Abstract
Green tea polyphenols are chemopreventive in several cancer models but their use as adjunctive therapeutic agents for cancer is unknown. Cholangiocarcinomas respond poorly to chemotherapeutic agents, and our aims were to assess the utility of green tea polyphenols as adjuncts to chemotherapy for cholangiocarcinoma. We assessed the effect of purified green tea catechins on chemotherapy-induced apoptosis in KMCH, CC-LP-1 and Mz-ChA-1 human cholangiocarcinoma cells. Epigallocatechin-gallate (EGCG), but not the structurally related catechin epigallocatechin, sensitized cells to apoptosis induced by gemcitabine, mitomycin C, or 5-fluorouracil in vitro. Mitochondrial membrane depolarization, cytosolic cytochrome C expression and apoptosis were increased in cells incubated with EGCG and gemcitabine compared to either agent alone. Furthermore, EGCG decreased in vivo growth and increased the sensitivity to gemcitabine of Mz-ChA-1 cells xenografts in nude mice. In conclusion, the green tea polyphenol EGCG sensitizes human cholangiocarcinoma cells to chemotherapy-induced apoptosis and warrants evaluation as an adjunct to chemotherapy for the treatment of human cholangiocarcinoma.
Keywords: adjunct therapy, biliary tract cancers, green tea, chemotherapy, drug sensitivity
Introduction
Malignant tumors arising from the epithelial lining of the biliary tract, or cholangiocarcinomas, are rare but of considerable importance given their increasing incidence worldwide [18,26-27,33]. These cancers have a poor prognosis. In part, this is related to their late presentation, when curative surgery may not be possible. Unfortunately, the tumors are highly refractory to conventional chemotherapeutic agents and therefore improved treatments for this tumor are urgently needed.
Epidemiological studies showing an inverse association between increased green tea intake and relative risk for cancer suggest that green tea may be useful as a therapeutic agent for cancer [3,13]. Although their role as therapeutic agents for cancer remains undefined, green tea polyphenols have been shown to be chemopreventive for several different cancers. A green-tea derived compound, polyphenon E is currently in Phase I/II clinical trials for chemoprevention and treatment of several cancers. Moreover, green tea extracts are readily available, and often touted and promoted for a variety of health reasons, including many liver diseases [40].
Green tea polyphenols account for 30% of dry weight of green tea, and consist of several diverse catechins of which the most abundant is epigallocatechin-gallate (EGCG). EGCG has been extensively studied and has been reported to be chemopreventive for many different cancers such as liver, prostate, stomach, esophagus, colon, pancreas, bladder, skin, lung, and breast. In addition, EGCG has chemopreventive effects in carcinogenesis induced by UV light, chemical agents and genetic aberrations [19]. These observations suggest that EGCG may be potentially useful for the prevention or treatment of cancer, but this remains to be formally established.
Because of the growing literature on the potential benefits of green tea extracts and their ready availability, it is likely that they may be used by patients diagnosed with cancers seeking to improve their survival. However, the impact of green tea constituents on the growth or treatment of cholangiocarcinoma is unknown. Although published studies suggest that green tea extracts may be useful as adjunct treatments, the contributions of individual green tea constituent catechins to these effects are unclear. Our aims were to assess the effect of green tea polyphenols on chemotherapeutic responses in human cholangiocarcinoma cells to provide a rational basis for their use as adjuncts in the treatment of this otherwise poorly responsive cancer. We sought to address the following questions: Are green tea catechins toxic to cholangiocarcinoma cells? Do different catechins have similar effects on cholangiocarcinoma cells? What is their effect on chemosensitivity? Can they induce or protect against chemotherapy-induced apoptosis?
Experimental Procedures
Cells
KMCH-1 human malignant cholangiocytes were obtained as previously described and cultured in Dulbecco's Modified Eagle Medium, supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic solution, and 1% sodium pyruvate[24]. CC-LP-1 cells were kindly provided by Patricia Whiteside (University of Pittsburgh, Pittsburgh, PA) and were cultured exactly as KMCH-1 cells. Mz-ChA-1 cells, human malignant cholangiocytes, were kindly provided by Dr. J.G. Fitz (University of Colorado, Denver, CO) and were cultured in CMRL 1066 medium supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% antibiotic/antimycotic solution.
IC50 determinations
Cells were plated (10,000 per well) in 96-well plates (BD biosciences, Rockville, MD) and incubated at 37°C with varying concentrations of drug ranging from 10-3 to 10-9 M. Cell viability was assessed after 72 hours using the CellTiter 96 Aqueous assay (Promega, Madison, WI) and the IC50 was determined by curve fitting using the XLFit software (IDBS, Guildford, Surrey, U.K).
Apoptosis Assay
Cells with morphological changes indicative of cell death by apoptosis were identified and quantitated by fluorescence microscopy after staining with DAPI as previously described. Fluorescence was visualized using a Nikon Eclipse TE300 inverted fluorescence microscope (Nikon Corp., Tokyo, Japan). Apoptotic nuclei were identified by condensed chromatin as well as nuclear fragmentation.
Western blot analysis
Western blot analysis was performed as previously described[34]. Cells were grown to 70-80% confluency and then lysed. Protein samples were separated on 4-12% gradient polyacrylamide gels (Invitrogen, San Diego, CA) and electro-blotted to a positively charged 0.45 μM nitrocellulose membrane (Millipore, Bedford, CA). Membranes were then incubated sequentially with primary antibody, used at a 1:1000 dilution, and then with a secondary polyclonal goat anti-rabbit immunoglobulin-peroxidase conjugate (Zymed, San Francisco, CA) at a 1:2000 dilution. Membranes were washed and then visualized using an enhanced chemiluminescence kit (LumiGLO, Cell Signaling, Beverly, MA) and quantitated by densitometry using a CCD-camera based image analyzer (ChemiImager 4000, Alpha Innotech, San Leandro, CA).
Analysis of drug interactions
Quantitation of the interactions between EGCG and gemcitabine was performed as previously described[35]. Cytotoxicity was assessed using a cell viability assay (CellTiter 96AQueous; Promega Corp., Madison, WI) and the concentration at which 50% toxicity occurred (LD50) was determined. A median-effect analysis was performed and a Combination Index (CI) was derived, based on the multiple drug effect equation of Chou and Talalay and using the Calcusyn program (Biosoft, Ferguson, MO)[7]. A CI of <1, 1 and >1 define the interactions between two agents as synergistic, additive, or antagonistic, respectively.
