Abstract
The occurrence of CRPS after a snake bite was very rare, only two cases were reported worldwide. Here we report a case that the 44-year-old female patient bitten by snakes CRPS type 1 was treated consecutive intravenous regional block, lumbar sympathectomy and antiepileptic drug therapy, also discuss the possible pathophysiology.
Keywords: complex regional pain dyndromes, snake bite
Complex regional pain syndrome (CRPS) is defined as a series of symptoms such continuous pain, allodynia, hyperalgesia, vasomotor abnormality, usually after an injury or trauma [1]. It is completely unaware whether CRPS is caused by the nerve damage or a soft tissue injury. In recent years, pathophysiology of various mechanisms is thought to involve in the development of CRPS [2]. The occurrence of CRPS after snake bite was very rare, only two cases were reported worldwide [3,4]. De Mos et al. suggested that pathology of the autonomic and somatic nervous system, as well as the roles of neurogenic inflammation, hypoxia, and the contribution of psychological factors were involved [5]. This case is a rare example of CRPS after snake bites, we are to describe the possible pathophysiology at this condition and course of treatment.
CASE REPORT
A 44-year-old woman without any specific medical history presented to the emergency room of our hospital after a snake bite, (Agkistrodon ussuriensis, Chinese viper) near the right lateral malleolus, in a parking lot three months before being referred to the pain clinic. At that time, the patient complained of severe edema up to the right femoral region, acute pain, abdominal pain, muscle pain, double vision, dizziness, and nausea. Agkistrodon halys antivenin (6,000 IU) was intravenously injected, followed by the administration of antitetanus immunoglobulin, an antihistamine, a steroid, and antibiotics. At the time of the injury, the Visual Analogue Scale (VAS) pain intensity score was 100/100 (the patient felt as if her foot was exploding). The score gradually reduced up to 4 days after the injury as the swelling decreased.
When the patient was referred to our pain clinic during the 3rd month after the injury (Fig. 1), she had static allodynia on the 2nd, 3rd, and 4th digits of the right foot along with repetitive pain that felt like needle stabs. The patient also exhibited hyperalgesia in the entire foot as well as constant tingling sensation the upper lateral malleolus. The pain worsened during walking; she experienced stiffness and edema with a VAS intensity of approximately 50/100 on the lateral sole and the top of the foot in addition to pain in the foot at the slightest exertion, such as walking up or down the stairs. The patient felt a sickly cold sensation in the heel that was alleviated by using a hot or cold pack. An electrophysiologic study performed at the end of 1st month after the injury indicated findings suggestive of damage to the right superficial peroneal nerve or around peripheral nerve; however, no abnormal features were observed in a 3-phase bone scan or on thermographic examination.
Fig. 1.
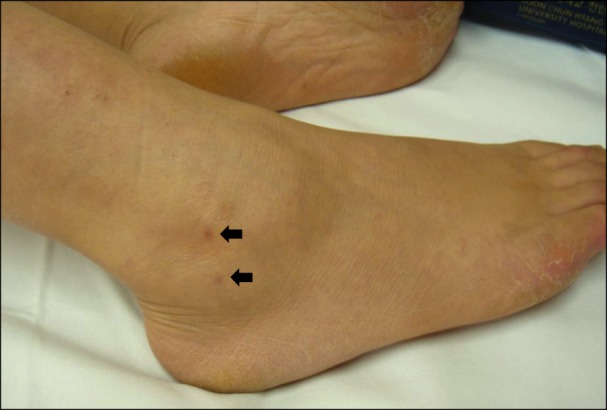
Bite marks of right lateral malleolus region. Black arrows show bite marks. There is no significant edema at this time (3 months after bite).
On the first day of the visit, an intravenous regional block was performed with 40 cc of 0.5% mepivacaine and 30 mg of ketorolac. In addition, 300 mg/day of gabapentin was administered in three divided dose. The constant tingling sensation and pain the upper lateral malleolus completely disappeared but she sporadically felt tingling pain near the bite area. The pain during walking decreased to a VAS intensity of 20/100, but the cold sensation in the heel persisted. On the 3rd day, when performing a secondary intravenous regional block with 40 cc 0.5% mepivacaine and 30 mg ketorolac, the patient developed urticaria in the entire calf with severe pain on the bite site and in the stiff and painful regions during walking. Therefore, dexamethasone 5 mg was injected through the same intravenous route as the mepivacaine injection. The severe pain diminished immediately after the injection.
The next day, the patient had minimal pain, when relaxed, and experienced pain with a VAS intensity of 15/100 on the lateral dorsal part of the foot while wearing shoes and walking. However, the sickly cold sensation was still present in the heel. On the 5th day, a lumbar sympathetic ganglion block was performed on the 2nd and 3rd vertebrae. The cold sensation in the heel reduced for 2-3 days after the block but returned at the same level as before; therefore, we decided to perform a sympathectomy using alcohol. The preoperative temperature of both heels was almost the same (left heel, 31.1℃; right heel, 31.0℃). 2 ml of contrast medium and 2 ml of 4% lidocaine were injected in the sympathetic ganglia at the L2 and L3 level. After a 10-min wait to confirm the absence of motor weakness and other abnormalities, 3 ml of 99% alcohol was injected on each side. The foot temperature at 15 min after the injection indicated a difference of 3.3℃ between the left (32.1℃) and right (35.4℃) side, and the patient felt a burning sensation on her foot.
