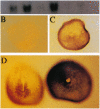
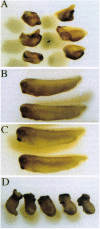
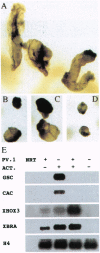
Abstract
The formation of ventral mesoderm has been traditionally viewed as a result of a lack of dorsal signaling and therefore assumed to be a default state of mesodermal development. The discovery that bone morphogenetic protein 4 (BMP4) can induce ventral mesoderm led to the suggestion that the induction of the ventral mesoderm requires a different signaling pathway than the induction of the dorsal mesoderm. However, the individual components of this pathway remained largely unknown. Here we report the identification of a novel Xenopus homeobox gene PV.1 (posterior-ventral 1) that is capable of mediating induction of ventral mesoderm. This gene is activated in blastula stage Xenopus embryos, its expression peaks during gastrulation and declines rapidly after neurulation is complete. PV.1 is expressed in the ventral marginal zone of blastulae and later in the posterior ventral area of gastrulae and neurulae. PV.1 is inducible in uncommited ectoderm by the ventralizing growth factor BMP4 and counteracts the dorsalizing effects of the dominant negative BMP4 receptor. Overexpression of PV.1 yields ventralized tadpoles and rescues embryos partially dorsalized by LiCl treatment. In animal caps, PV.1 ventralizes induction by activin and inhibits expression of dorsal specific genes. All of these effects mimic those previously reported for BMP4. These observations suggest that PV.1 is a critical component in the formation of ventral mesoderm and possibly mediates the effects of BMP4.
Full text
PDF

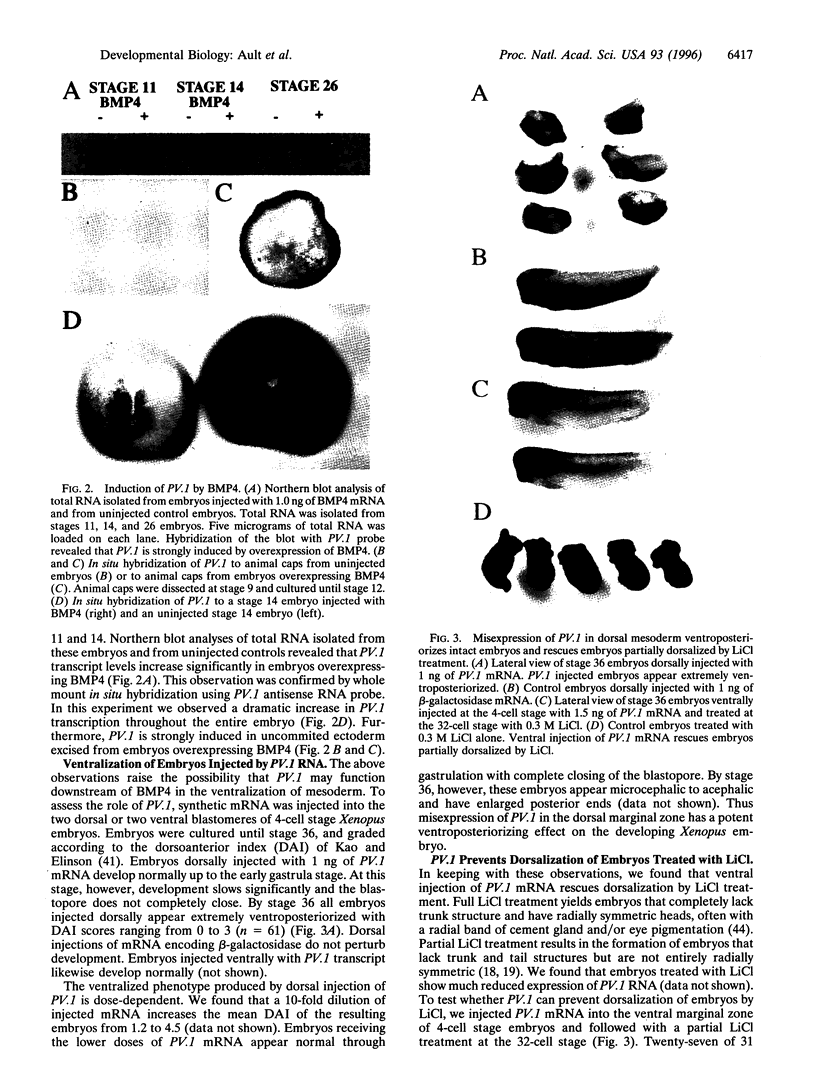


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Wright C. V., De Robertis E. M., Cho K. W. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991 Jul 12;253(5016):194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Moon R. T. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993 Jan;7(1):13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe V., Smith J. C. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature. 1992 Jul 30;358(6385):427–430. doi: 10.1038/358427a0. [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987 Apr;99(4):527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987 Jun;100(2):279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Steinbeisser H., De Robertis E. M. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994 Nov 1;13(21):5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawantka V., Delius H., Hirschfeld K., Blumenstock C., Niehrs C. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 1995 Dec 15;14(24):6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gont L. K., Steinbeisser H., Blumberg B., de Robertis E. M. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993 Dec;119(4):991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Kuehn M. R., Hogan B. L., Smith J. C., Wright C. V. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995 Nov;121(11):3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988 May;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Peptide growth factors and the regulation of early amphibian development. Biochim Biophys Acta. 1993 Aug 23;1155(2):227–237. doi: 10.1016/0304-419x(93)90006-x. [DOI] [PubMed] [Google Scholar]
