was to investigate the feasibility of such an intestinal segment in animal models. Hepatocytes isolated from luciferase-transgenic Lewis rats were transplanted into jejunal segments of wild-type Lewis rats with mucosa removal without PCS application. Luciferase-derived luminescence from transplanted hepatocytes was stably detected for 30 days. Then, we performed autologous hepatocyte transplantation into the submucosal layer of an isolated and vascularized small intestinal segment in pigs. Transplanted hepatocytes were isolated from the resected left-lateral lobe of the liver. On day 7, hepatocyte clusters and bile duct-like structures were observed histologically. To create an intestinal PCS system in pigs, an auto-graft of the segmental ileum and interposing vessel graft were anastomosed to the portal vein trunk and inferior vena cava. However, thrombi were observed in vessels of the intestinal PCSs. We measured the correlation between infusion pressure and flow volume in whole intestines ex vivo in both species and found that the high pressure corresponding to portal hypertension was still insufficient to maintain the patency of the intestinal grafts. In conclusion, we demonstrated the feasibility of the small intestine as a scaffold for hepatocyte transplantation in rat and pig models, but PCS using an intestinal graft failed to maintain patency in a pig model.
Keywords: liver cirrhosis, portal hypertension, portocaval shunt, organogenesis, intestinal scaffold, hepatocyte transplantation, ectopic liver, rat, pig
Introduction
End-stage liver diseases can evoke portal hypertension (PH) with various complications, including variceal bleeding, ascites, hydrothorax, hepatic encephalopathy and hepatorenal syndrome. In current clinical settings, transjugular intrahepatic portosystemic shunt (TIPS) is a well-known portocaval shunt (PCS) that is efficacious in reducing portal venous pressure and controlling complications.1-4 However, it is sometimes associated with serious problems, such as stenosis or dislocation of the stent, resulting in recurrence or deterioration of hepatic encephalopathy and hepatic dysfunction.3,4 Orthotopic liver transplantation is the only treatment for liver failure that is both effective and consented to by a high percentage of patients, and it also contributes to improvement of PH. Nevertheless, it is limited to selected patients based on detailed criteria due to graft shortages from both deceased and living donors. It also requires recipients to have lifelong immunosuppression, which increases the risk of infection as well as medical costs both for patients and society. Therefore, much effort has been focused on developing alternative approaches using regenerative and stem cell technologies.5-9
Hepatocyte transplantation has been considered an alternative therapy to liver transplantation, and it involves minimally invasive procedures.10-12 Yet it still has drawbacks such as poor engraftment of transplanted hepatocytes, pulmonary embolization of infused hepatocytes, portal vein (PV) thrombosis and PH. Thus, transplantation into extrahepatic sites has been considered.13,14
Transplanted hepatocytes can survive for variable periods in multiple heterologous sites, such as the sp.leen and peritoneal cavity.14,15 We have been focusing on small intestinal segments with denudation of the mucosa, whose submucosal layer can maintain transplanted tissues such as liver microfragments and fetal pancreas.13,16,17 In our previous work, we demonstrated a PCS system using a small intestinal segment in rats.7 Herein, we focused on the use of the small intestine as a scaffold for hepatocyte transplantation as well as a PCS system, in which we propose a strategy to develop a PCS with hepatic function (Fig. 1). In this report we demonstrate hepatocyte transplantation into small intestinal segments in both rat and pig models and also evaluate the feasibility of small intestinal segments as a PCS component in a pig model.
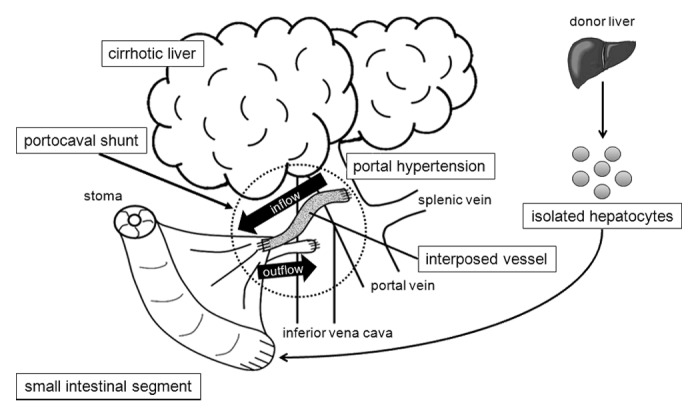
Figure 1. Schema of our strategy of small intestinal PCS with cell transplantation. The PCS is constructed using an autologous small intestinal segment. Isolated hepatocytes are transplanted into the small intestinal graft.