The cold sensation in the heel completely disappeared 2 days after the sympathectomy. The following day, the patient was discharged with a VAS score of 10/100 and similar levels of pain while walking and relaxing. The dose of gabapentin was increased to 600 mg tid. The decrease in the pain was maintained during the 2-month follow-up period. A reduced dose of gabapentin (300 mg tid) was administered for the next 2 months. When the pain disappeared, administration of the drug (gabapentin 300 mg tid) as well as follow-up were discontinued.
DISCUSSION
Complex regional pain syndrome (CRPS), characterized by continuous pain regardless of the inducing stimulus, manifests as at least 3 of 4 categories including sense, vascular mobility, edema, and motor function, and it is diagnosed as such when >2 positive symptoms of the 4 categories mentioned above are present [1]. In the present case, the patient complained of hyperalgesia, allodynia, edema, and decreased range of motion after receiving a noxious stimulus due to the snake bite. Physical examination also indicated hyperalgesia, allodynia, and edema as well as peripheral nerve damage. The electrophysiological examination confirmed the clinical features and the diagnosis of CRPS.
CRPS has several causes, including sympathetic mediated disorder, central sensitization, autoimmunity, ischemia, cortical reorganization, nerve damage, and inflammation; however, the underlying mechanisms are not yet clearly understood [6]. The development of CRPS due to a snake bite is very rare, with only 2 reported cases worldwide reported thus far [3,4]. Two million people worldwide experience snakebites each year, of whom 20,000 die [7]. Depending upon the type of snakes, bite cases are classified into those resulting in local edema and necrosis of the bitten limbs and those resulting in systemic neurological symptoms [7]. Although snakebites cause edema and pain that are sufficiently severe to induce compartment syndrome with life-threatening systemic symptoms, local symptoms are often insufficiently treated as they are hidden by systemic symptoms.
Of the cytotoxic enzymes in snake venom, phospholipases-A2 and metalloproteases result in inflammation and cause local symptoms including edema and pain [8-10]. Agkistrodon brevicaudus, A. ussuriensis, and A. saxatilis are mainly found in Korea, and common venom components among these snakes include the enzymes that belong to phospholipase-A2 and metalloprotease categories [11]. Animal studies reported the occurrence of hyperalgesia and allodynia when phospholipase-A2 was injected into subcutaneous tissues and muscle. Inflammatory and proinflammatory cytokines (interleukin-1, tumor necrosis factor-α, bradykinin, substance P, and calcitonin gene-related peptide) increased, and the development of hyperalgesia was inhibited when proinflammatory cytokine inhibitors were used [8-10]. Levels of inflammatory cytokines were also increased in patients with CRPS [12]. Another study reported that noticeable edema is observed in cases of snake bites, and that local edema due to inflammation causes ischemia and neural compression of the peripheral tissues, resulting in permanent tissue damage [13]. In the early stage of edema, patients with CRPS also experience plasma extravasation-induced edema due to an increase in substance P, which is a proinflammatory cytokine [2]. Edema due to extravasation by a 5% dextrose solution was reported to cause compartment-like syndrome, which results in CRPS [14]. In an animal study with reperfusion after 3 h of ischemia, CRPS symptoms manifested without any evidence of neural damage. In particular, the study found that free radical accumulation in ischemic conditions induced edema and inflammation because of blood vessel damage in the deep tissues during reperfusion, which could explain how CRPS type 1 might develop in the absence of clear neural damage [15].
Thus, in our case, hyperalgesia and allodynia due to excessive inflammation by snake venom, decreased movement to avoid pain, free radical accumulation due to edema-induced reperfusion, and constant pain induced by autonomic nervous system stimulation could result in CRPS.
Despite many studies on CRPS treatment methods, very few treatments have been found to be consistently effective [2]. Considering that the patient's pain was still localized in the ankle region and that local edema and inflammation were present, the authors performed an intravenous regional block (IVRB) utilizing local anesthetics and ketorolac. Many pain clinicians have conducted IVRB using various sympatholytics, anesthetics, and anti-inflammatory drugs to treat CRPS-affected limbs because of its convenience and reliability [16,17]. IVRB with Ketorolac is reported to be especially useful for the treatment of hyperalgesia, joint pain, and edema [18]. Ketorolac decreases the sensitization associated with prostaglandin E2-mediated bradykinin hyperalgesia, tissue ischemia, vasodilation, and k-opioid receptors, by inhibiting thromboxane [18,19].
