Abstract
Background
The recently known analgesic action mechanisms of nefopam (NFP) are similar to those of anticonvulsants and antidepressants in neuropathic pain treatment. It is difficult to prescribe high doses of oral neuropathic drugs without titration due to adverse effects. Unfortunately, there are few available intravenous analgesics for the immediate management of acute flare-ups of the chronic neuropathic pain. The aim of this study was to determine the additional analgesic effects for neuropathic pain of NFP and its adverse effects during the titration of oral medications for neuropathic pain among inpatients with postherpetic neuralgia (PHN).
Methods
Eighty inpatients with PHN were randomly divided into either the NFP or normal saline (NS) groups. Each patient received a 3-day intravenous continuous infusion of either NFP with a consecutive dose reduction of 60, 40, and 20 mg/d, or NS simultaneously while dose titrations of oral medications for neuropathic pain gradually increased every 3 days. The efficacy of additional NFP was evaluated by using the neuropathic pain symptom inventory (NPSI) score for 12 days. Adverse effects were also recorded.
Results
The median NPSI score was significantly lower in the NFP group from days 1 to 6 of hospitalization. The representative alleviating symptoms of pain after using NFP were both spontaneous and evoked neuropathic pain. Reported common adverse effects were nausea, dizziness, and somnolence, in order of frequency.
Conclusions
An intravenous continuous infusion of NFP reduces spontaneous and evoked neuropathic pain with tolerable adverse effects during the titration of oral medications in inpatients with PHN.
Keywords: anticonvulsants, antidepressants, nefopam, postherpetic neuralgia, titration
INTRODUCTION
Neuropathic pain is defined as "pain arising as a direct consequence of a lesion or disease affecting the somatosensory nervous system" [1]. In cases of a new patient with acute flare-ups or exacerbation of neuropathic pain, there are few available intravenous medications for neuropathic pain so far. A neural blockage may be an answer until dose titrations of oral medications for neuropathic pain reach beyond the pain threshold. A continuous neural blockage or neural ablation after hospitalization is the next procedure for a new patient who has already received a neural blockage and is referred from another pain clinic. However, titration of oral medications for neuropathic pain for preparing the patient's discharge is also needed even after a continuous neural blockage or neural ablation. It is difficult to prescribe high doses of oral neuropathic drugs from the beginning without titration due to their common adverse effects, therefore, it takes at least three days to raise and maintain the concentration of drugs beyond the pain threshold.
Nefopam (NFP) is a non-opioid clinically potent analgesic, whose mechanism of action is not fully understood. The known analgesic action mechanisms of NFP are the inhibitions of the synaptosomal uptakes of serotonin, norepinephrine and dopamine, known as serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI), or triple reuptake inhibitor (TRI) [2]. NFP's mode of action is similar to that of antidepressants in the treatment of neuropathic pain. In addition, it also inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels [3]. In contrast, in some reports, nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission [4-8]. However, there have been few compelling human studies of NFP in the management of neuropathic pain related to its descending inhibition of pain [9]. Most studies related to NFP have focused on analgesic effects for nociceptive pain related to acute postoperative pain and a comparison of its analgesic potency with morphine [10,11]. Several recent studies have been refocused on the prevention of postoperative shivering [12,13].
The aim of this study was to determine the additional analgesic effects for neuropathic pain using continuous intravenous infusion of NFP, as well as its adverse effects during the titration of oral neuropathic medications among inpatients with postherpetic neuralgia (PHN) based on the analgesic action mechanisms of NFP.
MATERIALS AND METHODS
1. Participants
After Institutional Review Board approval was obtained, 80 inpatients with intractable PHN and in need of titration of oral medications for neuropathic pain were enrolled into this prospective, randomized, double blinded study.
2. Inclusion criteria
The enrollment took place from January 2011 through December 2012 in a pain clinic of a university hospital. Inclusion criteria were the inpatients with PHN with an initial neuropathic pain symptom inventory (NPSI) [14] over 70%, aged between 20 and 80 years, and estimated glomerular filtration rate over 60 mg/dl. PHN was defined as pain persisting beyond 120 days from rash onset [15].
