Abstract
Background
Myofascial pain dysfunction syndrome (MPDS), otherwise called myofascial pain is one of the most common temporomandibular disorders, which in turn is the most common cause of orofacial pain of non-dental origin. Its etiology is multifactorial and still poorly understood. Psychological factors have been shown to play a role in the etiology. The aim of the study was to evaluate the association between anxiety and salivary cortisol levels in patients with myofascial pain.
Methods
Twenty patients suffering from myofascial pain were recruited as the study group. The same number of age and sex matched healthy individuals were taken as the control group. The salivary samples collected between 9-9:15 am from both groups were analyzed for cortisol levels with the competitive enzyme-linked immunosorbent assay method. Anxiety levels of 40 patients were measured using Hamilton's anxiety scale.
Results
The mean serum cortisol level of the MPDS group showed a highly significant difference (p < 0.001) from the controls. The mean anxiety scores of the MPDS group showed a highly significant difference (p < 0.001) from the controls. A positive correlation was found between anxiety and the salivary cortisol levels in MPDS patients.
Conclusions
These findings suggest that anxiety plays a vital role in the etio-pathogenesis of MPDS; thus, besides pharmacological treatment, psychological support is also needed.
Keywords: anxiety, ELISA, myofascial pain, salivary cortisol
INTRODUCTION
Common conceptions of clinical pain tend to emphasize such benign disorders as headache or back pain or to focus on pain associated with cancer. It surprises many individuals to learn that TMDs are the most common chronic orofacial condition and that they affect a very sizeable proportion of the population [1]. In the craniofacial region, TMDs affect 4-12% of the population, with masticatory muscle pain often labeled as myofascial pain dysfunction syndrome (MPDS) or myofascial pain (MFP) being the most frequent (66%) patient complaint [2]. TMDs are often not restricted to the TMJ, but frequently include pain and tenderness of the masticatory muscles designated as group I in the Research Diagnostic Criteria (RDC) for TMDs [3].
Myofascial pain syndrome is a trigger point induced regional musculoskeletal pain disorder affecting one or more muscles or group of muscles [4]. A myofascial trigger point is defined as a hyperirritable spot, usually within a taut band of skeletal muscle or in the muscle fascia, which is painful on compression and can give rise to characteristic referred pain, motor dysfunction and autonomic phenomenon [4].
For more than 40 years, the contributing factors and actual underlying etiology for temporomandibular joint (TMJ) pain and myofascial pain (MFP) have been the subject of debate. Chronic pain is the most serious problem in this patient clientele, making the prevention and management of persistent pain the most important clinical challenge [5]. The etiology of MPDS is multidimensional. Biomechanical, neuromuscular, biopsychosocial, and neurobiological factors may contribute to the disorder [6]. This suggests that interventions such as cognitive behavioral therapy (CBT) targeting cognitive, emotional, and behavioral factors may be effective in modifying the expression of pain and dysfunction. For the development of such treatments, a comprehensive insight into the complex interactions between physical symptoms and psychosocial factors is essential [5].
Psychometric tests, as well as endocrine measurements, have been used to evaluate some of these factors and have sometimes linked psychological features to certain subgroups. According to biopsychosocial models, future research should emphasize both physiological and psychological factors and the relationship between them [7]. Physiological stress is known to induce various adaptational responses of physiologic systems, including increased activity in the hypothalamic-pituitary-adrenocortical (HPA) system, which promotes cortisol secretion from the adrenal cortex [8], with many bodily effects. Cortisol, also known as a stress hormone, has been used as an indicator in stress evaluation studies [9]. The assessment of cortisol in saliva has gained interest in studies for evaluating anxiety, because it provides measurement of unbound cortisol compared to serum [10]. Collecting saliva is a relatively stress free and non - invasive procedure and does not require trained personnel, and changes in environmental conditions like temperature, motion and growth of organisms do not alter the concentration of cortisol in saliva samples [10].