Results
Hepatocyte transplantation to an intestinal segment in rat and pig
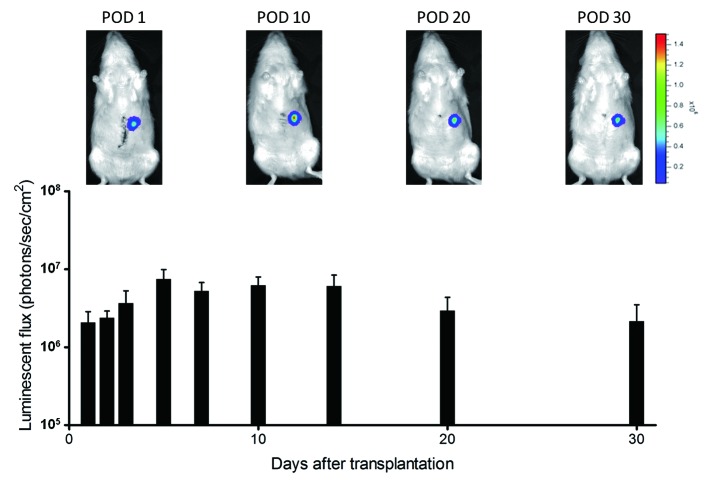
Fifteen wild-type Lewis rats underwent operational preparation of a blind segmental jejunum (2 cm long) with intact arteriovenous supply and removal of its mucosa 1 d before hepatocyte transplantation. They then received hepatectomy of the left-lateral lobe and primary hepatocytes (1 × 107 cells, mean viability 73.1 ± 3.3%) isolated from luciferase-transgenic Lewis rat into its lumen. All rats tolerated the procedure without complications. Luciferase-derived luminescence was observed for 30 d, indicating the prolonged maintenance of the transplanted hepatocytes (Fig. 2). However, no obvious hepatocyte cluster was observed histologically, and mucosa of the segmental jejunum was newly developed (data not shown).
Figure 2. Luminescence from transplanted hepatocytes in rat small intestinal segments. Luminescence was stably detected for 30 d after transplantation.
Eight Mexican hairless pigs underwent hepatectomy of the left-lateral lobe and preparation of the blind segmental ileum. Primary hepatocytes were isolated from resected liver immediately (mean viability 76.8 ± 2.9%), and 5 × 106 cells per site were transplanted into the submucosal layer of the segmental ileum during surgery. They survived for 7 d without any complication. In 3 pigs, hematoxylin and eosin (HE) staining showed that clusters of hepatocytes (Fig. 3A) and bile-duct-like structures (Fig. 3B) were in the submucosal and muscular layers of the intestine. The hepatocyte-like clusters were positive for periodic acid-Schiff (PAS) staining (Fig. 3C) and immunohistochemically positive for albumin (Fig. 3D), cytochrome P450 subtype 1A1 (CYP1A1) (Fig. 3E) and OV-6 (Fig. 3F).
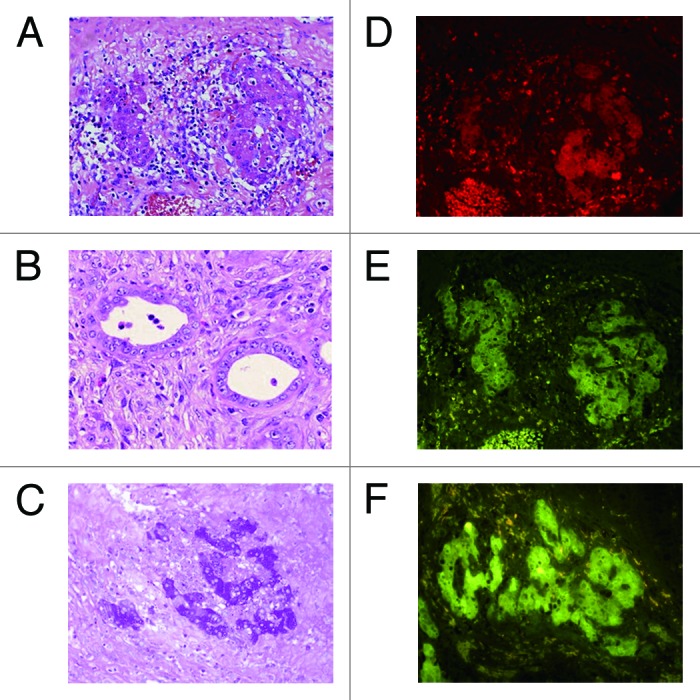
Figure 3. Histological analyses of intestinal segments in pigs. Intestinal segments were sampled on postoperative day 7. (A) Hepatocyte cluster in the submucosa (HE, x400). (B) Bile duct-like structure in the submucosa (HE, x400). (C) Glycogen in the hepatocytes (PAS, x200). (D) Immunohistochemical staining for albumin (x400). (E) Immunohistochemical staining for CYP1A1 (x400). (F) Immunohistochemical staining for OV-6 (x400).