3. Exclusion criteria
Exclusion criteria included patients with contraindications of NFP administration, such as a history of epilepsy, myocardial infarction, convulsion, risk of urinary retention due to urethra or prostate problems, closed angle glaucoma, monoamine oxidase inhibitors administrator, and pregnancy or breast feeding. The patients with previous oral administration for PHN over the dose of pregabalin 150 mg, nortrityline 25 mg, and tramadol 100 mg per day were also excluded in this study. Other excluded patients were those who could not understand or fill in the NPSI score.
4. Randomization-sequence generation and randomization-allocation concealment
Using a computer-generated random allocations sequence, 80 patients with PHN were randomized and assigned into 2 equal groups: a NFP group and a normal saline (NS) group.
5. Blinding (masking)
The doses of oral medications for PHN, which escalated every 3 days, were decided by the same investigator and all follow-ups were performed by another investigator. Both the participants and the care providers did not know whether intravenous drugs contained NFP or not.
6. Interventions
All 80 participants had already completed basic laboratory examination including complete blood count, liver and renal function test, and electrolyte levels before hospitalization. They also had filled out the NPSI questionnaire before hospitalization. Both groups received the same 3-day-dose escalating with pregabalin for anticonvulsants, nortrityline for antidepressants, and tramadol for weak opioids. All patients received a 3-day intravenous continuous infusion of 72 ml containing either a mixture of NFP and normal saline with a consecutive dose reduction of 60, 40, and 20 mg at a 3-day interval, or NS only.
A rescue analgesic, 10 mg of oral codeine, was given at each NPSI score VAS > 40, with a maximum limit of 5 times per day to prevent overdose.
The neuropathic pain symptom inventory (NPSI) score and adverse effects were evaluated every day (Fig. 1). Basic laboratory examinations were performed every 3 days.
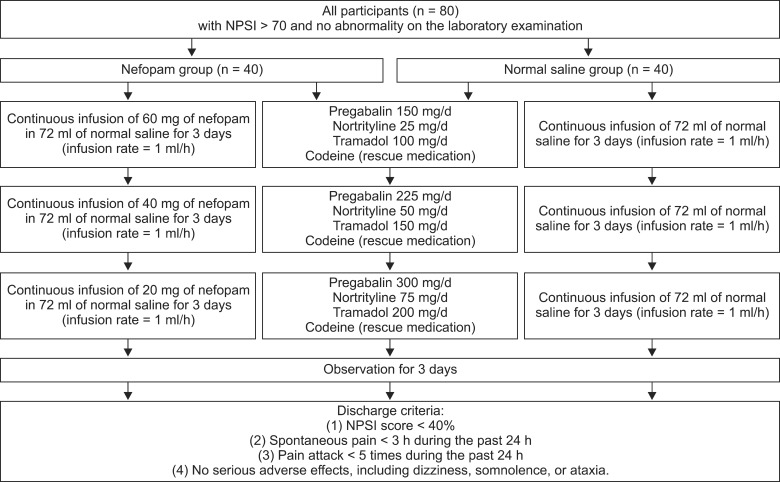
Fig. 1.
A schedule for the continuous infusion of nefopam with a consecutive dose reduction in hospitalized patients with postherpetic neuralgia while dose-escalating of oral medications. Each patient received a 3-day intravenous continuous infusion of either nefopam (NFP) with a consecutive dose reduction of 60, 40, and 20 mg/d or NS simultaneously while dose titrations of oral medications for neuropathic pain gradually increased every 3 days. A rescue analgesic, 10 mg of oral codeine, was given at each NPSI score VAS > 40 less than 5 times a day. The efficacy of additional NFP was evaluated by using the neuropathic pain symptom inventory (NPSI) score for 12 days. Adverse effects were also recorded. Discharge criteria after the 12-day-admission included: (1) NPSI score < 40%, (2) spontaneous pain < 3 h during the past 24 h, (3) pain attack < 5 times during the past 24 h, and (4) no serious adverse effects, including dizziness, somnolence, or ataxia.
