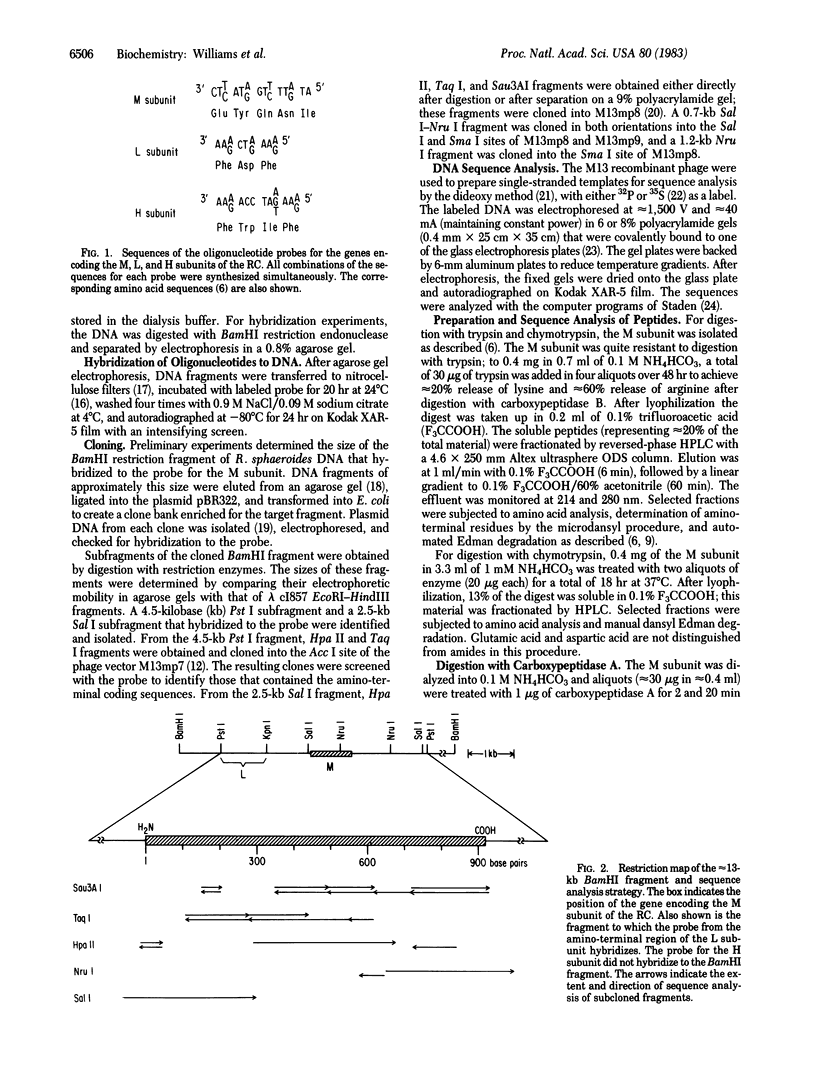
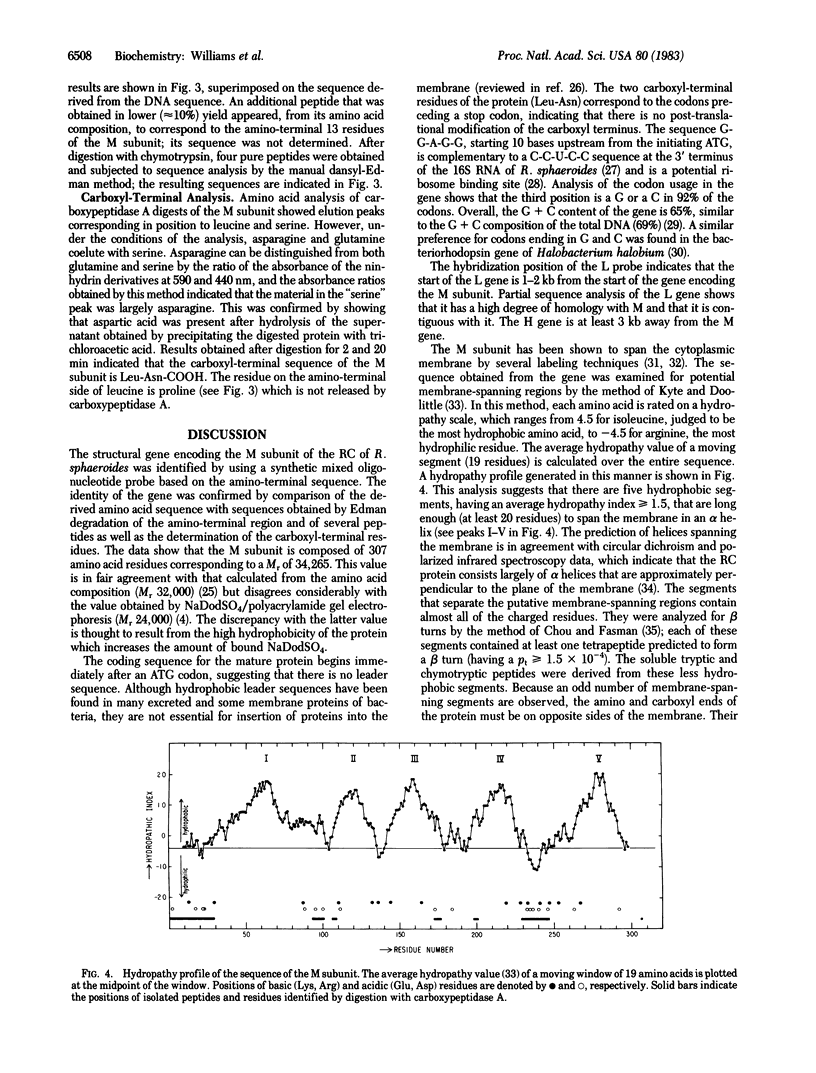
Abstract
The reaction center is a membrane-bound bacteriochlorophyll-protein complex that mediates the primary photochemical events in the photosynthetic bacterium Rhodopseudomonas sphaeroides. The previously determined amino-terminal sequences of the three subunits of the reaction center protein were used to design synthetic mixed oligonucleotide probes for the structural genes encoding the subunits. One of these probes was used to isolate and clone a fragment of DNA from R. sphaeroides that contained the gene encoding the M subunit. The nucleotide sequence of this gene was determined by the dideoxy method. In addition, a number of tryptic and chymotryptic peptides from the M protein were isolated and subjected to sequence analysis, and the sequence of the carboxyl terminus was determined. Together with the amino-terminal sequence, the data establish the primary structure of the M protein. The distribution of hydrophobic residues in the amino acid sequence suggests the presence of five membrane-spanning segments. A significant homology was found between the amino acid sequence of the M subunit and a thylakoid membrane protein (Mr 32,000) from spinach that has been implicated in herbicide and quinone binding.
Keywords: photosynthesis, oligonucleotide probe, nucleotide sequence, membrane protein
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann R. C., Gillies K., Takemoto J. Y. Membrane topography of the photosynthetic reaction center polypeptides of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Aug 4;20(16):4590–4596. doi: 10.1021/bi00519a012. [DOI] [PubMed] [Google Scholar]
- Beatty J. T., Cohen S. N. Hybridization of cloned Rhodopseudomonas capsulata photosynthesis genes with DNA from other photosynthetic bacteria. J Bacteriol. 1983 Jun;154(3):1440–1445. doi: 10.1128/jb.154.3.1440-1445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K., SMITH C. Rhodopseudomonas spheroides: high catalase and blue-green double mutants. Biochem Biophys Res Commun. 1960 Aug;3:143–145. doi: 10.1016/0006-291x(60)90210-2. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chory J., Kaplan S. The in vitro transcription-translation of DNA and RNA templates by extracts of Rhodopseudomonas sphaeroides. Optimization and comparison of template specificity with Escherichia coli extracts and in vivo synthesis. J Biol Chem. 1982 Dec 25;257(24):15110–15121. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Ogden R. C., Abelson J. N., Baldwin T. O., Nealson K. H., Simon M. I., Mileham A. J. Cloning of the Vibrio harveyi luciferase genes: use of a synthetic oligonucleotide probe. Proc Natl Acad Sci U S A. 1983 Jan;80(1):120–123. doi: 10.1073/pnas.80.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marinetti T. D., Okamura M. Y., Feher G. Localization of the primary quinone binding site in reaction centers from Rhodopseudomonas sphaeroides R-26 by photoaffinity labeling. Biochemistry. 1979 Jul 10;18(14):3126–3133. doi: 10.1021/bi00581a033. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Huang T., Itakura K. Solid-phase synthesis of polynucleotides. III. Synthesis of polynucleotides with defined sequences by the block coupling phosphotriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5491–5505. doi: 10.1093/nar/8.22.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- Rosen D., Okamura M. Y., Abresch E. C., Valkirs G. E., Feher G. Interaction of cytochrome c with reaction centers of Rhodopseudomonas sphaeroides R-26: localization of the binding site by chemical cross-linking and immunochemical studies. Biochemistry. 1983 Jan 18;22(2):335–341. doi: 10.1021/bi00271a016. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Okamura M. Y., Lopes A. D., Moskowitz E., Feher G. Characterization of reaction centers from photosynthetic bacteria. II. Amino acid composition of the reaction center protein and its subunits in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1403–1410. doi: 10.1021/bi00704a014. [DOI] [PubMed] [Google Scholar]
- Steiner L. A., Pardo A. G., Margolies M. N. Amino acid sequence of the heavy-chain variable region of the crystallizable human myeloma protein Dob. Biochemistry. 1979 Sep 18;18(19):4068–4080. doi: 10.1021/bi00586a003. [DOI] [PubMed] [Google Scholar]
- Sutton M. R., Rosen D., Feher G., Steiner L. A. Amino-terminal sequences of the L, M, and H subunits of reaction centers from the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26. Biochemistry. 1982 Aug 3;21(16):3842–3849. doi: 10.1021/bi00259a019. [DOI] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Hilmen M., Simon M. Regulation of gene expression by site-specific inversion. Cell. 1978 Sep;15(1):237–244. doi: 10.1016/0092-8674(78)90098-3. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]