Feasibility of ileal auto-graft as a portocaval shunt component in pig
Five pigs underwent the PCS operation. Auto-grafts of 30- to 40-cm-long segmental ileum with corresponding first branches of the mesenteric artery were isolated, and 5- to 10-cm-long interposing vessel grafts were harvested from either the sp.lenic vein or the left internal carotid artery (Fig. 4A). Under normal PV pressure, intraoperative portography suggested poor blood inflow into the ileal segment from the host’s PV. Even under high PV pressure induced by clamping the PV trunk at the hepatic hilus, patent vasculature of the engrafted ileal segment and interposed vessel graft were shown only in 1 pig by intraoperative portography (Fig. 4B). The other pigs showed some inflow from the PV into ileal segments, but no outflow to the inferior vena cava (IVC).
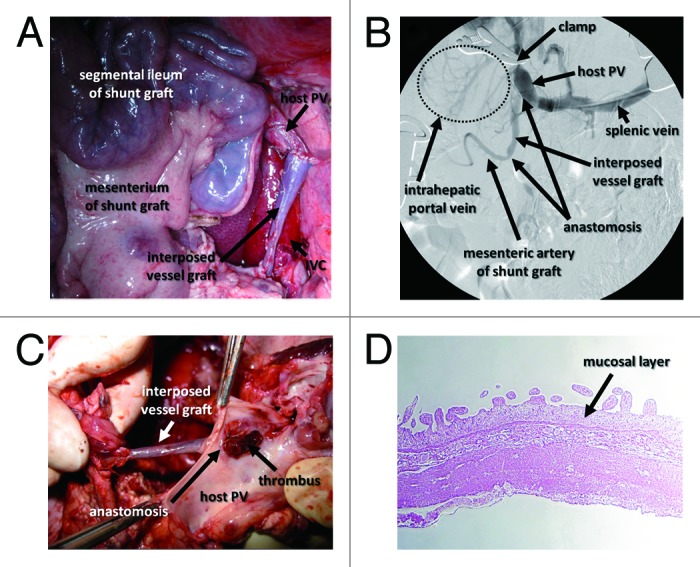
Figure 4. Intraoperative and postoperative findings of intestinal PCS in pigs. (A) Intraoperative image of the ileal graft and interposed vessel graft anastomosed to the host PV. (B) Intraoperative portography shows that temporary PH created by clamping the PV led to the visualization of the mesenteric artery of the intestinal graft. (C) The graft vessel interposed between the portal vein and small intestinal segment is occupied with a thrombus. (D) HE staining shows shortened villi and a thinned mucosal layer of the small intestinal segment.
One of the 5 pigs was in poor condition and was sacrificed on day 1. The others were sacrificed on day 7 after PCS operation. All pigs had a thrombus in the mesenteric vessels of the ileal segment or the interposed vessel grafts (Table 1 and Fig. 4C). Histological analysis showed shortened villi and a thinned mucosal layer in the ileal grafts, suggesting an ischemic influence after PCS operation (Fig. 4D).
Table 1. Operative procedure and findings of the PCS experiment in pigs.
| Number | Gender | BW (kg) |
Interposing vessel graft | Intraoperative portography* | Location of thrombus on sacrification |
|---|---|---|---|---|---|
| 1 | Female | 43.5 | Splenic vein | N/A | vein graft |
| 2 | Female | 35.5 | Splenic vein | MA: detected IVC: not detected |
MV of ileal graft |
| 3 | Female | 24.9 | Splenic vein | MA: not detected IVC: not detected |
vein graft |
| 4† | Female | 23.6 | Internal carotid artery | MA: not detected IVC: not detected |
arterial graft |
| 5 | Female | 26.8 | (-) | MA: not detected IVC: not detected |
MA of ileal graft |
Performed with clumping PV at hepatic hilus. †Euthanized on postoperative day 1 because of severe general condition. BW, body weight; MA, mesenteric artery; MV, mesenteric vein; N/A, not available.
Ex vivo perfusion analyses of the whole small intestine in rat and pig
The entire small intestine of rats and pigs was harvested and perfused using 0.9% saline under 7, 15 or 30 mmHg pressure for 1 min. The correlation between perfusion pressure applied to the small intestine and outflow volume was measured in each animal (Fig. 5). At each pressure point, the mean outflow volume standardized by intestinal weight was larger in rat entire intestines than in pig entire intestines. Outflow volumes increased with perfusion pressure in both animals, and a significant difference was observed between the outflow volumes at 15 and 30 mmHg in rat intestine. Comparing the two species, the mean outflow volume of rat intestine at 30 mmHg (10.2 ± 2.0 ml/min/100 g) was significantly larger than that of pig intestine (1.9 ± 0.6 ml/min/100 g).