- Mathers P. H., Miller A., Doniach T., Dirksen M. L., Jamrich M. Initiation of anterior head-specific gene expression in uncommitted ectoderm of Xenopus laevis by ammonium chloride. Dev Biol. 1995 Oct;171(2):641–654. doi: 10.1006/dbio.1995.1311. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Christian J. L. Competence modifiers synergize with growth factors during mesoderm induction and patterning in Xenopus. Cell. 1992 Nov 27;71(5):709–712. doi: 10.1016/0092-8674(92)90545-n. [DOI] [PubMed] [Google Scholar]
- Moos M., Jr, Wang S., Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995 Dec;121(12):4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Steinbeisser H., De Robertis E. M. Mesodermal patterning by a gradient of the vertebrate homeobox gene goosecoid. Science. 1994 Feb 11;263(5148):817–820. doi: 10.1126/science.7905664. [DOI] [PubMed] [Google Scholar]
- Richter K., Grunz H., Dawid I. B. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Involvement of the Xenopus homeobox gene Xhox3 in pattern formation along the anterior-posterior axis. Cell. 1989 Apr 21;57(2):317–326. doi: 10.1016/0092-8674(89)90969-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Jamrich M., Dawid I. B. Cell interactions and the control of gene activity during early development of Xenopus laevis. Dev Biol. 1986 Mar;114(1):238–246. doi: 10.1016/0012-1606(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995 Jul 27;376(6538):333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. E., Suzuki A., Ueno N., Kimelman D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995 May;169(1):37–50. doi: 10.1006/dbio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Simeone A., Gulisano M., Acampora D., Stornaiuolo A., Rambaldi M., Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992 Jul;11(7):2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith W. C., McKendry R., Ribisi S., Jr, Harland R. M. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995 Jul 14;82(1):37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Taira M., Otani H., Saint-Jeannet J. P., Dawid I. B. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994 Dec 15;372(6507):677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- Wilson P. A., Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995 Jul 27;376(6538):331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Zaraisky A. G., Lukyanov S. A., Vasiliev O. L., Smirnov Y. V., Belyavsky A. V., Kazanskaya O. V. A novel homeobox gene expressed in the anterior neural plate of the Xenopus embryo. Dev Biol. 1992 Aug;152(2):373–382. doi: 10.1016/0012-1606(92)90144-6. [DOI] [PubMed] [Google Scholar]
- von Dassow G., Schmidt J. E., Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev. 1993 Mar;7(3):355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]