Mitochondrial Membrane Potential (Δym) Analysis
Alterations in Δym were analyzed using the mitochondrial membrane potential sensitive cationic dye JC-1 (Cell Technology, Mountain View, CA) and using the manufacturer's protocol. Cells were incubated with the agents at the indicated conditions, then were harvested and washed with PBS. 1 × 106 cells were then incubated with JC-1 (10 μg/ml) at 37°C for 15 minutes in the dark. Stained cells were then washed with PBS and analyzed using a fluorescence microplate reader to measure red fluorescence (excitation 530 nm, emission 620 nm) and green fluorescence (excitation 485 nm, emission 530 nm). JC-1 accumulates in mitochondria, whereupon J–aggregates form that are red fluorescent. Loss of the mitochondrial membrane potential is accompanied by a loss of mitochondrial accumulation and an increase in green fluorescence due to the monomeric cytoplasmic form of JC-1. A reduction in red:green fluorescence thus indicates loss of Δym and mitochondrial membrane depolarization.
Immunocytochemistry
Immunocytochemistry was performed as outlined in the SelectFX Alexa Fluor 488 Cytochrome C Apoptosis Detection Kit (Molecular Probes, Eugene, OR). Anti-cytochrome C primary antibody was used at a dilution of 1:1000 and applied to samples for 2 hours at room temperature. Alexa Fluor 488 goat anti-mouse secondary antibody was used at a dilution of 1:1000 and applied to samples for 30 minutes at room temperature. Slides were mounted with ProLong Gold antifade reagent with DAPI (Molecular Probes). Imaging was performed using a Nikon Eclipse TE300 inverted fluorescence microscope equipped with a CCD camera. Fluorescence intensity in cytoplasmic regions was quantitated in at least 10 cells/treatment group using the MetaMorph 6.2 (Universal Imaging Corp, Downingtown, PA).
Transient Transfections
The Bcl-2 expression plasmid was kindly provided by Dr. Junying Yuan (Boston, MA). Mz-ChA-1 cells (3 × 105) were plated in a 35-mm dish in culture media for 24 hours prior to transient transfection with control or Bcl-2 expression plasmid using TransIT-LT1 (Mirus Corp, Madison, WI) as previously described [32].
In vivo chemosensitivity
Studies were approved by the Institutional Animal Care and Use Committee. All animals received humane care according to the NIH Guide for the Care and Use of Laboratory Animals. Male athymic nu/nu mice, 8 weeks old, were obtained from Harlan Teklad (Indianapolis, IN). The mice were housed 3 per cage and fed ad libitum. Fluorescent light was controlled to provide alternate light and dark cycles of 12 h each, and temperature and humidity were controlled. Mz-ChA-1 cells (5×106 viable cells suspended in 250 μL of extracellular matrix (ECM) gel) were injected subcutaneously into the right and left flanks as previously described[21]. The size of the resulting tumors was estimated three times/week by tri-axial tumor measurements, and tumor volume was estimated using the following equation: Tumor volume (mm3) = 4/3 π [length (mm) × width (mm) × height (mm)]3. Once xenografts had formed in all mice, mice were randomly assigned to one of four groups. Group A received intraperitoneal (i.p.) injections of saline daily for 10 days. Group B received i.p. injections of EGCG (20 mg/kg) daily for 10 days. The dose of EGCG used per body weight is similar to that used in human clinical studies of EGCG. Group C received i.p injections of gemcitabine (120 mg/kg) on days 1, 4, and 7 only. Group D received i.p. injections of EGCG (20 mg/kg) daily for 10 days in addition to i.p. injections of gemcitabine (120 mg/kg) 3 hours following EGCG injections on days 1, 4, and 7 only.
Cell Proliferation Assay
Cell proliferation was measured using the CellTiter 96 AQueous assay kit (Promega, Madison, WI). Mz-ChA-1 cells were plated (10,000 cells/well) in 96-well plates (BD Biosciences, Rockville, MD) and incubated at 37°C, and cell proliferation was assessed after 72 hours as previously described [32]. The study was repeated four times, each with five replicates.
Statistical analysis
Data are expressed as the mean ± 95% confidence intervals from at least three separate experiments performed in quadruplicate, unless otherwise noted. The differences between two groups were analyzed using a two-sided Student's t test. Statistical significance was considered as p < 0.05. Statistical analyses were performed with the Graph Pad In-Stat statistical software program (Graph Pad, San Diego, CA).
Materials
CMRL 1066 and DMEM media, L-glutamine, sodium pyruvate, fetal bovine serum, and antibiotic-antimycotic solutions were from Gibco BRL (Grand Island, New York). Gemcitabine was obtained from Eli Lilly (Indianapolis, IN). Antibody to the 67 kD Laminin receptor was obtained from Abcam (Cambridge, MA), and antibody to caspase-8 and caspase-9 were obtained from New England Biolabs (Ipswich, MA). 5-Fluorouracil, camptothecin, EGCG, EGC, and all other catechins were obtained from Sigma Aldrich (St. Louis, MO). Mitomycin C and DAPI were obtained from Calbiochem (La Jolla, CA). All other reagents were of analytical grade from the usual commercial sources.