The present cross-sectional study aimed to conduct an investigation by evaluating the relationship between anxiety and salivary cortisol in patients with myofascial pain dysfunction syndrome (MPDS) using both a psychological testing instrument (Hamilton's anxiety scale) and a physiological testing instrument (salivary cortisol).
MATERIALS AND METHODS
The study was conducted in the department of Oral Medicine and Radiology, Kamineni Institute of Dental Sciences, India, after approval by the institutional ethics committee. The study and control population of approximately 40 subjects were considered for this study after informed consent was given. Twenty subjects fulfilling the research diagnostic criteria for temporomandibular disorders (RDC/TMD) Axis I, group I criteria [11] without any other joint symptoms and radiographic findings (11 females 55%, 9 males 45%) were recruited as the study group. Twenty age and sex matched healthy subjects were recruited as the control group. None of the above patients had any systemic disease (endocrine and metabolic) nor were they using any type of medication including corticosteroids and oral contraceptives. All patients were non-smokers. None of the patients had any type of parafunctional habits and malocclusion.
MPDS patients were diagnosed only based on clinical findings (RDC/TMD Axis I group I) and also subjected to transcranial projections to rule out any internal joint problems. Such patients were given an appointment at 9:00 AM. Saliva samples were collected for cortisol and anxiety questionnaire forms were filled.
Saliva samples from both the study and control groups were collected between 9 to 9:15 am, before their meal without stimulation by spitting method directly into a sterile glass tube. Patients were allowed to spit saliva until 5 ml were collected. All participants were asked to wash their mouth properly before sample collection. The collected salivary samples were centrifuged for 15 minutes at 3,000 rpm and frozen at -20℃ until shortly before assay. During the assay, the samples were thawed at 37℃.
Salivary cortisol was measured by competitive enzyme linked immunosorbent assay (ELISA) method, with cortisol EIA (Diametra kit, Korea). The normal cortisol concentrations that were given as a guideline according to the kit are in the range of 3-10 ng/ml in the morning time and 0.6-2.5 ng/ml in the evening collected samples.
After saliva collection, the patients were subjected to psychological evaluation. Anxiety levels were measured with the "Hamilton's anxiety scale [12]" that provides the measures of overall anxiety, psychic anxiety (mental agitation and psychological distress) and somatic anxiety (physical complaints related to anxiety).