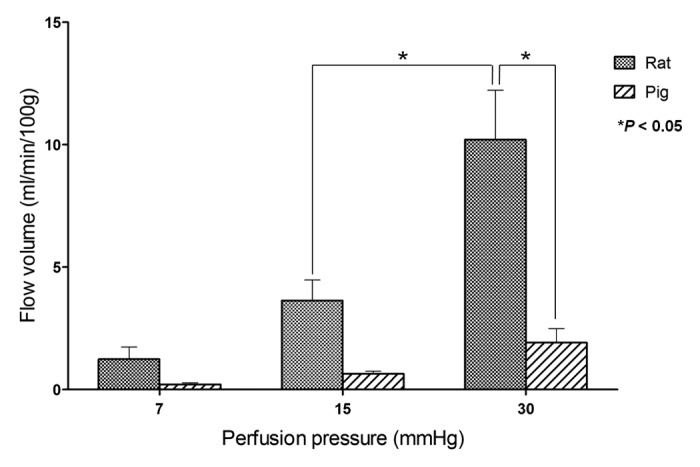
Figure 5. Relationship between flow volume and perfusion pressure in whole intestine ex vivo in rats and pigs. The mean flow volumes of the rat small intestine were larger than those of the pig under each pressure. *P value < 0.05.
Discussion
Many groups have performed hepatocyte transplantation, with various transplant sites, including liver, spleen and peritoneum.18 For clinical hepatocyte transplantation, intraportal injection is the preferred and most physiological site.19 However, PV thrombosis and subsequent PH are frequent complications of the intraportal infusion.20 In liver cirrhosis, transplanted cells cannot be delivered to the liver due to hepatofugal portal flow caused by PH, and the risk of pulmonary embolism is increased by cells migrating through portocaval collaterals.19 Moreover, cells may have difficulty surviving in cirrhotic liver, which is a severe environment with inflammatory cells, extensive apoptosis and high oxidative stress.21
The sp.leen can be used as an alternative site for hepatocyte transplantation in cirrhotic recipients. Though long-term engraftment of the hepatocytes in the sp.lenic pulp has led to marked improvement of liver function in cirrhotic animals,22 intrasplenic or intra-arterial (sp.lenic artery) injection has the risk of arterial thrombosis, sp.lenic infarction and necrosis, and severe perisplenic inflammation.23,24 The peritoneal cavity is also an attractive site because it can yield a large number of cells, but the engraftment and long-term survival rates are low.25
As an ectopic site for hepatocyte transplantation, the small intestine could be a potential scaffold. Joseph et al.13 reported the successful engraftment of liver microfragments in rats, and we have also demonstrated fetal pancreatic transplantation into the small intestine in a rat model.17 In both models, intestinal mucosa was surgically removed, and the exposed submucosal layer functioned as a transplant site. The small intestine of humans has abundant sites that might be amenable to such transplantation, and it is also advantageous under the conditions of impaired hemostasis in liver failure because the liver and sp.leen are prone to bleed massively. Therefore, we hypothesized that the vascular-rich wall of the small intestine would provide a safe scaffold for transplanted hepatocytes.
One alternative to liver transplantation is addressing the complications of liver failure. PH is an important complication that leads to a variety of diseases that impair patients’ quality of life and can cause lethal events. Radiological PCS, such as TIPS, can effectively improve PH and related disease, whereas the complications of PCS may result in aggravation of PH and liver function. Moreover, reduced detoxification of PV blood in native liver sometimes worsens encephalopathy.26 We hypothesized that a shunt component functioning as an auxiliary liver would facilitate the prevention of encephalopathy as well as improve PH. We investigated candidate organs that could act as an appropriate scaffold for liver tissue or hepatocytes as well as have a surgically transplantable vessel system as a PCS component. Because our previous work suggested the potential use of a small intestinal segment as a PCS component in a rat model,7 we investigated the possibility of using the small intestine as both the PCS component and the scaffold for transplanted hepatocytes in this paper. The scaffold function of the small intestine with intact vasculature was first verified in rat and pig models. Then, PCS installation was performed in pigs. Finally, perfusion pressure and flow volume were measured ex vivo.
As the rat model of intestinal scaffold for hepatocyte transplantation, we used luciferase transgenic rats, which ubiquitously expresses the luciferase gene,27 as a hepatocyte donor. Because the generation of luminescence depends on the cellular ATP activity and incorporation of its substrate, detectable luminescence indicated the survival of hepatocytes and a secure blood supply for 30 d after transplantation. However, we could not identify obvious hepatocyte clusters. In contrast, transplanted hepatocytes were detected in the pig intestinal wall on day 7 after transplantation. In the procedure of transplantation, although we just injected the hepatocytes into the lumen of the rat intestinal segment, cells were transplanted into certain sites of the intestinal wall in the pigs. Therefore, we could identify the injected sites at sacrifice and isolated the appropriate specimens. Moreover, the optimal period between transplantation and sampling might be different between species. However, we found OV-6-positive cells in the pig intestinal wall, suggesting hepatic clusters were included the stem cell population.28 It might be possible to induce cell proliferation from this population under certain conditions, which was not investigated in our study.