Results
Chemotherapeutic agents induce apoptosis in human cholangiocarcinoma cell lines
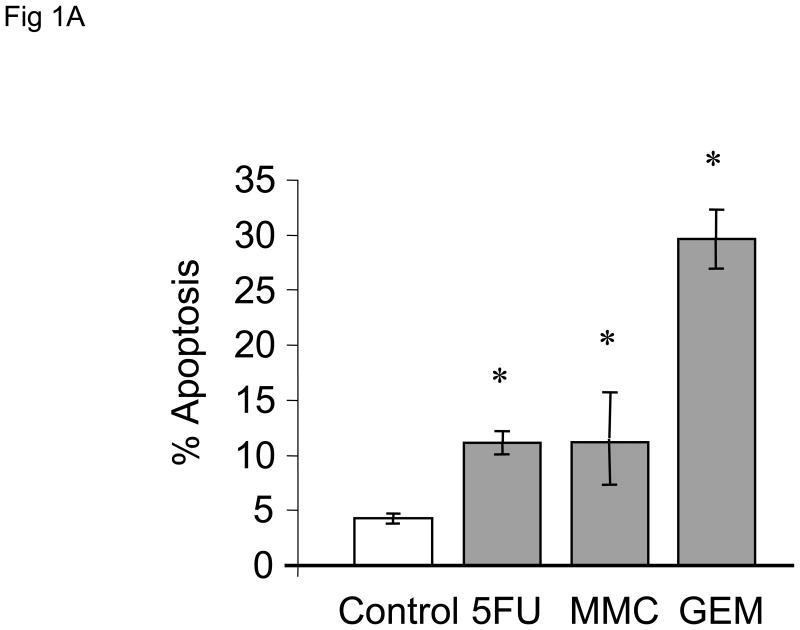
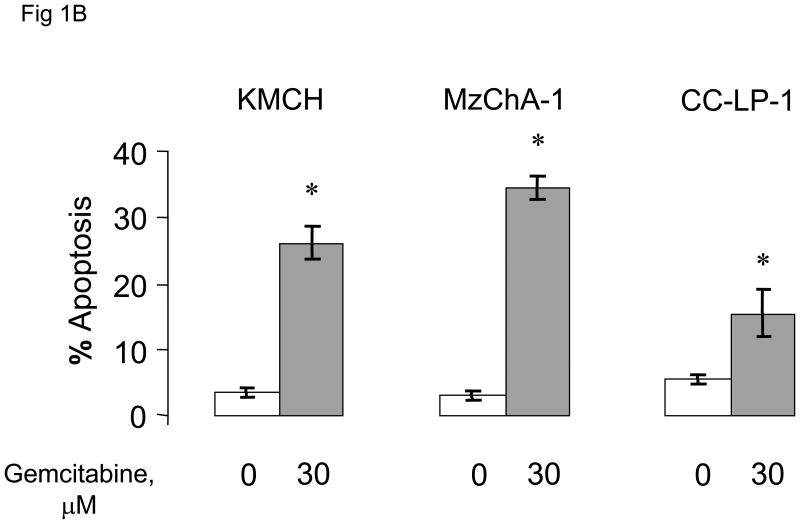
We began by assessing the effect of chemotherapeutic agents on apoptosis in human cholangiocarcinoma cell lines. First, we evaluated the effect of the chemotherapeutic agents gemcitabine, 5-fluorouracil and mitomycin C on apoptosis in Mz-ChA-1 cells derived from metastatic gall bladder cancer. All agents induced apoptosis, with the effect being greatest for gemcitabine (Fig 1A). These in vitro data parallel the clinical experience of these agents for the treatment of biliary tract cancers in which the best survival rates for therapy with a single agent has been observed with gemcitabine[8,25]. Next, we assessed the effect of gemcitabine on other cell types, CC-LP-1 and TFK-1 derived from an intrahepatic and extrahepatic biliary tract, respectively. Gemcitabine induced apoptosis to a similar extent in all cell lines (Fig 1B).
Figure 1. Chemotherapeutic agents induce apoptosis in human cholangiocarcinoma cell lines.
Panel A: The response of Mz-ChA-1 cells to diverse chemotherapeutic agents was assessed. Using a viable cell assay, the IC50 for MzChA-1 cells at 72 hours for 5-fluorouracil (5FU), mitomycin C (MMC) and gemcitabine (GEM) was determined to be 42, 51 and 28 μM respectively. Cells were incubated at their IC50 concentrations and the extent of apoptosis was assessed after 24 hours. All three chemotherapeutic agents increased apoptosis in Mz-ChA-1 cells in vitro. The data represents mean ± 95% confidence intervals of three experiments, *p < 0.05 compared to controls. Panel B: KMCH, Mz-ChA-1 and CC-LP-1 human cholangiocarcinoma cell lines were incubated in the presence or absence of gemcitabine 30 μM. Apoptosis was assessed after 48 hrs. Gemcitabine induced apoptosis in all three cell lines. The data represents mean ± 95% confidence intervals of three separate studies. * p < 0.05 compared to untreated controls.
Plant-derived catechins have diverse effects on apoptosis
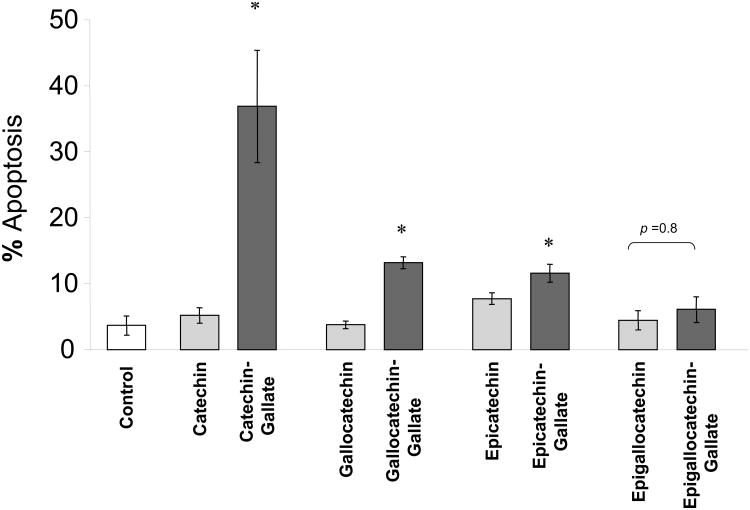
In order to assess whether the addition of green tea-derived polyphenols could modulate chemosensitivity, we initially assessed the effect of selected purified catechins and the corresponding catechin-gallates on apoptosis (Fig 2). The most abundant catechins, epigallocatechin (EGC) and epigallocatechin-gallate (EGCG), did not significantly induce apoptosis in Mz-ChA-1 cells compared to controls. Furthermore, there was no difference observed between these two compounds. In contrast, other compounds such as catechin-gallate, gallocatechin-gallate and epicatechin-gallate induced apoptosis to variable extents. Surprisingly, despite their structural similarities, significantly greater apoptosis was observed in the presence of a gallate compared to the corresponding catechin. These findings suggest that the presence of a gallate moiety can promote apoptosis. We chose EGCG for further investigation because it is the most abundant catechin in green tea, is readily available in purified form, and has been extensively investigated as a chemopreventive agent. Moreover, EGCG was not cytotoxic to non-malignant human cholangiocytes at the concentrations studied (data not shown). The fact that EGC showed very comparable apoptosis results to EGCG prompted its use as a structurally-related negative control for our studies.
Figure 2. Plant-derived catechins have diverse effects on apoptosis.
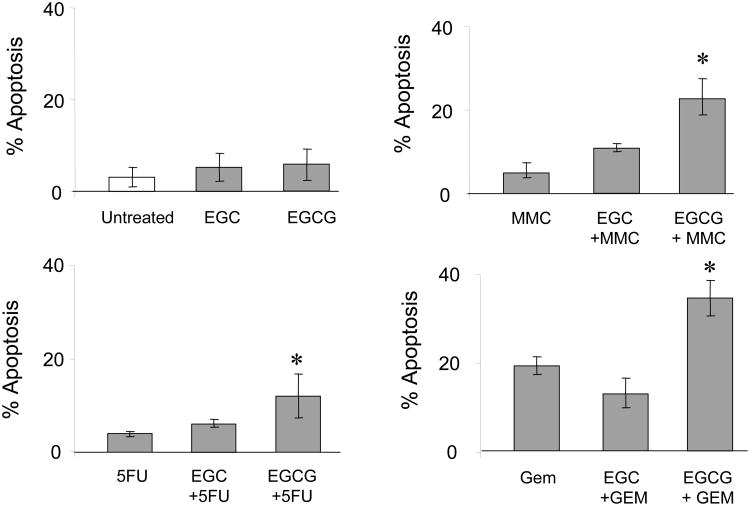
The effect of pre-incubation with several plant-derived catechins on gemcitabine-induced apoptosis was assessed in Mz-ChA-1 cells. Cells were pre-incubated with 1 μM of each catechin for 1 hour. The media was then replaced with media containing 30 μM gemcitabine. After 24 hours, the number of cells undergoing apoptosis was counted after staining with DAPI. The data represents mean ± 95% confidence intervals from 3-4 separate studies.