T-test was used to compare the anxiety and salivary cortisol levels between patients with MPDS and the control group. Pearson's correlation analysis was used to study the correlation among anxiety and salivary cortisol levels in patients with MPDS. Logistic regression analysis was used to assess the variables related with MPDS. Logistic regression analysis was used to calculate each variable's independent contribution to the dependent variable. The dependent variable must always be dichotomous, as group membership. MPDS was a dependent variable, and salivary cortisol and anxiety levels were independent variables in this model.
RESULTS
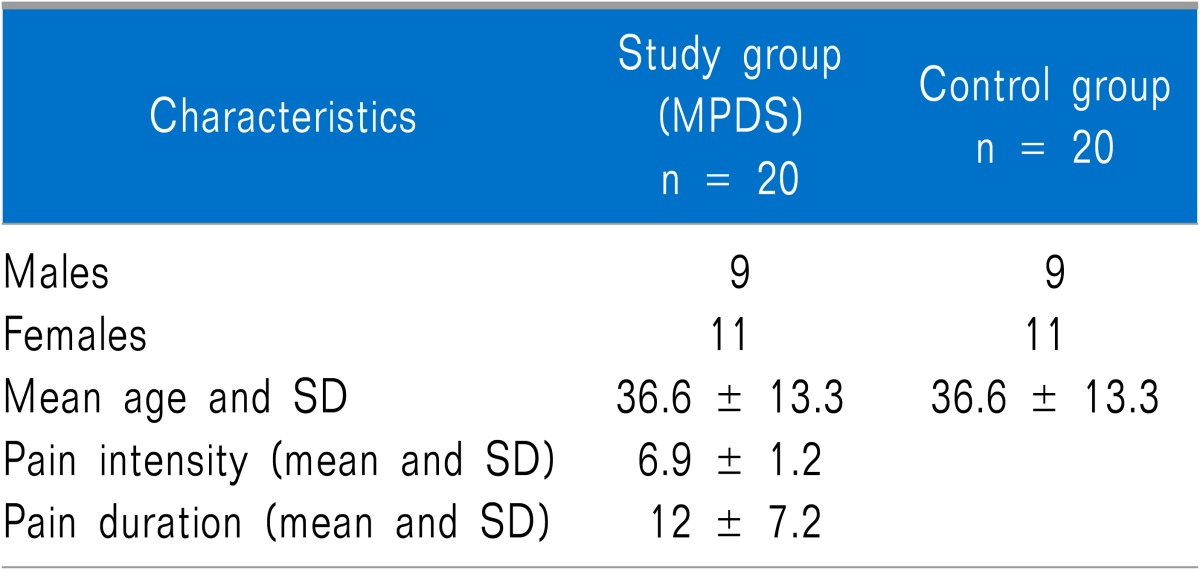
Forty patients were enrolled in this trial. The MPDS group was comprised of 9 males and 11 females and the mean age was 36.6 years (range 18-51 years). The control group was comprised of the same number of age and sex matched individuals (Table 1).
Table 1.
Demographic Data of the Study and Control Groups
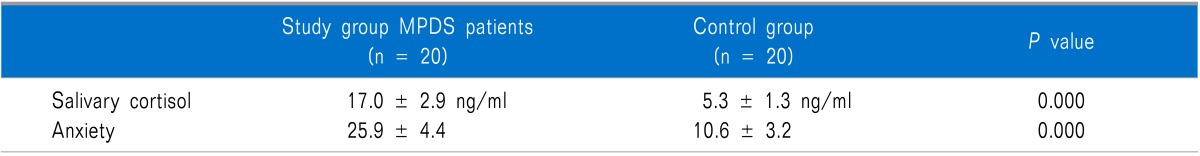
The mean salivary cortisol levels were 17.0 ± 2.9 ng/ml in the MPDS patients and 5.3 ± 1.3 ng/ml in the controls with a P value of 0.000. The mean anxiety levels in the MPDS group were 25.9 ± 4.4 and 10.6 ± 3.2 in the control group (p < 0.001) presented in Table 2.
Table 2.
Comparison of the Salivary Cortisol Levels and Anxiety Scores (Mean ± S.D.) of Patients
t-test.
We found that salivary cortisol and anxiety levels were significantly higher in the MPDS group compared to the control group. There was a highly significant positive correlation (p < 0.001) between the salivary cortisol levels and anxiety.
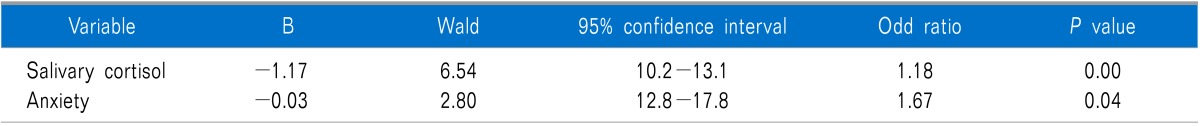
A logistic regression model in which the MPDS was taken as the dependent variable and salivary cortisol and anxiety levels were taken as the independent variables were performed. Salivary cortisol levels and anxiety scores were found significantly related with MPDS. Group membership of each individual can be predicted correctly with 89 % probability in this model (Table 3).
Table 3.
Logistic Regression Analysis, in Which the Group Membership (Myofascial Pain Dysfunction Syndrome or Control) Was the Dependent Variable
DISCUSSION
The results of this study suggest that both salivary cortisol and anxiety levels were increased in the MPDS patients compared to the control group. Literature data on TMDs clearly support the existence of an association with a number of psychosocial disorders [13]. In particular, some early works suggested that populations of patients with the muscular form of TMDs were the most compromised from a psychosocial viewpoint [14,15].