In this study, we transplanted syngeneic or autologous primary hepatocytes in both animals to avoid immunosuppression. However, an immediate application of cells after isolation might include potentially damaged cells. The current candidate cell sources include autologous and allogeneic primary hepatocytes.10-12 Isolated hepatocytes need to proliferate effectively and increase in number in vitro. One solution might be to apply allogeneic hepatocytes cryopreserved in a cell bank system, consisting of hepatocytes isolated from non-transplantable human livers and resected livers. Advances in culture methods for primary hepatocytes would also solve this issue (e.g., bioreactor options, including encapsulation technology and spherical aggregate culture systems, and humanized animal livers).9,29
In the PCS experiments, we harvested an intestinal graft of segmental ileum from a pig and transplanted it to a same pig. To secure the blood circulation in the remnant intestine, we cut the first branches of the superior mesenteric artery and vein corresponding to the segmental ileum. Unlike humans, the pig superior mesenteric artery branches into many arteries, and the first branches were quite short except in one case, resulting in insufficient length to anastomose with the PV trunk. Although we needed to interpose vessel grafts to supplement the length, these additional anastomoses might have influenced the maintenance of patency and the formation of thrombi. To confirm the patency after installation, we performed portography during the operation. We found little inflow from the PV into intestinal PCSs without clamping the PV trunk at the hepatic hilus. Because we did not induce PH surgically or pharmaceutically in this pig model, the PV pressure was insufficient to overcome the vascular resistance of the mesenteric arteries in the graft. One pig was sacrificed on day 1 due to its poor general condition, and a thrombus had already developed. The lower pressure of PV flow, dehydration and hemostatic disorder might have induced this early thrombus formation. Both macroscopic appearance and histological analysis showed no obvious necrotic lesions, but shortened villi and a thinned wall were observed. We speculate that weak blood inflow from the PV into the intestinal graft persisted for several days, whereas this inflow should be examined in samples harvested sooner after operation than was performed here.
We measured the correlation between infusion pressure and flow volume in whole intestine ex vivo in both rats and pigs. Infusion pressures of 7, 15, and 30 mmHg were used as low, normal and PH pressures, respectively. Both sp.ecies showed increasing volumes with increasing pressure, especially rat intestine. However, pig intestine, which we expected to be closer to human, showed significantly less response to 30 mmHg pressure, suggesting that it had a vessel resistance that was too high for use as a PCS component in pig and most likely human. These problems in utilizing the small intestine as a PCS component suggest that intestinal grafts as PCSs will be difficult to apply to humans under our current strategy.
The liver PV flow has a low infusion pressure. Hence, the liver could be considered a candidate for a PCS component. Recent rat and pig studies successfully transplanted the recellularized liver using decellularized scaffold.30-32 This recellularized liver graft might be transplanted with anastomoses to the PV and IVC in PH patients, like the classical application of auxiliary liver transplantation suggested by Calne et al.,33 and could be expected to function as a PCS.
Our strategy was designed for patients with PH who are waiting for or will not benefit from liver transplantation. We suppose that with more appropriate components providing the scaffolding and shunt functions, our strategy could also be applied as a “bridge therapy” to liver transplantation and thereby reduce the waiting-list mortality rate.
Conclusions
In this study, we demonstrated the feasibility of the small intestine, with intact vasculature, as a scaffold for hepatocyte transplantation in both rat and pig models. To investigate the possibility of using the small intestine as a portocaval shunt component, we performed transplantation of an auto-intestinal graft in a pig model, resulting in unsuccessful outcomes. From our ex vivo measurement of the infusion pressure and flow volume in the intestine, we concluded that small intestinal grafts are inappropriate as a shunt component in pig. We speculate the decellularized new grafts will be hopeful as a shunt component in pig.
Materials and Methods
Animals
Eight male luciferase-transgenic Lewis rats (MHC haplotype: RT1l) developed by us27 and 15 male wild-type Lewis rats (RT1l) commercially purchased from Charles River Japan were used as hepatocyte donors and recipients, respectively. Four wild-type Lewis rats were also used as the donors of whole small intestine for ex vivo perfusion analysis. All rats were 8 to 10 weeks old and weighed 200–250 g.
Female Mexican hairless pigs (10 to 15 mo old, weighing 30–40 kg) were purchased from the National Livestock Breeding Center Ibaraki Station. Eight pigs were used for autologous hepatocyte transplantation into the submucosal layer of the ileal segment. Five pigs underwent surgical installation of a PCS using an autologous ileal segment. Four pigs were also used as donors of whole small intestine for ex vivo perfusion analysis.
All animal experiments were performed in accordance with the Jichi Medical University Guide for Laboratory Animals.
Rat model of an intestinal scaffold for transplanted hepatocytes
Preparation of small intestinal segment
A day before transplantation of isolated hepatocytes, recipient rats, anesthetized with isoflurane, underwent surgical preparation of a jejunal segment as described previously.17 Briefly, 2-cm-long jejunal segments with intact arteriovenous supply were isolated. After end-to-end anastomosis of the jejunal cut-ends using 7–0 Nylon (Ethicon), the mucosa of the jejunal segment was removed with a surgical knife. Then, one end of the jejunal segment was brought to the body surface as a jejunostomy, and the other end was closed. After the operation, the rats were carefully observed until they completely recovered from anesthesia and were maintained under free access to water and food and controlled temperature.