EGCG increases chemotherapy-induced apoptosis
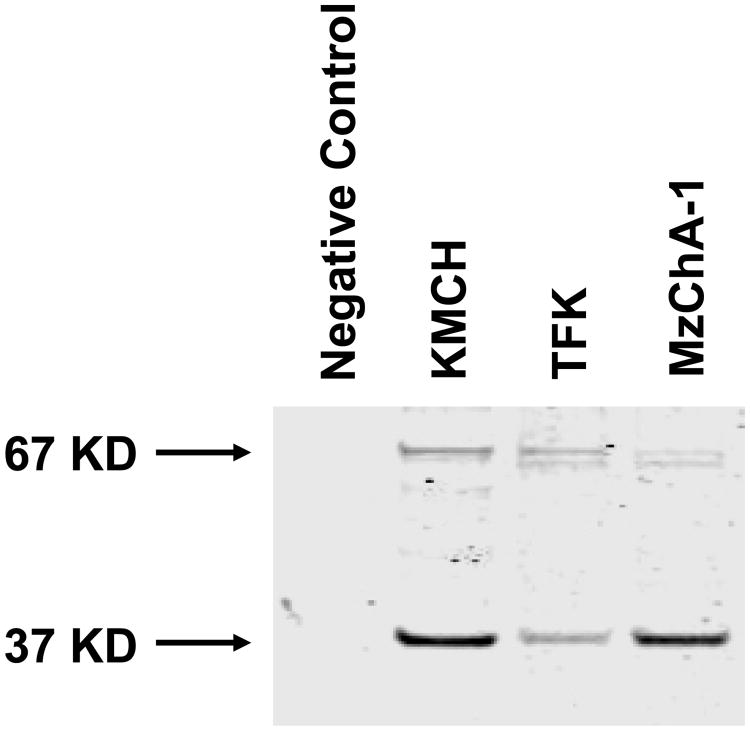
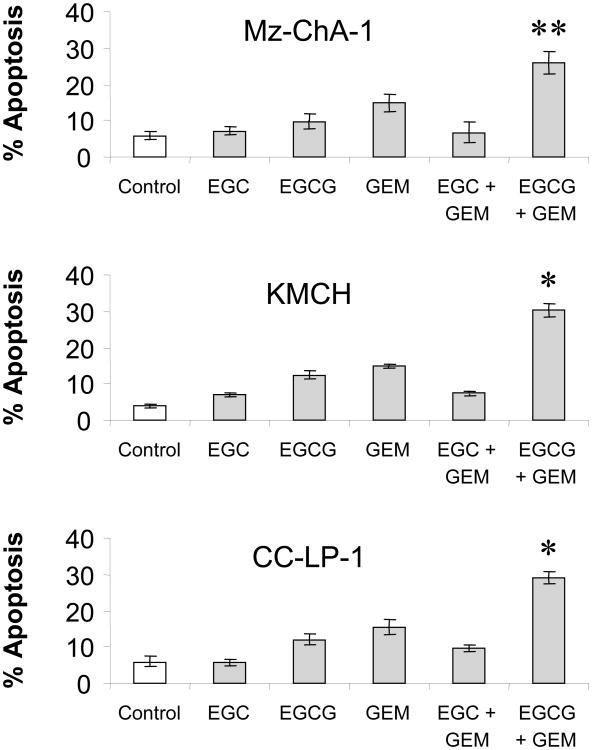
Binding to the 67-kDa laminin receptor has been shown to mediate the anti-cancer effects of EGCG [30]. We began by assessing the expression of this protein in human cholangiocarcinoma cell lines by immunoblot analysis. The laminin receptor was expressed on all three cell lines (Fig 3). Next, we assessed the effect of pre-incubation with either EGCG or EGC on chemotherapy-induced apoptosis in Mz-ChA-1 cells. Although EGCG by itself did not appreciably enhance apoptosis, EGCG significantly increased chemotherapy-induced apoptosis (Fig 4). This effect was specific for EGCG since pre-incubation with EGC did not significantly increase chemotherapy-induced apoptosis. Similar results were observed for all three agents studied - gemcitabine, 5-fluorouracil and mitomycin C. Next, we assessed if these effects also occurred in other human cholangiocarcinoma cell lines. KMCH, Mz-ChA-1, and CC-LP-1 cells were pre-incubated with catechin alone, gemcitabine alone, or the combination of catechin and gemcitabine. Similar to our observations with the Mz-ChA-1 cells, EGCG, but not EGC increased chemotherapy-induced apoptosis (Fig 5). Taken together, these data suggest that EGCG may act on a common pathway involved in modulating apoptosis rather than on a drug-specific pathway.
Figure 3. The 67kDa laminin receptor is expressed in human malignant cholangiocytes.
Lysates were obtained from human cholangiocarcinoma cells grown to near confluency. 50 μg protein was separated by SDS-PAGE electrophoresis, and immunoblotted using an antibody that recognizes both the 67kDa laminin receptor and its 37kDa precursor. Expression of the 67kDa laminin receptor was detected in all cell lines.
Figure 4. EGCG, but not EGC, increases chemotherapy-induced apoptosis.
MzChA-1 cells were pre-incubated with the plant catechins EGC (1 μM), EGCG (1 μM), or diluent controls for 1 hour prior to treatment with (A) diluent (Controls), (B) 40 μM 5-fluorouracil (5FU) (C) 50 μM Mitomycin C (MMC), or (D) 30 μM Gemcitabine (GEM). Apoptosis was assessed after 24 hrs. For all the agents, EGCG, but not EGC, increased chemotherapy-induced apoptosis. The data represents mean ± 95% confidence intervals from 4 separate studies. *p < 0.05 compared to chemotherapeutic drug alone.
Figure 5. EGCG increases gemcitabine-induced apoptosis in cholangiocarcinoma cell lines.
KMCH, MzChA-1, or CC-LP-1 cells were pre-incubated with the plant catechins EGC (1 μM), EGCG (1 μM), or diluent (control) for 1 hour prior to treatment with 30 μM gemcitabine (GEM) or diluent. Apoptosis was assessed after 24 hours. EGCG, but not EGC, increased gemcitabine-induced apoptosis in all three human cholangiocarcinoma cell lines. The data represents mean ± 95% confidence intervals from 3 studies. *p < 0.01, **p < 0.05 compared to gemcitabine alone.