It is considered that persons exposed to stress are under increased risk of the occurrence and progression of MPDS, as stress and unpleasant life experiences are more frequent in patients with dysfunctions [16]. One of the most frequently suggested mechanisms causing myofascial pain associated with TMDs is hyperactivity of the masticatory muscles [8]. When exposed to stress, these patients respond with increased masticatory muscle activity, rather than with a general increase in body muscle tonus. Such activity whether centrally generated or peripherally manifested as parafunctional habits, or both, can result in muscular fatigue and spasm, leading to MPDS [17].
The main system in the human body associated with stress psychobiology is the HPA axis, and the major marker found in saliva associated with the HPA axis is cortisol. Cortisol activates various energy sources within the body to initiate an automatic fight or flight response [18]. This process takes place through the breakdown of proteins, conversion of amino acids and lactic acid to glucose and through the breakdown of triglycerides [19]. This activation of energy sources eventually results in fatigue, wear and tear on bodily muscles and steroid diabetes [20].
Jones et al. [7] showed a significantly higher cortisol response to experimental stress in TMDs group than the control group. Korszun et al. [21] evaluated plasma cortisol levels in patients with muscular TMDS and compared with controls concluded that increased cortisol levels were found in the muscular group of TMDs patients than in the controls. These studies are supported by another study conducted by Yoshihara et al. [8] who concluded that plasma cortisol, adrenaline, nor adrenaline were significantly higher in patients suffering from MFP than in healthy controls. Our study results also showed that anxiety and salivary cortisol levels were statistically significantly higher in MFP patients compared to the control population, matching with the results of previous studies. In contrast to these studies, Nilsson et al. [22] concluded that patients with TMDs, irrespective of diagnosis, appeared to be more psychologically distressed than controls, but did not find any corresponding differences in salivary cortisol levels.
Some studies evaluated only psychosocial profiles in TMD patients by using different questionnaires and showed the existence of a close association between pain and psychosocial disorders in TMDs patients and also showed that myofascial pain patients differ from those with other TMDs in relation to some psychopathological symptoms and MFP patients were the most compromised from a psychosocial view point compared to any other group of TMDS [5,13,15,23-25]. Our study also showed statistically significant higher anxiety levels compared to the controls matching with the results of these studies.
In conclusion, this study showed a relationship between psychological and endocrine variables in MPDS patients; therefore, it is important to take an integrated approach that covers the whole biopsychosocial spectrum. These results suggest that teamwork between clinicians, psychiatrists and dentists is needed for a successful outcome.
References
- 1.Rollman GB, Gillespie JM. The role of psychosocial factors in temporomandibular disorders. Curr Rev Pain. 2000;4:71–81. doi: 10.1007/s11916-000-0012-8. [DOI] [PubMed] [Google Scholar]
- 2.Machado LP, Nery Cde G, Leles CR, Nery MB, Okeson JP. The prevalence of clinical diagnostic groups in patients with temporomandibular disorders. Cranio. 2009;27:194–199. doi: 10.1179/crn.2009.029. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 4.Chen CK, Nizar AJ. Myofascial pain syndrome in chronic back pain patients. Korean J Pain. 2011;24:100–104. doi: 10.3344/kjp.2011.24.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reissmann DR, John MT, Wassell RW, Hinz A. Psychosocial profiles of diagnostic subgroups of temporomandibular disorder patients. Eur J Oral Sci. 2008;116:237–244. doi: 10.1111/j.1600-0722.2008.00528.x. [DOI] [PubMed] [Google Scholar]