Isolation of rat hepatocytes
Rat hepatocytes were isolated from luciferase-transgenic Lewis rats using a standard collagenase perfusion method34 with 0.05% collagenase (COLLAGENASE S-1; Nitta-gelatin). The cell suspension was centrifuged 3 times at 4 °C and 50 g for 5 min. The final concentration of the hepatocyte suspension was 1 × 107 cells/ml in Williams E medium buffer (Life Technologies).
Partial hepatectomy and transplantation of rat hepatocytes into segmental jejunum
Recipient rats, anesthetized with isoflurane, underwent hepatectomy of the left-lateral lobe to stimulate proliferation of the transplanted hepatocytes. One milliliter of hepatocyte suspension was injected into the lumen of the jejunal segment through the jejunostomy, followed by the surgical closure of the stoma with 3–0 Vicryl (Ethicon). After the operation, the recipient rats received 2.0 ml of lactate ringer solution intravenously and were observed until they completely recovered from anesthesia. They were maintained under free access to water and food and controlled temperature until sacrifice.
Evaluation of luminescence from transplanted hepatocytes
The survival of the transplanted hepatocytes was evaluated by luciferase-derived luminescence. Because luciferase-derived photon emission requires cellular ATP, luminescence reflects cellular viability and tissue expansion.27,35 To detect luminescence from luciferase-introduced hepatocytes, 30 mg/kg D-luciferin (potassium salt; Biosynth) was injected into the penile vein of recipient rats, and the photon count was detected using the non-invasive bioimaging system IVIS™ (Caliper Life Sciences) and analyzed using the software packages Igor (WaveMetrics) and IVIS Living Image (Caliper Life Sciences). The signal intensity (photon flux) was quantified as photons/sec/cm2/steradian in the region of interest.
Pig model of an intestinal scaffold for transplanted hepatocytes
Partial hepatectomy and transplantation of autologous pig hepatocytes to ileal segment without PCS
All pigs were intubated and anesthetized with isoflurane, and a veterinarian monitored them during surgical procedures. After laparotomy, the PV trunk and hepatic artery were exposed, and the left-lateral lobe of the liver was resected using electrocautery and vessel ligation. Immediately after hepatectomy, resected liver was processed for hepatocyte isolation. A 30- to 40-cm ileal segment with intact arteriovenous supply was isolated as a scaffold of hepatocyte transplantation. After the end-to-end anastomosis of the jejunal cut-ends using 3-0 Vicryl, one end of the ileal segment was brought to the body surface as an ileostomy, and the other end was closed.
Hepatocytes were isolated from the resected lobe according to a published perfusion method36 with 0.1% collagenase (COLLAGENASE S-1). The left PV was perfused using a peristaltic pump (ROLLER PUMP RP-2000; Tokyo Rikakikai), and effluents from the hepatic veins were drawn and re-circulated. The hepatic parenchymal cells were collected and purified in the same way as in the rat hepatocyte isolation procedure. The final concentration of hepatocyte suspension was 1 × 107 cells/ml in Williams E medium buffer. During the operation, the hepatocyte suspensions (0.5 ml) were slowly injected through a 20-gauge needle from outside the intestinal wall into the submucosal layer of the ileal segment. After the surgery, we removed the tracheal tube, a veterinarian monitored the pigs intensively for heart rate and body temperature, and the pigs were then housed under free access to water in a temperature-controlled cage. Pigs were infused intravenously with 2000 ml of 10% glucose solution per day until their regular diet was started on day 3.
Histological analyses of transplanted hepatocytes in ileal segments
The pigs were sacrificed on day 7, and ileal segments were sampled. Tissue samples were fixed in 10% formalin, embedded in paraffin, and cut into 4-μm-thick sections. They were stained with HE and PAS (PAS kit; Sigma-Aldrich). We also examined the tissues immunohistochemically using anti-pig albumin (RASW/ALB; Nordic Immunological Laboratories), CYP1A1 (SC-101828PE, Santa Cruz Technology) and OV-6 antibodies (SC-101863PE, Santa Cruz Technology).