We next examined the interactions between EGCG and gemcitabine on drug-induced cytotoxicity in Mz-ChA-1 cells. The nature of the interaction was quantitated by using the median effects analysis to derive a combination index. Combinations of EGCG and gemcitabine were synergistic with a CI of 0.32 ± 0.19 for gemcitabine at an effective dose for 50% cytotoxicity (ED50). Similar results were observed with EGCG, with a CI of 0.69 ± 0.17. Thus, EGCG has a synergistic effect to gemcitabine in malignant cholangiocytes in vitro.
EGCG increases gemcitabine-induced loss of mitochondrial membrane potential (MMP)
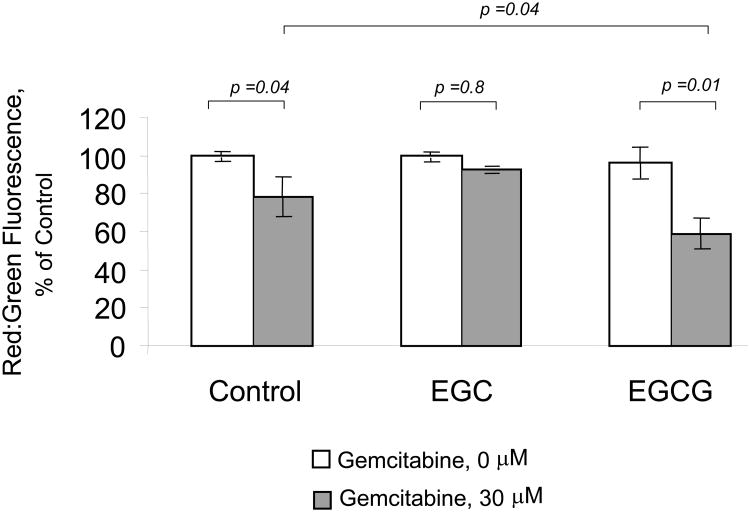
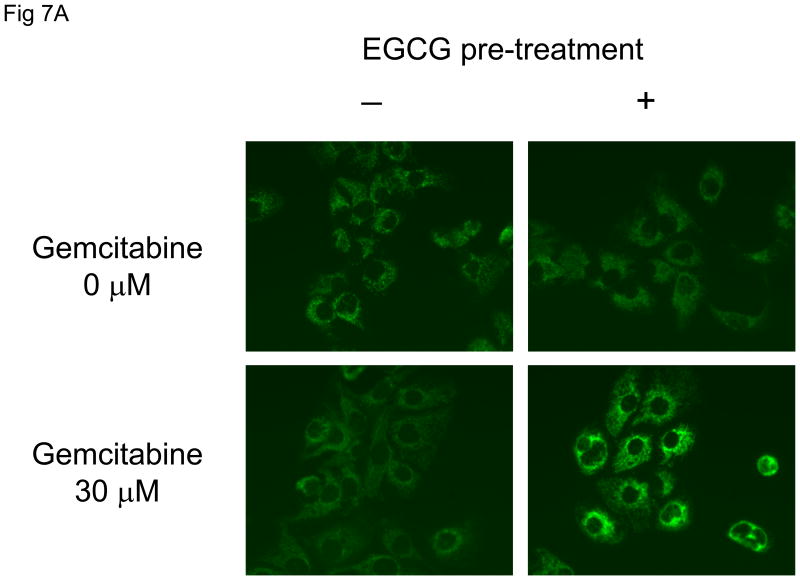
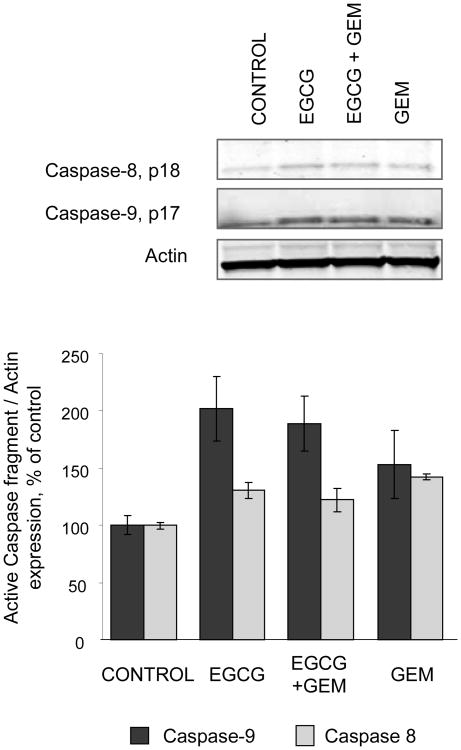
Our focus now turned to understanding the mechanism by which EGCG could enhance chemotherapy-induced apoptosis. Several non-mutually exclusive pathways leading to apoptosis have been described. We postulated that EGCG modulated the mitochondrial pathway of apoptosis, which involves mitochondrial membrane depolarization in response to an apoptosis-inducing stimulus, with subsequent release of factors such as cytochrome C that are capable of activating downstream effector caspases and inducing apoptosis. To test this hypothesis, we assessed the effect of incubation with EGCG, or EGC as a control, on loss of mitochondrial membrane potential (MMP) in response to gemcitabine. MMP was assessed as the ratio of red to green fluorescence resulting from the addition of a JC-1 dye to the cultures. A significant loss of the MMP was seen only in cells treated with the EGCG/gemcitabine combination (Fig 6). Next, we assessed the effect of EGCG and gemcitabine on cytochrome C release. Immunocytochemistry for cytochrome C was performed, and cytoplasmic cytochrome C quantitated (Fig 7). An increase in cytoplasmic cytochrome C was observed only in cells treated with the combination of EGCG and gemcitabine. Loss of cytochrome C can be inhibited by over-expression of the anti-apoptotic protein Bcl-2 which can modulate mitochondrial membrane permeabilization [5]. In Mz-ChA-1 cells transiently transfected to over-express Bcl-2, there was an inhibition of EGCG/gemcitabine cytotoxicity by 40.1 ± 9.7%. These findings indicate that EGCG can act on mitochondria to enhance cytochrome C release and thereby modulate gemcitabine-induced apoptosis. Upon release from mitochondria, cytochrome c forms a complex with pro-caspase-9 and Apaf 1 resulting in activation of procaspase-9 by cleavage to an active 35 or 17 kDa fragment. Consistent with this effect, we observed a predominant increase in expression of the 17 kDa active caspase-9 cleavage product, but not the 18 kDa active caspase-8 cleavage product by immunoblot analysis (Fig 8).
Figure 6. EGCG increases gemcitabine-induced loss of mitochondrial membrane potential.