In the present case, the patient experienced significant pain relief with only 2 trials of IVRB. The short-term effects of IVRB have been reported in a case series, whereas the evidence regarding its long-term effects has not yet been sufficiently provided [18]. However, because multimodal treatment is generally preferred to single treatment for addressing CRPS and since CRPS is an obstinate disease that is difficult to cure, the application of multimodal treatment for its short-term effects (if any) seems reasonable, even though its therapeutic effects are not well understood.
Although the thermographic findings in our patient were normal, we performed a sympathetic ganglion block as her complaints of a cold sensation persisted. To maintain the reduced pain level, a chemical sympathectomy was performed by using alcohol, and the pain was remarkably reduced. In the current case, intense treatments appear to have been helpful because of the relatively short duration of the symptoms.
In conclusion, clinicians should acknowledge that the development of inflammation and edema due to snake bites can result in CRPS. To reduce the occurrence of CRPS, aggressive treatment of early-stage symptoms is necessary.
References
- 1.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 2.Maihöfner C, Seifert F, Markovic K. Complex regional pain syndromes: new pathophysiological concepts and therapies. Eur J Neurol. 2010;17:649–660. doi: 10.1111/j.1468-1331.2010.02947.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhattarai B, Shrestha BP, Rahman TR, Sharma SK, Tripathi M. Complex regional pain syndrome (CRPS) type-1 following snake bite: a case report. Nepal Med Coll J. 2008;10:278–280. [PubMed] [Google Scholar]
- 4.Ergan SA, Yoleri Ö, Yavaşi S, Ölmez N, Memiş A. Complex regional pain syndrome caused by snake bite: a case report. Turk J Phys Med Rehabil. 2012;58:69–71. [Google Scholar]
- 5.de Mos M, Sturkenboom MC, Huygen FJ. Current understandings on complex regional pain syndrome. Pain Pract. 2009;9:86–99. doi: 10.1111/j.1533-2500.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 6.Goebel A. Current concepts in adult CRPS. Br J Pain. 2011;5:3–11. doi: 10.1177/204946371100500202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Del Brutto VJ. Neurological complications of venomous snake bites: a review. Acta Neurol Scand. 2012;125:363–372. doi: 10.1111/j.1600-0404.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 8.Chacur M, Gutiérrez JM, Milligan ED, Wieseler-Frank J, Britto LR, Maier SF, et al. Snake venom components enhance pain upon subcutaneous injection: an initial examination of spinal cord mediators. Pain. 2004;111:65–76. doi: 10.1016/j.pain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Chacur M, Milligan ED, Sloan EM, Wieseler-Frank J, Barrientos RM, Martin D, et al. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain. 2004;108:180–191. doi: 10.1016/j.pain.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Chacur M, Longo I, Picolo G, Gutiérrez JM, Lomonte B, Guerra JL, et al. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: pharmacological mediation and molecular determinants. Toxicon. 2003;41:667–678. doi: 10.1016/s0041-0101(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 11.Seong WK, Chung KT, Kim HH, Park JG, Park YM, Oh HB. Immunological characterization of the venoms from Korean snakes of the genus Agkistrodon (2) Rep Natl Ins Health. 1998;35:32–33. [Google Scholar]
- 12.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 13.Otero R, Gutiérrez J, Beatriz Mesa M, Duque E, Rodríguez O, Luis Arango J, et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon. 2002;40:1107–1114. doi: 10.1016/s0041-0101(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 14.Subedi A, Bhattarai B, Biswas BK, Khatiwada S. Complex regional pain syndrome (CRPS type-1) in an adolescent following extravasation of dextrose containing fluid-an underdiagnosed case. Korean J Pain. 2011;24:112–114. doi: 10.3344/kjp.2011.24.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coderre TJ, Bennett GJ. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010;11:1224–1238. doi: 10.1111/j.1526-4637.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lake AP. Intravenous regional sympathetic block: past, present and future? Pain Res Manag. 2004;9:35–37. doi: 10.1155/2004/657141. [DOI] [PubMed] [Google Scholar]
- 17.Tran de QH, Duong S, Bertini P, Finlayson RJ. Treatment of complex regional pain syndrome: a review of the evidence. Can J Anaesth. 2010;57:149–166. doi: 10.1007/s12630-009-9237-0. [DOI] [PubMed] [Google Scholar]
- 18.Vanos DN, Ramamurthy S, Hoffman J. Intravenous regional block using ketorolac: preliminary results in the treatment of reflex sympathetic dystrophy. Anesth Analg. 1992;74:139–141. doi: 10.1213/00000539-199201000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Uphouse LA, Welch SP, Ward CR, Ellis EF, Embrey JP. Antinociceptive activity of intrathecal ketorolac is blocked by the kappa-opioid receptor antagonist, nor-binaltorphimine. Eur J Pharmacol. 1993;242:53–58. doi: 10.1016/0014-2999(93)90009-7. [DOI] [PubMed] [Google Scholar]