Pig model of a portocaval shunt using segmental ileum and vessel graft
Preparation of ileal segment and installation of PCS without hepatectomy
After anesthetized pigs underwent laparotomy, an intravenous catheter was placed in the PV for portography. Following systemic heparinization with 1000 IU heparin sodium (Novo-Heparin), an ileal segment (30 to 40 cm long) with corresponding first branches of the mesenteric artery and vein was isolated. Intestinal integrity was restored by end-to-end anastomosis of ileum cut-ends using 3-0 Vicryl. Then, a vessel graft was obtained from either the splenic vein or the left internal carotid artery (5 to 10 cm long). Both intestinal and vessel grafts were stored in ET-Kyoto solution (Otsuka Pharmaceutical) at 4°C. Then, the vessel graft was anastomosed to the mesenteric artery of the graft with 8–0 Prolene (Ethicon) ex vivo. The anastomosed vessel graft and the mesenteric vein of the ileal graft were anastomosed in an end-to-side fashion to the host PV trunk and IVC, respectively, using 8–0 Prolene. After the anastomosis of vessels, one end of the ileal segment was brought to the body surface as an ileostomy, and the other end was closed (Fig. 1). After the surgery, pigs were infused with 2000 ml of 10% glucose solution per day until their regular diet was started on day 3. All pigs were sacrificed on day 7, except one, which was diagnosed with poor general condition and sacrificed at day 1.
Intraoperative portography and histological analysis
Intraoperative portography was performed in 4 pigs. Ten milliliters of Iopamidol (Iopamiron Inj.; Bayer HealthCare) was injected through the PV catheter, and images were captured on a monitor. To induce temporary PH, the PV trunk at the hepatic hilus was clamped by a vessel clamp. On day 7, tissue samples of the ileal segment and vessel graft were fixed in 10% formalin, embedded in paraffin, and cut into 4-μm-thick sections. They were stained with HE and analyzed morphologically.
Ex vivo analysis of perfusion pressure and volume in small intestine of rat and pig
Harvesting whole intestines from rat and pig donors
To investigate the relationship between perfusion pressure and flow volume in the small intestine, an ex vivo perfusion experiment was performed in rats and pigs. In both animal models, the entire small intestine was harvested with the vascular pedicle, consisting of the superior mesenteric artery and portal vein from anesthetized animals.
Measurement of infusion pressure and flow volume in whole small intestine
The superior mesenteric artery of the intestine was cannulated and perfused by dripped infusion with 0.9% saline under a perfusion pressure of 7, 15 or 30 mmHg for 1 min using a pressure monitor (Manometer M-382; As One). The flow volume per 100 g of small intestine under each pressure was measured.
Statistical analysis
The results are expressed as the mean values ± standard error of the mean (S.E.M). Statistical analysis was performed using the Mann-Whitney U test and one-way analysis of variance. p < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism ver.5 (GraphPad Software).
Glossary
Abbreviations:
- PCS
portocaval shunt
- PH
portal hypertension
- TIPS
transjugular intrahepatic portosystemic shunt
- PV
portal vein
- IVC
inferior vena cava
- HE
hematoxylin and eosin
- PAS
periodic acid-Schiff
Disclosure of Potential Conflicts of Interest
EK is a chief scientific advisor to Otsuka Pharmaceutical Factory Inc.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25968
References
- 1.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK., Jr. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746–55. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 2.Corbett C, Mangat K, Olliff S, Tripathi D. The role of Transjugular Intrahepatic Portosystemic Stent-Shunt (TIPSS) in the management of variceal hemorrhage. Liver Int. 2012;32:1493–504. doi: 10.1111/j.1478-3231.2012.02861.x. [DOI] [PubMed] [Google Scholar]
- 3.Funes FR, Silva RdeC, Arroyo PC, Jr., Duca WJ, Silva AA, Silva RF. Mortality and complications in patients with portal hypertension who underwent transjugular intrahepatic portosystemic shunt (TIPS) - 12 years experience. Arq Gastroenterol. 2012;49:143–9. doi: 10.1590/S0004-28032012000200009. [DOI] [PubMed] [Google Scholar]
- 4.Barney EJ, Little EC, Gerkin RD, Ramos AX, Kahn J, Wong M, Kolli G, Manch R. Coated transjugular intrahepatic portosystemic shunt does not improve thrombocytopenia in patients with liver cirrhosis. Dig Dis Sci. 2012;57:2430–7. doi: 10.1007/s10620-012-2162-z. [DOI] [PubMed] [Google Scholar]
- 5.Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, Tsuruyama T, Uemoto S, Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6:e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hata T, Uemoto S, Fujimoto Y, Murakami T, Tateno C, Yoshizato K, Kobayashi E. Transplantation of engineered chimeric liver with autologous hepatocytes and xenobiotic scaffold. Ann Surg. 2013;257:542–7. doi: 10.1097/SLA.0b013e31825c5349. [DOI] [PubMed] [Google Scholar]
- 7.Hata T, Iwasaki J, Hishikawa S, Fujimoto Y, Uemoto S, Kobayashi E. Development of a portocaval shunt using a small intestinal segment in rats. Microsurgery. 2010;30:302–6. doi: 10.1002/micr.20751. [DOI] [PubMed] [Google Scholar]