7. Outcome measures
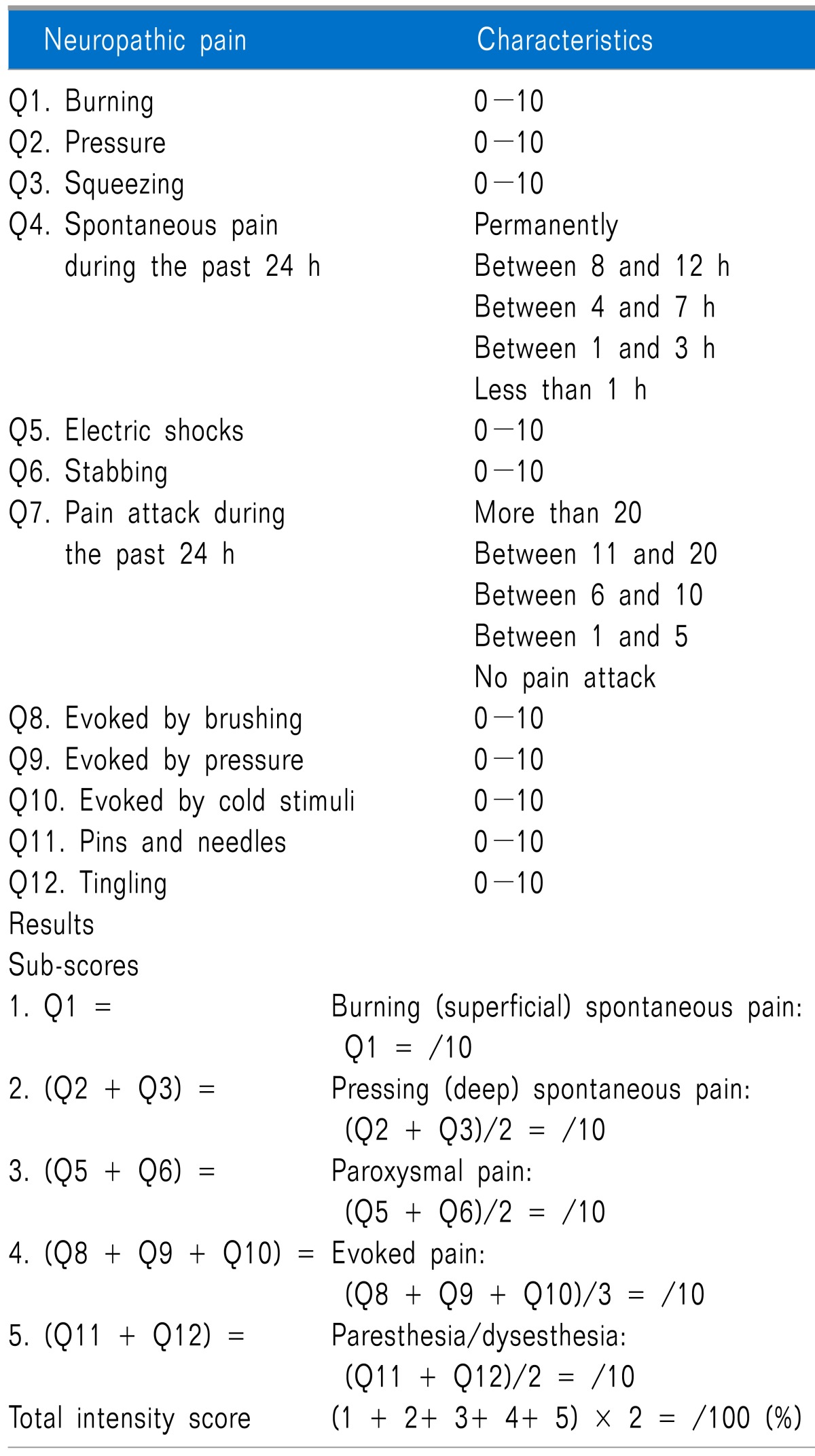
The NPSI was self-evaluated by the patient every day using both visual analogue scale (VAS) score (from 0 to 10, "no pain" to "worst pain imaginable") of neuropathic pain including burning, pressure, squeezing, electric shocks, stabbing, evoked by brushing, evoked by pressure, evoked by cold stimuli, pins and needles, and tingling, along with duration of spontaneous pain and frequency of proved pain [14]. The median of both 5 sub-scores, including burning (superficial) spontaneous pain, pressing (deep) spontaneous pain, evoked pain, paresthesia/dysesthesia, and pins and needles/tingling, and total scores were compared in both groups during the study days (Table 1).
Table 1.
Neuropathic Pain Symptom Inventory
The average daily dosage (mg/d) of oral codeine as a rescue medication for pain control at VAS > 4 was recorded during the study.
Adverse effects in both groups were also recorded every day and compared.