- 6.Oral K, Bal Küçük B, Ebeoğlu B, Dinçer S. Etiology of temporomandibular disorder pain. Agri. 2009;21:89–94. [PubMed] [Google Scholar]
- 7.Jones DA, Rollman GB, Brooke RI. The cortisol response to psychological stress in temporomandibular dysfunction. Pain. 1997;72:171–182. doi: 10.1016/s0304-3959(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara T, Shigeta K, Hasegawa H, Ishitani N, Masumoto Y, Yamasaki Y. Neuroendocrine responses to psychological stress in patients with myofascial pain. J Orofac Pain. 2005;19:202–208. [PubMed] [Google Scholar]
- 9.Koray M, Dülger O, Ak G, Horasanli S, Uçok A, Tanyeri H, et al. The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis. 2003;9:298–301. doi: 10.1034/j.1601-0825.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 10.Safarzadeh E, Mostafavi F, Haghi Ashtiani MT. Determination of salivary cortisol in healthy children and adolescents. Acta Med Iran. 2005;43:32–36. [Google Scholar]
- 11.Truelove E, Pan W, Look JO, Mancl LA, Ohrbach RK, Velly AM, et al. The research diagnostic criteria for temporomandibular disorders. III: validity of Axis I diagnoses. J Orofac Pain. 2010;24:35–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Manfredini D, Marini M, Pavan C, Pavan L, Guarda-Nardini L. Psychosocial profiles of painful TMD patients. J Oral Rehabil. 2009;36:193–198. doi: 10.1111/j.1365-2842.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 14.Kight M, Gatchel RJ, Wesley L. Temporomandibular disorders: evidence for significant overlap with psychopathology. Health Psychol. 1999;18:177–182. doi: 10.1037//0278-6133.18.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Manfredini D, Bandettini di Poggio A, Cantini E, Dell'Osso L, Bosco M. Mood and anxiety psychopathology and temporomandibular disorder: a spectrum approach. J Oral Rehabil. 2004;31:933–940. doi: 10.1111/j.1365-2842.2004.01335.x. [DOI] [PubMed] [Google Scholar]
- 16.Uhac I, Kovac Z, Valentić-Peruzović M, Juretić M, Moro LJ, Grzić R. The influence of war stress on the prevalence of signs and symptoms of temporomandibular disorders. J Oral Rehabil. 2003;30:211–217. doi: 10.1046/j.1365-2842.2003.01030.x. [DOI] [PubMed] [Google Scholar]
- 17.Mercuri LG, Olson RE, Laskin DM. The specificity of response to experimental stress in patients with myofascial pain dysfunction syndrome. J Dent Res. 1979;58:1866–1871. doi: 10.1177/00220345790580090401. [DOI] [PubMed] [Google Scholar]
- 18.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 19.Tortora GJ, Grabowski SR. Introduction to the human body: the essentials of anatomy and physiology. 5th ed. New York (NY): Wiley; 2000. [Google Scholar]
- 20.Breedlove SM, Rosenzweig MR, Watson NV. Biological psychology: an introduction to behavioral, cognitive, and clinical neuroscience. 5th ed. Sunderland (MA): Sinauer Associates cop; 2007. [Google Scholar]
- 21.Korszun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81:279–283. doi: 10.1177/154405910208100411. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson AM, Dahlström L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk-related temporomandibular disorders. Acta Odontol Scand. 2010;68:284–288. doi: 10.3109/00016357.2010.494620. [DOI] [PubMed] [Google Scholar]
- 23.Yap AU, Chua EK, Hoe JK. Clinical TMD, pain-related disability and psychological status of TMD patients. J Oral Rehabil. 2002;29:374–380. doi: 10.1046/j.1365-2842.2002.00822.x. [DOI] [PubMed] [Google Scholar]
- 24.Yap AU, Dworkin SF, Chua EK, List T, Tan KB, Tan HH. Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. J Orofac Pain. 2003;17:21–28. [PubMed] [Google Scholar]
- 25.Nifosì F, Violato E, Pavan C, Sifari L, Novello G, Guarda Nardini L, et al. Psychopathology and clinical features in an Italian sample of patients with myofascial and temporomandibular joint pain: preliminary data. Int J Psychiatry Med. 2007;37:283–300. doi: 10.2190/PM.37.3.f. [DOI] [PubMed] [Google Scholar]