Mz-ChA-1 cells were pre-incubated with diluent controls, 1μM EGC, or 1 μM EGCG for one hour prior to incubation with either diluent alone or 30 μM gemcitabine for 24 hours. 1 × 105 cells were then labeled with 10 μg/ml JC-1 for 30 minutes, and fluorescence measured. The loss of mitochondrial membrane potential was assessed as the change in ratio of red to green fluorescence with loss of mitochondrial membrane potential, causing an increase in green fluorescence. EGCG decreases the red:green fluorescence ratio during incubation with gemcitabine consistent with enhanced loss of mitochondrial membrane potential.
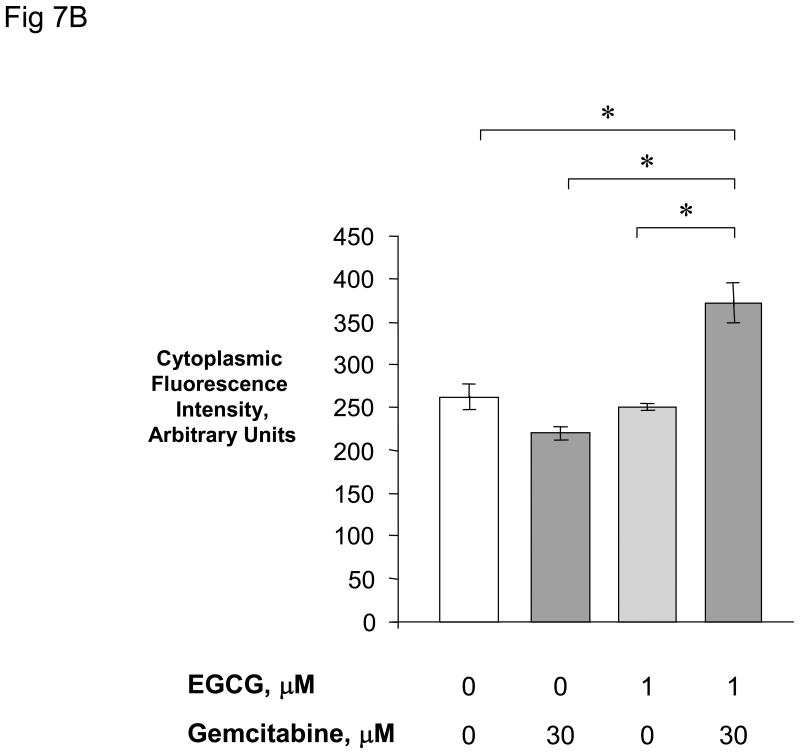
Figure 7. EGCG + gemcitabine increase cytoplasmic cytochrome C release.
Panel A: Mz-ChA-1 cells were pre-incubated for 1 hour with 1 μM EGCG or diluent control prior to treatment with either 0 or 30 μM gemcitabine for 24 hours. Immunocytochemistry was performed for cytochrome C, and images obtained by fluorescence microscopy. Panel B: The fluorescence intensity was measured in cellular cytoplasmic regions in all cells in a randomly selected field using the Metamorph 6.2 image analysis software. The data represents mean ± 95% confidence intervals from 11-13 cells in each group. *p < 0.01.
Figure 8. EGCG enhances cleavage of caspase 9.
MzChA-1 cells were incubated with 1 μM EGCG, 30 μM GEM or the combination of 1 μM EGCG and 30 μM GEM in serum free medium. After 24 hours cell lysates were obtained and evaluated for the expression of caspase 8 and caspase 9 by western blotting. Representative blots of the cleaved form of the caspases are shown along with quantitative data representing the average and the standard error of expression of active caspase cleavage products relative to the expression in control cells from four separate determinations.
EGCG decreases cholangiocarcinoma xenograft growth and enhances the sensitivity to gemcitabine
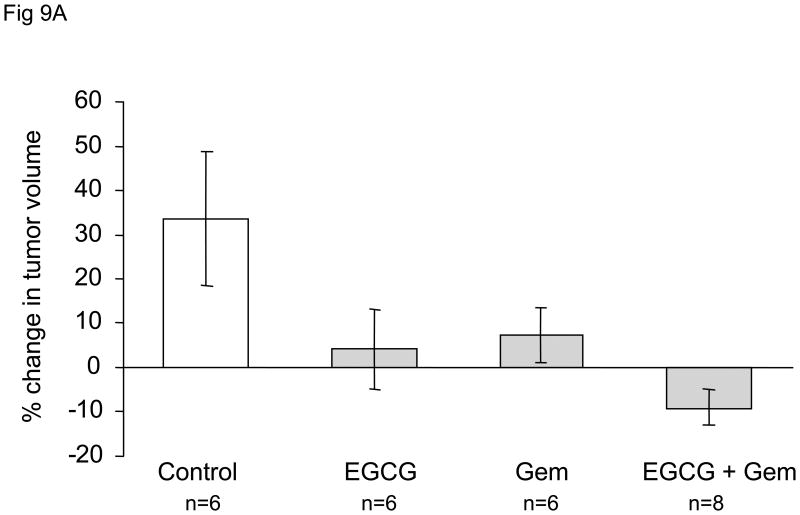
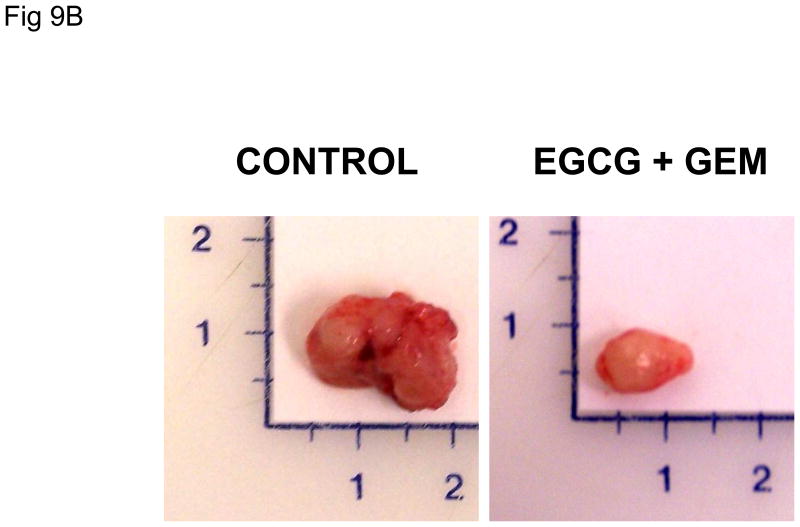
In order to evaluate these effects in vivo, we studied the effect of administration of EGCG and gemcitabine on the growth of Mz-ChA-1 cell xenografts in athymic nude mice. Once xenografts had grown to a pre-determined size, the animals received a course of saline (control group), or treatment with EGCG alone, gemcitabine alone, or the combination of EGCG and gemcitabine for 10 days. When compared to the control group, there was a significant decrease in tumor growth in each group over a two-week period (Fig 9). The combination of EGCG and gemcitabine decreased tumor growth to the greatest degree, thus corroborating our results found in vitro and supporting the hypothesis that EGCG enhances the sensitivity of Mz-ChA-1 cells to gemcitabine treatment. Of note, treatment with EGCG alone also showed a significant reduction in tumor growth, even though EGCG did not induce apoptosis or increase cytotoxicity in our in vitro studies. Incubation with 10 μM EGCG decreased Mz-ChA-1 cell proliferation in vitro by 21 ± 3% after 72 hours. Thus, it is likely that EGCG has anti-tumor effects through other mechanisms, and provides a stronger rationale for the use of EGCG as an adjunct in combination with gemcitabine for the treatment of cholangiocarcinoma.