Discharge criteria after the 12-day admission included: (1) NPSI score < 40%, (2) spontaneous pain < 3 h during the past 24 h, (3) pain attack < 5 times during the past 24 h, and (4) no serious adverse effects, including dizziness, somnolence, or ataxia (Fig. 1).
8. Sample size
On the basis of a pilot study, we determined that a sample size of 40 participants per group was sufficient for this study using a desired power of 0.8 and a α level of 0.05. The primary outcome for power analysis was the pain score. The calculations were made for NPSI based on the VAS, using a clinically significant difference in 2 groups with a rating of a 10% reduction in VAS and assuming a standard error of 5%.
9. Statistical analysis
The data were presented as a mean ± standard deviation or the median ± standard error. Demographic characteristics, including age and sex, were analyzed using the Student t test and the chi-square test in the intergroup comparison. Changes in NPSI score in both groups were used Chi-squared test. Fisher's exact test was used to test the differences in the proportions of each adverse effect between the before and after treatments. In all comparisons, a P value less than 0.05 was considered statistically significant. The statistical analyses were performed using SPSS 12.0 (IBM Corporation, Somers, New York).
RESULTS
1. Patient demography and baseline data, participant flow, recruitment, and numbers analyzed
The patients' demographic characteristics were similar and had no obvious effect on the outcome (Table 2). There were no drop-out patients who stopped taking medicine or receiving intravenous NFP. The therapeutic and adverse effects of NFP on the 40 patients in each group were analyzed (Fig. 1).
Table 2.
Baseline Characteristics of the Study Participants
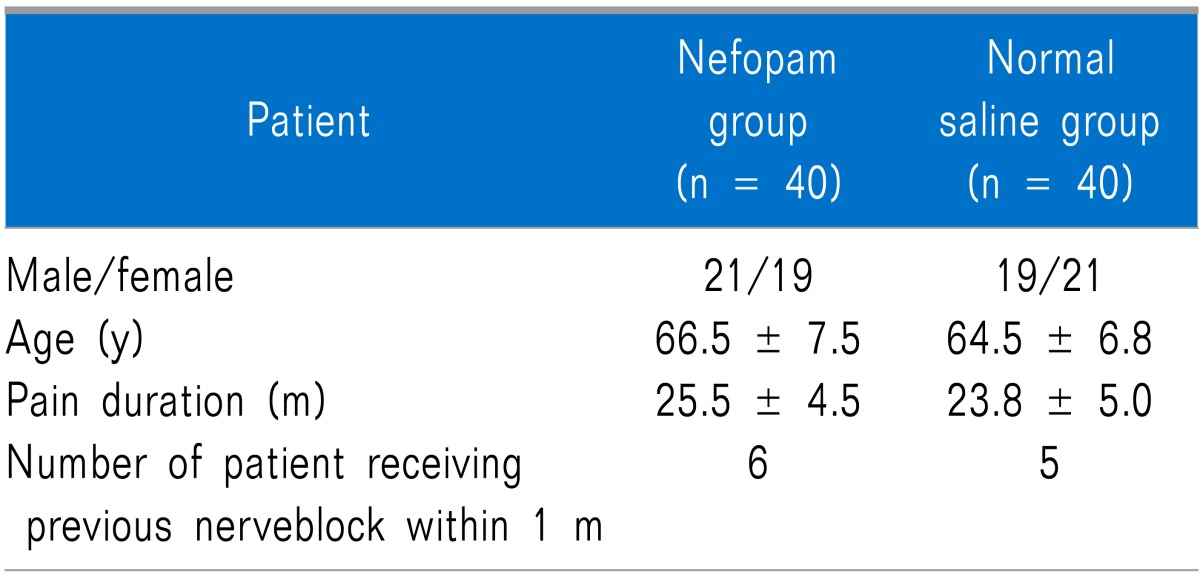
Data are mean ± SD or numbers.
2. Outcomes and estimation
The daily median score of total NPSI was significantly lower in the NFP group from day 1 to 6 of hospitalization (P < 0.05). However, the median scores of each of the 5 components of NPSI did not show statistical differences between both groups during the study days (Table 3).
Table 3.
