Abstract
The neural cell adhesion molecule (NCAM) is a membrane-associated member of the immunoglobulin superfamily capable of both homophilic and heterophilic binding. To investigate the significance of this binding, a gene targeting strategy in embryonic stem (ES) cells was used to replace the membrane-associated forms of NCAM with a soluble, secreted form of its extracellular domain. Although the heterozygous mutant ES cells were able to generate low coat color chimeric mice, only the wild-type allele was transmitted, suggesting the possibility of dominant lethality. Analysis of chimeric embryos with high level of ES cell contribution revealed severe growth retardation and morphological defects by E8.5-E9.5. The second allele was also targeted, and embryos derived almost entirely from the homozygous mutant ES cells exhibited the same lethal phenotype as observed with heterozygous chimeras. Together, these results indicate that dominant lethality associated with the secreted NCAM does not require the presence of membrane-associated NCAM. Furthermore, the data indicate that potent bioactive cues or signals can be generated by NCAM.
Full text
PDF

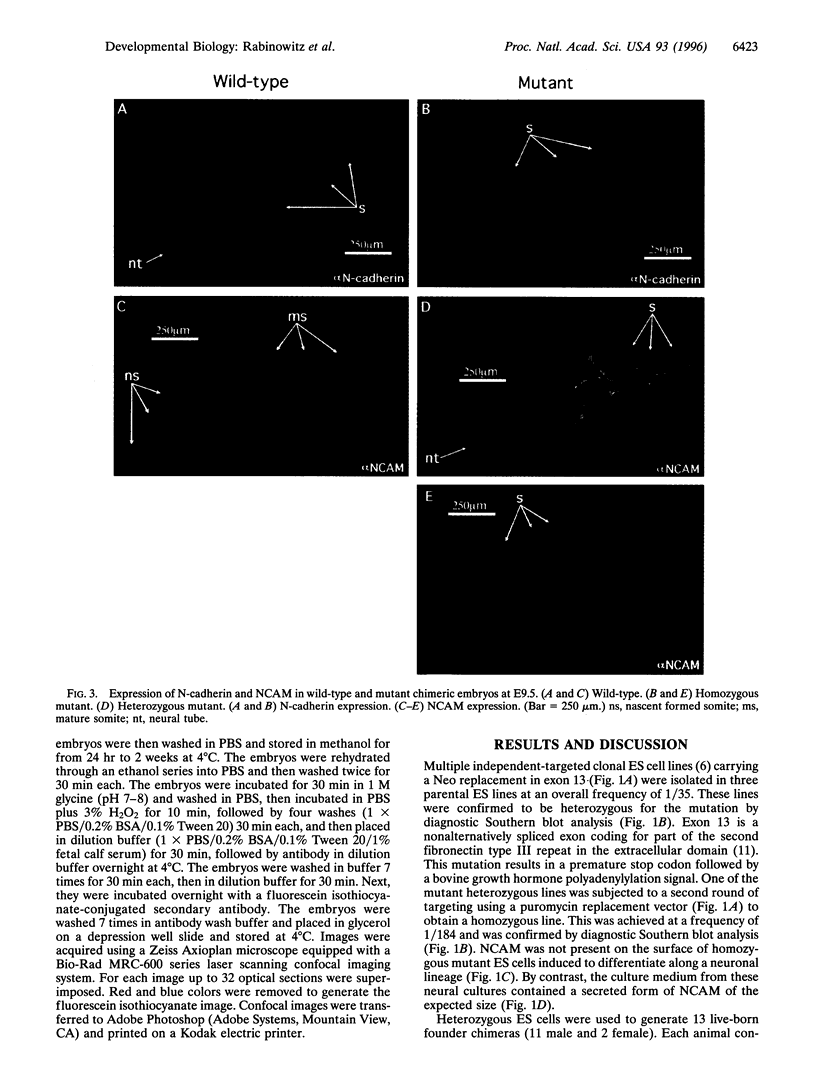

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bain G., Kitchens D., Yao M., Huettner J. E., Gottlieb D. I. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995 Apr;168(2):342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Brusés J. L., Oka S., Rutishauser U. NCAM-associated polysialic acid on ciliary ganglion neurons is regulated by polysialytransferase levels and interaction with muscle. J Neurosci. 1995 Dec;15(12):8310–8319. doi: 10.1523/JNEUROSCI.15-12-08310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. J., Akeson R. Identification of a heparin binding domain of the neural cell adhesion molecule N-CAM using synthetic peptides. Neuron. 1989 Feb;2(2):1157–1165. doi: 10.1016/0896-6273(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Loewy A., Glaser L. Neuronal cell-cell adhesion depends on interactions of N-CAM with heparin-like molecules. Nature. 1986 Apr 3;320(6061):445–447. doi: 10.1038/320445a0. [DOI] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994 Feb 3;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Duband J. L., Dufour S., Hatta K., Takeichi M., Edelman G. M., Thiery J. P. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987 May;104(5):1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander D. R., Milev P., Karthikeyan L., Margolis R. K., Margolis R. U., Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994 May;125(3):669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadmon G., Kowitz A., Altevogt P., Schachner M. The neural cell adhesion molecule N-CAM enhances L1-dependent cell-cell interactions. J Cell Biol. 1990 Jan;110(1):193–208. doi: 10.1083/jcb.110.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. Effects of altered expression of the neural cell adhesion molecule, N-CAM, on early neural development in Xenopus embryos. Neuron. 1988 Sep;1(7):545–555. doi: 10.1016/0896-6273(88)90104-3. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Sutherland L. C., Adra C. N., Leclair B., Rudnicki M. A., Jardine K. The mouse Pgk-1 gene promoter contains an upstream activator sequence. Nucleic Acids Res. 1991 Oct 25;19(20):5755–5761. doi: 10.1093/nar/19.20.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probstmeier R., Bilz A., Schneider-Schaulies J. Expression of the neural cell adhesion molecule and polysialic acid during early mouse embryogenesis. J Neurosci Res. 1994 Feb 15;37(3):324–335. doi: 10.1002/jnr.490370305. [DOI] [PubMed] [Google Scholar]
- Probstmeier R., Kühn K., Schachner M. Binding properties of the neural cell adhesion molecule to different components of the extracellular matrix. J Neurochem. 1989 Dec;53(6):1794–1801. doi: 10.1111/j.1471-4159.1989.tb09245.x. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H., Ono K., Yee D., Thompson C., Goridis C., Rutishauser U., Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993 Dec;11(6):1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Dickson G. Generation of multiple N-CAM polypeptides from a single gene. Bioessays. 1989 Oct;11(4):83–88. doi: 10.1002/bies.950110402. [DOI] [PubMed] [Google Scholar]
- Williams E. J., Furness J., Walsh F. S., Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994 Sep;13(3):583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Yagi T., Ikawa Y., Yoshida K., Shigetani Y., Takeda N., Mabuchi I., Yamamoto T., Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]