- 8.Hata T, Uemoto S, Kobayashi E. Transplantable liver production plan: “Yamaton”-liver project, Japan. Organogenesis. 2013;9 doi: 10.4161/org.25760. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. Cell therapies for liver diseases. Liver Transpl. 2012;18:9–21. doi: 10.1002/lt.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. Methods Mol Biol. 2010;640:525–34. doi: 10.1007/978-1-60761-688-7_29. [DOI] [PubMed] [Google Scholar]
- 11.Pietrosi G, Vizzini GB, Gruttadauria S, Gridelli B. Clinical applications of hepatocyte transplantation. World J Gastroenterol. 2009;15:2074–7. doi: 10.3748/wjg.15.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sgroi A, Serre-Beinier V, Morel P, Buhler L. What clinical alternatives to whole liver transplantation? Current status of artificial devices and hepatocyte transplantation. Transplantation. United States, 2009:457-66. [DOI] [PubMed] [Google Scholar]
- 13.Joseph B, Berishvili E, Benten D, Kumaran V, Liponava E, Bhargava K, Palestro C, Kakabadze Z, Gupta S. Isolated small intestinal segments support auxiliary livers with maintenance of hepatic functions. Nat Med. 2004;10:749–53. doi: 10.1038/nm1057. [DOI] [PubMed] [Google Scholar]
- 14.Kusano M, Mito M. Observations on the fine structure of long-survived isolated hepatocytes inoculated into rat spleen. Gastroenterology. 1982;82:616–28. [PubMed] [Google Scholar]
- 15.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–2. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 16.Berishvili E, Liponava E, Kochlavashvili N, Kalandarishvili K, Benashvili L, Gupta S, Kakabadze Z. Heterotopic auxiliary liver in an isolated and vascularized segment of the small intestine in rats. Transplantation. 2003;75:1827–32. doi: 10.1097/01.TP.0000065297.56712.C1. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki J, Hata T, Hishikawa S, Fujimoto Y, Uemoto S, Murakami T, Kobayashi E. Use of rat segmental intestine for fetal pancreatic transplantation. Microsurgery. 2010;30:296–301. doi: 10.1002/micr.20771. [DOI] [PubMed] [Google Scholar]
- 18.Selden C, Hodgson H. Cellular therapies for liver replacement. Transpl Immunol. 2004;12:273–88. doi: 10.1016/j.trim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201–23. doi: 10.1111/j.1365-2796.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani U, Adani GL, Sanna A, Avellini C, Sainz-Barriga M, Lorenzin D, Montanaro D, Gasparini D, Risaliti A, Donini A, et al. Portal vein thrombosis after intraportal hepatocytes transplantation in a liver transplant recipient. Transpl Int. 2005;18:750–4. doi: 10.1111/j.1432-2277.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi N, Ito M, Nakamura J, Cai J, Hammel JM, Fox IJ. Hepatocyte transplantation improves liver function and prolongs survival in rats with decompensated liver cirrhosis. Transplant Proc. 1999;31:428–9. doi: 10.1016/S0041-1345(98)01691-1. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal RJ, Chen SC, Hewitt W, Wang CC, Eguchi S, Geller S, Phillips EH, Demetriou AA, Rozga J. Techniques for intrasplenic hepatocyte transplantation in the large animal model. Surg Endosc. 1996;10:1075–9. doi: 10.1007/s004649900243. [DOI] [PubMed] [Google Scholar]
- 24.Nagata H, Ito M, Shirota C, Edge A, McCowan TC, Fox IJ. Route of hepatocyte delivery affects hepatocyte engraftment in the spleen. Transplantation. 2003;76:732–4. doi: 10.1097/01.TP.0000081560.16039.67. [DOI] [PubMed] [Google Scholar]
- 25.Pilichos C, Perrea D, Demonakou M, Preza A, Donta I. Management of carbon tetrachloride-induced acute liver injury in rats by syngeneic hepatocyte transplantation in spleen and peritoneal cavity. World J Gastroenterol. 2004;10:2099–102. doi: 10.3748/wjg.v10.i14.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggio O, Nardelli S, Moscucci F, Pasquale C, Ridola L, Merli M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16:133–46. doi: 10.1016/j.cld.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Hakamata Y, Murakami T, Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation. 2006;81:1179–84. doi: 10.1097/01.tp.0000203137.06587.4a. [DOI] [PubMed] [Google Scholar]
- 28.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher JE, Lillegard JB, McKenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl. 2013;19:328–35. doi: 10.1002/lt.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, Itano O, Kawachi S, Tanabe M, Coudriet GM, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22:231–42. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11–25. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–6. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 34.Enosawa S, Suzuki S, Li XK, Okuyama T, Fujino M, Amemiya H. Higher efficiency of retrovirus transduction in the late stage of primary culture of hepatocytes from nontreated than from partially hepatectomized rat. Cell Transplant. 1998;7:413–6. doi: 10.1016/S0963-6897(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X-D, Tokiwa T, Kano J, Kodama M. Isolation and primary culture of adult pig hepatocytes. Methods Cell Sci. 1998;19:277–84. doi: 10.1023/A:1009795728288. [DOI] [Google Scholar]