Total and Subtotal Neuropathic Pain Symptom Inventory (NPSI) Scores During Study Days
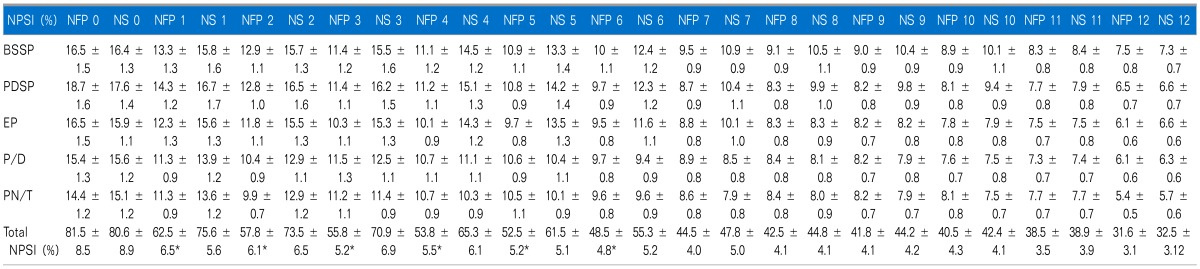
*NFP group showed significantly lower total NPSI scores from study day 1 to 6 (P < 0.05 compared to those of NS group). However, 5 each subtotal NPSI median scores did not show any statistical difference in both groups, respectively. All data are expressed SD ± error. NFP: nefopam, NS: normal saline, BSSP: burning (superficial) spontaneous pain, PDSP: pressing (deep) spontaneous pain, EP: evoked pain, PDSP: paresthesia/dysesthesia, PN/T: pIns and needles/tingling.
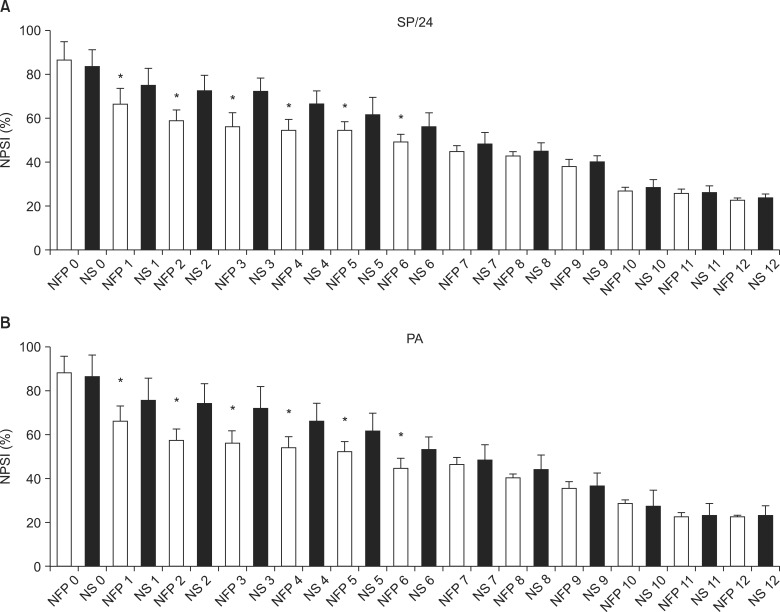
In the NFP group, the duration of spontaneous pain during the previous 24 h was shorter, and the number of pain attacks during the past 24 h was less frequent from day 1 to 6 of hospitalization (P < 0.05) (Fig. 2).
Fig. 2.
The median scores of the grade of duration of spontaneous pain (SP) and number of pain attack (PA) during study days. (A) *The grade by the duration of SP during the past 24 h was lower, and (B) *the grade of the number of PA during the past 24 h was lower in NFP group from the day 2 to 6 of hospitalization (P < 0.05 compared to those of NS group). All data are expressed SD ± error. NFP: nefopam, NS: normal saline. Grade by duration of SP: grade 1 (less than 1 h), grade 2 (between 1 and 3 h), grade 3 (between 4 and 7 h), grade 4 (8 and 12 h), and grade 5 (permanently). Grade by frequency of PA: grade 0 (no pain attack), grade 1 (between 1 and 5), grade 2 (between 6 and 10), grade 3 (between 11 and 20), and grade 4 (more than 20).