Figure 9. EGCG decreases cholangiocarcinoma xenograft growth and enhances the sensitivity to gemcitabine.
Panel A: Nude mice with tumor cell xenografts were treated with (a) intraperitoneal (i.p.) injections of saline daily for 10 days (3 animals), (b) i.p injections of gemcitabine (120 mg/kg) every third day for 3 doses (3 animals), (c) i.p. injections of EGCG (20 mg/kg) daily for 10 days (3 animals), or (d) i.p. injections of EGCG (20 mg/kg) daily for 10 days in addition to i.p. injections of gemcitabine (120 mg/kg) 3 hours following EGCG injections on days 1, 4, and 7 only. Tumor growth was assessed as percentage change in tumor volume compared to initial volume for each tumor over a two-week period. Data represents mean ± 95% confidence intervals from 6-8 tumors for each group. * p < 0.05 compared to controls, ** p < 0.05 compared to gemcitabine alone. Panel B: Representative tumors from control or EGCG + gemcitabine-treated tumors, excised 25 days after completion of treatment.
Discussion
In this study, we have demonstrated an effect of the green tea polyphenol EGCG on enhancing apoptosis induced by chemotherapeutic agents in malignant human cholangiocarcinoma cells. Induction of apoptosis is a mechanism by which chemotherapeutic agents may exert an anti-tumoral effect. EGCG enhanced the effect of gemcitabine and, moreover, decreased the growth of cholangiocarcinoma xenografts in vivo. After oral administration, ECGC in plasma is mostly in the free form. The major route of excretion is biliary. Bioavailability is poor and oral administration of EGCG in rats resulted in recovery of 3.28% of the dose in bile. However, of this, 80.1% was free EGCG, and the remainder represented various methylated metabolites [37-39]. Although the biological activity of the methylated metabolites is unknown, cholangiocarcinoma can be exposed to EGCG in both bile and blood. These studies provide a strong rationale for proceeding to patient-based studies to evaluate and define the therapeutic utility of EGCG, and in particular its use as an adjunct to gemcitabine and other chemotherapeutic agents for the treatment of cholangiocarcinoma.
Many chemotherapeutic drugs induce apoptosis via activation of the intrinsic mitochondrial pathway, which is negatively regulated by the apoptosis regulators Bcl-2 and Bcl-XL[16]. Ample evidence exists supporting a critical role for these two anti-apoptotic molecules in mediating broad-spectrum resistance to chemotherapy. The mechanism of chemosensitization by EGCG involves modulation of apoptosis via mitochondrial membrane depolarization and release of cytochrome C. The involvement of mitochondria is additionally supported by observations that EGCG can bind to and strongly inhibit Bcl-XL, and that the effects of EGCG are decreased by over-expression of Bcl-2. Moreover, EGCG has recently been shown to directly induce apoptosis in some cancer cell lines by modulating mitochondrial release of cytochrome C. In contrast to these studies we did not observe a significant pro-apoptotic effect of EGCG alone in any of the three cholangiocarcinoma cell lines studied. Cholangiocarcinomas are highly resistant to most chemotherapeutic agents, likely due to the presence of constitutively active intrinsic survival mechanisms. The presence of such mechanisms may attenuate direct induction of apoptosis by EGCG in cholangiocarcinoma cells. Nevertheless EGCG enhances apoptosis in response to chemotherapeutic stress, suggesting that EGCG may sensitize cells and bring them closer to the tipping point at which intrinsic survival mechanisms cannot afford protection to cytotoxic agents. Although EGCG acts as an antioxidant by inhibiting lipid peroxidation or trapping reactive oxygen species, EGCG can also enhance oxidative stress by the generation of hydrogen peroxides [25]. We speculate that the relative amounts of endogenous antioxidants may contribute to direct damage to mitochondrial membranes and activation of cellular stress mechanisms by EGCG, and to the varying ability of EGCG to directly induce apoptosis. Elucidation of the precise mechanisms by which EGCG modulates mitochondrial membrane depolarization and cytochrome C release may provide new insights into mechanisms by which tumor chemosensitivity may be enhanced.
EGCG is a major component of green tea. Several green tea extracts are available over-the-counter, which contain mixtures of EGCG and other catechins. However, green tea catechins differed in their ability to induce apoptosis. For most agents, these differences reflected the presence or absence of a gallate moiety, with the catechin-gallates inducing a greater degree of apoptosis compared to the respective catechins alone. Gallic acid has been reported to induce apoptosis in several cancer cell lines by a variety of mechanisms, including generation of reactive oxygen species, and modulation of calcium influx and activation of calmodulin [29]. Thus, the differences in the ability of green tea catechins to induce apoptosis may reflect hydrolysis with the generation of gallic acid. However, neither EGC nor EGCG induced apoptosis by themselves, but differed in their chemo-sensitizing effect in cholangiocarcinoma cells. EGCG and EGC dramatically differ in their ability to bind to Bcl-XL, and the presence of the gallate group in EGCG enhances docking in the BH3 domain [20]. Thus, the differences between EGC and EGCG are more likely to reflect true structure-function effects.
While we have focused on studies to evaluate the effect of EGCG and other green tea catechins on chemotherapy-induced apoptosis, there are other potential mechanisms by which these compounds can potentiate the effects of chemotherapeutic agents. EGCG can modulate the expression of the multi-drug resistance proteins [22]. Moreover, EGCG and its metabolites are substrates for drug efflux pumps and can compete with chemotherapeutic drugs [11,12]. By saturating these efflux mechanisms, the effect of chemotherapeutic drugs can be increased. Studies to explore the mechanisms by which EGCG can decrease tumor growth in vivo are also warranted. EGCG can affect several different biological pathways [23]. The p38 MAPK signaling is aberrantly activated in cholangiocarcinoma, and inhibition of p38 MAPK signaling decreases cholangiocarcinoma growth both in vivo or in vitro [31] However, EGCG has been shown to decrease activation of p38 MAPK in some but not all studies [1,2,6]. EGCG has been shown to modulate growth-factor signaling pathways, and additionally has effects on cell-cycle progression, angiogenesis, and tumor cell invasion [15,17,23,28]. Furthermore, EGCG can inhibit DNA methyltransferase activity in protein extracts and in human cancer cell lines, and reactivate methylation silenced genes [9]. Thus, there are multiple mechanisms by which EGCG may have an anti-tumor effect.
