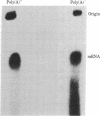
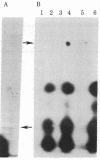
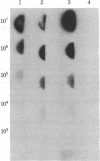
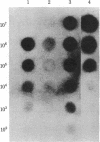
Abstract
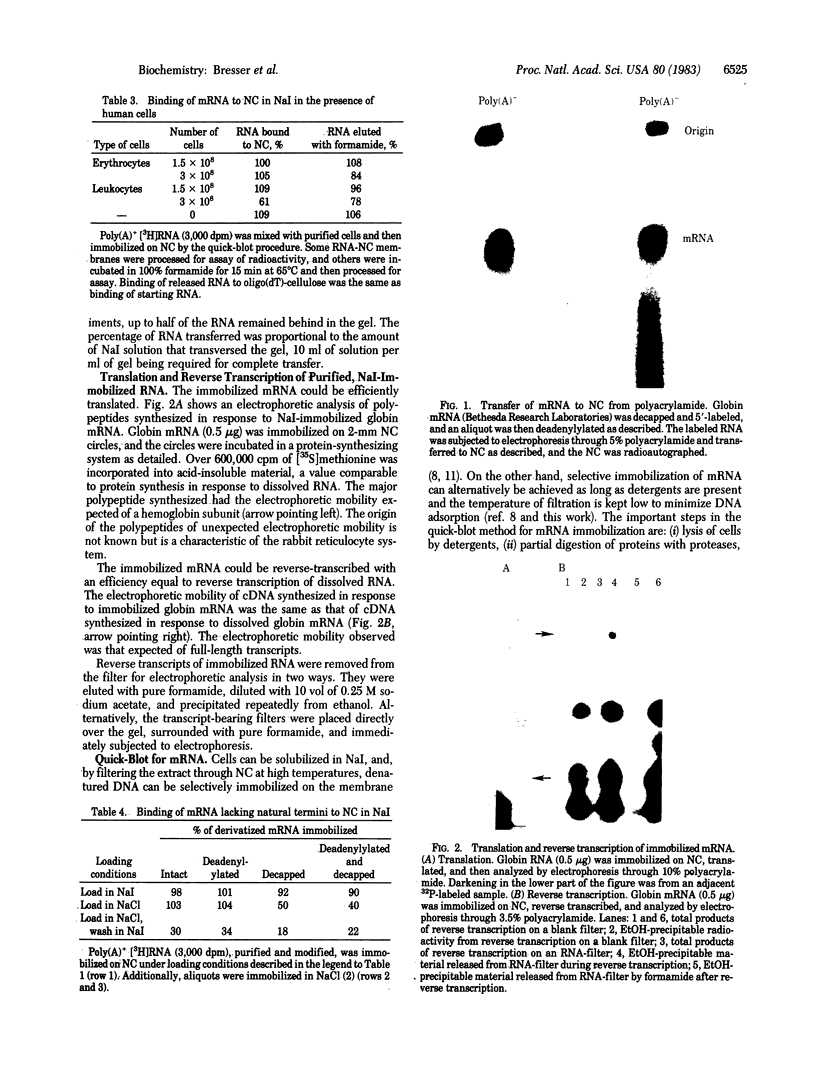
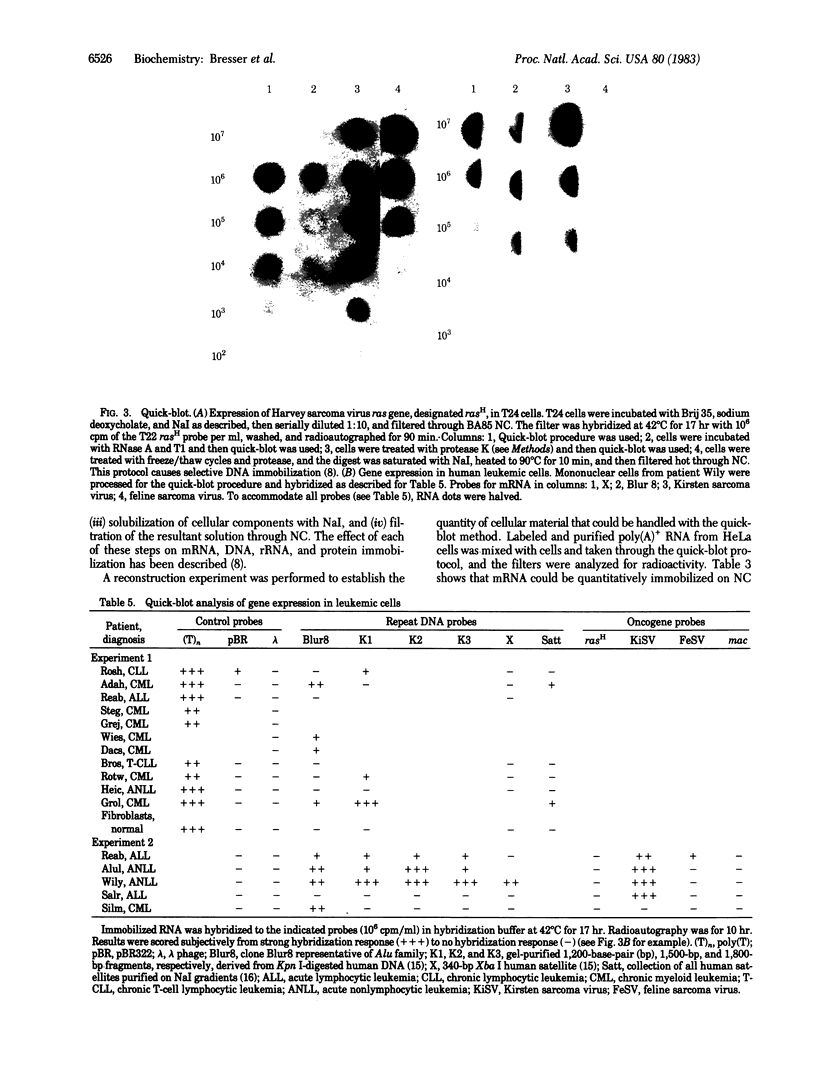
In 12.2 molal NaI and at 25 degrees C or below, mRNA bound to nitrocellulose while DNA and rRNA did not. Neither the poly(A) tract nor the cap were required for binding. The immobilized RNA could be translated, reverse transcribed, hybridized with radioactive probes, or released for further manipulation. mRNA was efficiently transferred from polyacrylamide to nitrocellulose in NaI. Baking was not required to fix NaI-immobilized mRNA to nitrocellulose. When cells dissolved in 12.2 molal NaI were filtered through nitrocellulose, mRNA became selectively bound (quickblot). The quick-blot system utilizing protease and detergents to prepare cells for NaI solubilization was especially suitable in quantitative, rapid screening of cells for expression of specific genes. Expression of highly repeated DNA sequences was detected in human leukemia cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Bresser J., Gillespie D. Quantitative binding of covalently closed circular DNA to nitrocellulose in NaI. Anal Biochem. 1983 Mar;129(2):357–364. doi: 10.1016/0003-2697(83)90562-6. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Guroff G. The binding of RNA to various celluloses. Biochem Biophys Res Commun. 1973 Jan 23;50(2):486–492. doi: 10.1016/0006-291x(73)90866-8. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Kato K., Noguchi M., Matsushima T., Sugimura T. A novel phosphodiesterase from cultured tobacco cells. Biochemistry. 1976 May 18;15(10):2185–2190. doi: 10.1021/bi00655a024. [DOI] [PubMed] [Google Scholar]
- Strayer D., Heintz N., Roeder R., Gillespie D. Three organizations of human DNA. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4770–4774. doi: 10.1073/pnas.80.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Uesugi S. Structure and functions of the Kirsten murine sarcoma virus genome: molecular cloning of biologically active Kirsten murine sarcoma virus DNA. J Virol. 1981 May;38(2):720–727. doi: 10.1128/jvi.38.2.720-727.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vournakis J. N., Efstratiadis A., Kafatos F. C. Electrophoretic patterns of deadenylylated chorion and globin mRNAs. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2959–2963. doi: 10.1073/pnas.72.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]