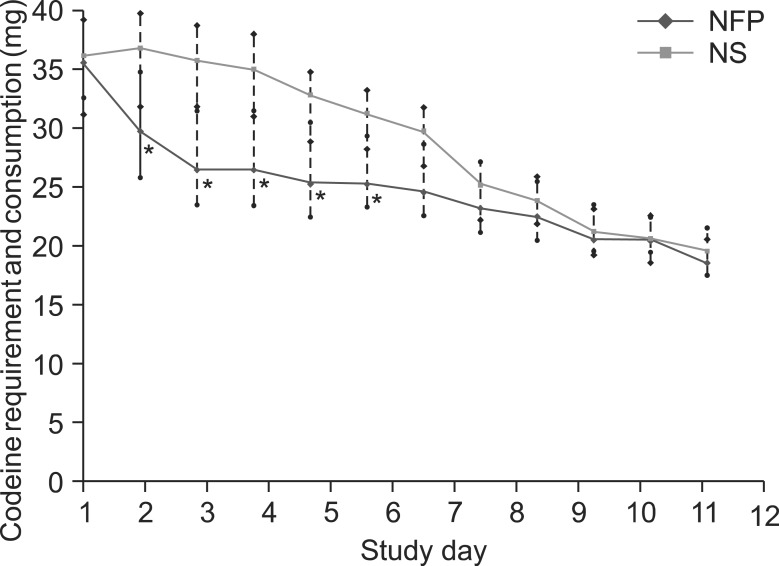
The NS group required higher consumption of additional rescue medication of codeine per person from day 2 to 6 of hospitalization (P < 0.05) (Fig. 3). The numbers of patients who did not require rescue medication during the study period were 9 and 3 in the NFP and NS group, respectively.
Fig. 3.
Rescue medication requirement and consumption. *Higher requirement and consumption of average additional rescue medication showed in NS group from the day 1 to 6 of hospitalization (P < 0.05 compared to those of NFP group). All data are expressed mean ± SD.
3. Adverse events
NFP increased the frequency of dry mouth, dizziness, nausea and ataxia, in order of frequency, during the initial period of the study days 1 to 6. There was no difference between the groups in overall frequencies of occurrence for each adverse effect. The numbers of patients who reported adverse effects were 30 and 23 in the NFP and NS group respectively on day 1. Dry mouth in both groups was an intolerable adverse effect which never decreased, and even increased until the end of the study period. No patient was prescribed medicine for the adverse effects (Fig. 4).
Fig. 4.
Adverse effects. *Nefopam (NFP) increased the frequency of dry mouth, dizziness, nausea and ataxia, in order of frequency, during the initial period of the study days 1 to 6 (P < 0.05 compared to those of normal saline [NS] group). There was no difference between the groups in overall frequencies of occurrence for each adverse effect. Dry mouth in both groups was an intolerable adverse effect which showed a never-decreasing and even-increasing symptom till the end of the study days.
The numbers of patients who did not meet the discharge criteria were 3 and 3 in both groups. The causes of delayed discharge were serious adverse effects including dizziness, somnolence, or ataxia. Only 1 patient in the NS group could not go back home due to uncontrolled pain. However, all patients who delayed discharge returned home within 16 days of admission.
DISCUSSION
1. Key results
All 80 patients had completed the 12-day study related to efficacy and adverse effects of intravenous NFP infusion during the titration of oral medications such as anticonvulsant, antidepressant, and opioid. The additional continuous intravenous infusion of NFP in hospitalized patients with PHN significantly reduced total NPSI scores, but increased the frequency of dry mouth, dizziness, nausea and ataxia during the initial period of the study days 1 to 6.
2. Interpretation
The additional continuous intravenous infusion of NFP reduced pain significantly until study day 6, even though each component - burning superficial spontaneous pain, pressing deep spontaneous pain, evoked pain, paresthesia/dysesthesia, and pins and needles - did not show statistical differences. It also significantly reduced the spontaneous pain duration and the frequency of pain attack from the study day 2 to 6. It also reduced the requirement for and usage of additional analgesic.
Seven patients could not discharge due to adverse effects and uncontrolled pain on the anticipated discharge day. Six patients (3 and 3 in both groups) could not discharge due to adverse effects of oral medication. One patient in the NS group could not discharge due to uncontrolled pain. However, all discharged within 16 days of hospitalization.
The most common adverse effect in both groups was dry mouth, which continued and even increased until the end of study. This adverse effect, even after discontinuation of NFP, was due to increased dosage of the antidepressant. Other adverse effects, such as dizziness, nausea, and ataxia, decreased after continuation of NFP infusion. These adverse effects seemed to originate from NFP infusion.