EGCG has been extensively studied and shown to be pharmacologically safe. However, cholestasis is a common feature of biliary tract malignancies associated with obstruction and the effects of cholestasis on toxicity are unknown. Recent case reports have alerted to the possibility of acute hepatitis in patients taking supplements containing EGCG or green tea extracts [4,10,14]. Although EGCG can not be definitely incriminated due to the presence of several other compounds in these preparations, these reports emphasize the need for monitoring for potential hepatotoxicity and for Phase I studies with escalating doses of EGCG, to evaluate the safety and efficacy and to identify tolerable doses in patients with cholestasis. Chemotherapeutic regimens are limited by non-specific toxicity towards normal cells and tissues. The use of adjunct EGCG may allow lower doses of conventional chemotherapeutic agents to be used, and hopefully improve outcomes for cholangiocarcinoma by reducing toxicity and improving effectiveness.
Acknowledgments
This study was supported by the Scott & White Hospital Foundation, and Grant DK069370 from the National Institutes of Health. The technical assistance provided by Valorie Chiasson, Linda Brooks, and Janna Mize-Berge and administrative assistance provided by Melinda Freed are gratefully acknowledged.
Abbreviations
- EGCG
Epigallocatechin-gallate
- EGC
Epigallocatechin
- 5-FU
5-fluoro-uracil
- GEM
Gemcitabine
- MMC
mitomycin C
References
- 1.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-raf and NF-{kappa}B, but not PI3K, in induction of hemeoxygenase (HO-1) by dietary polyphenols. Mol Pharmacol. 2005 Dec 14; doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002 Jan 18;277(3):1828–1836. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, Chow WH, McLaughlin JK. Tea and cancer: a review of the epidemiological evidence. Eur J Cancer Prev. 1996 Dec;5(6):425–438. [PubMed] [Google Scholar]
- 4.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann Intern Med. 2006 Jan 3;144(1):68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 5.Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004 Dec;16(6):647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Dong Z, Valcic S, Timmermann BN, Bowden GT. Inhibition of ultraviolet B-induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (-)-epigallocatechin gallate in a human keratinocyte cell line. Mol Carcinog. 1999 Feb;24(2):79–84. doi: 10.1002/(sici)1098-2744(199902)24:2<79::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 8.Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005 Dec;19(12):711–716. doi: 10.1155/2005/565479. [DOI] [PubMed] [Google Scholar]
- 9.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003 Nov 15;63(22):7563–7570. [PubMed] [Google Scholar]
- 10.Gloro R, Hourmand-Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salame E, et al. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005 Oct;17(10):1135–1137. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003 Oct 10;310(1):222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002 Dec 15;62(24):7241–7246. [PubMed] [Google Scholar]
- 13.Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997 Jan 27;70(3):255–258. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Saenz M, Martinez-Sanchez MD. Acute hepatitis associated with the use of green tea infusions. J Hepatol. 2005 Dec 27; doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001 Mar 23;84(6):844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann SH, Vaux DL. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene. 2003 Oct 20;22(47):7414–7430. doi: 10.1038/sj.onc.1206945. [DOI] [PubMed] [Google Scholar]
- 17.Kazi A, Smith DM, Daniel K, Zhong S, Gupta P, Bosley ME, et al. Potential molecular targets of tea polyphenols in human tumor cells: significance in cancer prevention. In Vivo. 2002 Nov;16(6):397–403. [PubMed] [Google Scholar]
- 18.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002 Dec;37(6):806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 19.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003 Oct;133(10):3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 20.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003 Dec 1;63(23):8118–8121. [PubMed] [Google Scholar]
- 21.Marienfeld C, Tadlock L, Yamagiwa Y, Patel T. Inhibition of cholangiocarcinoma growth by tannic acid. Hepatology. 2003 May;37(5):1097–1104. doi: 10.1053/jhep.2003.50192. [DOI] [PubMed] [Google Scholar]
- 22.Mei Y, Qian F, Wei D, Liu J. Reversal of cancer multidrug resistance by green tea polyphenols. J Pharm Pharmacol. 2004 Oct;56(10):1307–1314. doi: 10.1211/0022357044364. [DOI] [PubMed] [Google Scholar]
- 23.Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004 May;62(5):204–211. doi: 10.1111/j.1753-4887.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999 Nov;30(5):1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Oh SY, Kim SH, Kwon HC, Kim JS, Jin-Kim H, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005 Feb;35(2):68–73. doi: 10.1093/jjco/hyi021. [DOI] [PubMed] [Google Scholar]
- 26.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006 Jan;3(1):33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 27.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001 Jun;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 28.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002 May 3;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, et al. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002 Aug;132(8):2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 30.Stoner GD, Casto BC. Chemoprevention by fruit phenolic compounds. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Promising Cancer Chemoprevention. Agents Totowa, New Jersey: Humana Press; 2004. pp. 419–435. [Google Scholar]
- 31.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004 Apr;11(4):380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 32.Tadlock L, Patel T. Involvement of p38 mitogen-activated protein kinase signaling in transformed growth of a cholangiocarcinoma cell line. Hepatology. 2001 Jan;33(1):43–51. doi: 10.1053/jhep.2001.20676. [DOI] [PubMed] [Google Scholar]
- 33.Tadlock L, Yamagiwa Y, Hawker J, Marienfeld C, Patel T. Transforming growth factor-{beta} inhibition of proteasomal activity. A potential mechanism of growth arrest. Am J Physiol Cell Physiol. 2003 Mar 19; doi: 10.1152/ajpcell.00550.2002. [DOI] [PubMed] [Google Scholar]
- 34.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001 Jun;48(6):816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagiwa Y, Marienfeld C, Tadlock L, Patel T. Translational regulation by p38 mitogen-activated protein kinase signaling during human cholangiocarcinoma growth. Hepatology. 2003 Jul;38(1):158–166. doi: 10.1053/jhep.2003.50257. [DOI] [PubMed] [Google Scholar]
- 36.Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytes. Life Sci. 2005 Dec 3; doi: 10.1016/j.lfs.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998 Oct;19(10):1771–6. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 38.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003 Dec;133(12):4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 39.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003 Feb-Mar;523-524:201–8. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, Zheng RH, Li YM. Green tea consumption and liver disease: a systematic review. Liver Int. 2008 Aug;28(7):990–996. doi: 10.1111/j.1478-3231.2008.01776.x. [DOI] [PubMed] [Google Scholar]