3. Generalizability
This study was conducted for the patients with intractable PHN who needed titration of oral medication in the university hospital. To decide whether NFP may be helpful to treat PHN, a kind of representative neuropathic pain, the most ideal candidates: 1) have already finished titration of oral medication, and 2) it was difficult to escalate the doses of anticonvulsants, antidepressants, and opioids due to their general conditions, but 3) had continuous or breakthrough pain. The study's greatest limitation is that it is difficult to determine the origin of the therapeutic and adverse effects while escalating the doses of oral medications for neuropathic pain. However, most patients with intractable PHN who visit a university hospital referred from local clinic or other departments need titration of the previous medications. For the rapid titration of these drugs, most patients need hospitalization with cautious observation for adverse effects. It is not uncommon that most family or guardians let the patients who are titrating medications for neuropathic pain stop administration of drugs due to informed adverse effects, such as dizziness, dry mouth, somnolence, and ataxia.
It is difficult to find a proper intravenous drug for continuous pain during hospitalization before titration. Intravenous opioids or non-steroidal anti-inflammatory drugs alleviate pain slightly, but not completely in clinical practice. Few intravenous anticonvulsants or antidepressants are available now. If the patients had already received a nerve block or pulsed radiofrequency ablation of involved dorsal root ganglion, then a continuous epidural block is the next procedure before finishing titration of the medications. However, the continuous epidural catheterization cannot place over 1 week due to potential epidural infection even though the minimum period of titration needs at least 9 to 12 days. And the catheterization makes it difficult to recognize how much the PHN gets better by titration of oral medications.
4. Overall evidence
All kinds of neuropathic pain symptoms decreased within 30 minutes after infusion of NFP. Alleviation of these symptoms and maintenance of relief of pain were nearly complete. These therapeutic effects of NFP were worthy to compare when non-steroidal anti-inflammatory drugs (NSAIDs) or opioids used to show incomplete relief for neuropathic pain in our experience. Improvement of these symptoms caused by NFP included both positive and negative symptoms. The positive symptoms, including burning, pressing, squeezing, stabbing, evoked pain, pins and needles, and tingling, are usually considered to be controlled by anticonvulsants. The negative symptoms, paresthesia/dysesthesia and hypoesthesia, are considered to be controlled by antidepressants [16-18]. The characteristic dual analgesic activity of NFP may originate from the known mixed mechanisms of anticonvulsants and antidepressants.
There are a limited number of currently available effective intravenous analgesics for neuropathic pain. Some intravenous analgesics showed good responses, but it not possible to change to the orally administered form of these analgesics for use after discharge because an effective form is not yet available. Both formulae of NFP are available. In this study, intravenous dosage of NFP was reduced while the dosage of oral medications for neuropathic pain was increased simultaneously. However, if the oral medications for neuropathic pain reach the maximum recommended dosage, or cannot escalate the dosage due to general conditions including hepato-renal problems, then oral formula of NFP is a substitute after hospitalization if the intravenous NFP are not harmful for laboratory examination during the hospitalization.
There was a great limitation that NPSI score based on the VAS scores with spontaneous pain and pain attack in both groups was compared by differences by 10%. Clinically, if the control group showed severe pain (≥ 70%) and NFP group showed moderate (< 70% and > 30%) or mild pain (≤ 30%), NFP could be considered apparently effective to the neuropathic pain. However, NFP decreased the codeine consumption for rescue medication apparently.
Reported adverse effects were confused or masked with oral medications for neuropathic pain in this study at least during the infusion of NFP. The common adverse effects were in this study were dry mouth, dizziness, nausea, and ataxia, in order. The frequently reported adverse effects during intravenous NFP administration in a previous study were nausea and sweating, sedation, pain at the site of intravenous or intramuscular injection, skin rash, dizziness, lightheadedness, dry mouth, and tachycardia [2]. After discontinuation of NFP infusion after 6 days, the other 3 adverse effects, except dry mouth, showed a tendency to decrease, but dry mouth tended to increase until the end of the study. Dry mouth is thought to be caused by the anticonvulsant, nortriptyline, which was used at the dose of 75 mg from the 7th day to the end of the study. The adverse effects, such as dizziness, nausea, and ataxia, might originate from the infusion of NFP. The frequently reported adverse effect, sweating, in a study [19], was prevented and reduced by continuous infusion of NFP.
Future studies related to chronic neuropathic pain need a comparative study to the patients with PHN who have already finished the titration of the oral medications. It also will need a comparative study related to the prevalence of acute neuropathic pain in operations of limb amputation, ganglion, or nerve entrapment syndrome. If the intravenous administration of NFP was effective to treat neuropathic pain, it might be changed to oral NFP administration.
In conclusion, an intravenous continuous infusion of NFP reduces neuropathic pain during the titrations for oral neuropathic medications among inpatients. It may facilitate rapid titration of oral medications for neuropathic pain with tolerable adverse effects in patients with intractable PHN.
ACKNOWLEDGEMENTS
This study was partially supported by a 2-year study of Pusan National University.
IRB approval and clinical trials registration number: L-2011-517.
References
- 1.Turk DC, Okifuji A. Pain terms and taxonomies. In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica's management of pain. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2010. p. 16. [Google Scholar]
- 2.Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980;19:249–267. doi: 10.2165/00003495-198019040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005;28:183–191. doi: 10.1007/s00726-005-0166-0. [DOI] [PubMed] [Google Scholar]
- 4.Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004;1013:249–255. doi: 10.1016/j.brainres.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003;20:593–601. doi: 10.1089/089771503767168519. [DOI] [PubMed] [Google Scholar]
- 6.Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007;32:323–332. doi: 10.1007/s00726-006-0419-6. [DOI] [PubMed] [Google Scholar]
- 7.Czuczwar M, Czuczwar K, Cięszczyk J, Kiś J, Saran T, Łuszczki JJ, et al. Nefopam enhances the protective activity of antiepileptics against maximal electroshock-induced convulsions in mice. Pharmacol Rep. 2011;63:690–696. doi: 10.1016/s1734-1140(11)70580-1. [DOI] [PubMed] [Google Scholar]
- 8.Löscher W, Schmidt D. New Horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marazziti D, Rotondo A, Ambrogi F, Cassano GB. Analgesia by nefopam: does it act through serotonin? Drugs Exp Clin Res. 1991;17:259–261. [PubMed] [Google Scholar]
- 10.Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008;101:610–617. doi: 10.1093/bja/aen267. [DOI] [PubMed] [Google Scholar]
- 11.Sunshine A, Laska E. Nefopam and morphine in man. Clin Pharmacol Ther. 1975;18:530–534. doi: 10.1002/cpt1975185part1530. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi Y, Ali SZ, Kimberger O, Zmoos S, Lauber R, Markstaller M, et al. The effects of nefopam on the gain and maximum intensity of shivering in healthy volunteers. Anesth Analg. 2010;111:409–414. doi: 10.1213/ANE.0b013e3181e332bb. [DOI] [PubMed] [Google Scholar]
- 13.Alfonsi P, Passard A, Gaude-Joindreau V, Guignard B, Sessler DI, Chauvin M. Nefopam and alfentanil additively reduce the shivering threshold in humans whereas nefopam and clonidine do not. Anesthesiology. 2009;111:102–109. doi: 10.1097/ALN.0b013e3181a979c1. [DOI] [PubMed] [Google Scholar]
- 14.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Thakur R, Kent JL, Dworkin RH. Herpes zoster and postherpetic neuralgia. In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica's management of pain. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2010. pp. 338–357. [Google Scholar]
- 16.Magrinelli F, Zanette G, Tamburin S. Neuropathic pain: diagnosis and treatment. Pract Neurol. 2013;13:292–307. doi: 10.1136/practneurol-2013-000536. [DOI] [PubMed] [Google Scholar]
- 17.Attal N. Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneap Minn) 2012;18:161–175. doi: 10.1212/01.CON.0000411564.41709.2d. [DOI] [PubMed] [Google Scholar]
- 18.Costigan M, Woolf CJ. Pain: molecular mechanisms. J Pain. 2000;1(3 Suppl):35–44. doi: 10.1054/jpai.2000.9818. [DOI] [PubMed] [Google Scholar]
- 19.Durrieu G, Olivier P, Bagheri H, Montastruc JL French Network of Pharmacovigilance Centers. Overview of adverse reactions to nefopam: an analysis of the French pharmacovigilance database. Fundam Clin Pharmacol. 2007;21:555–558. doi: 10.1111/j.1472-8206.2007.00499.x. [DOI] [PubMed] [Google Scholar]