Operative hysteroscopy intravascular absorption (OHIA) syndrome is caused by intravascular absorption of fluid distention/irrigation medium during hysteroscopic surgery [1]. In order to reduce the occurrence of the OHIA syndrome caused by monopolar resectoscopy, which uses 1.5% glycine, bipolar resectoscopy using 0.9% saline is commonly performed [2]. No case involving OHIA caused by 0.9% saline have been reported. However, OHIA may be possible if the duration of the operation is prolonged and too much fluid is absorbed intravascularly, as we experienced in this case.
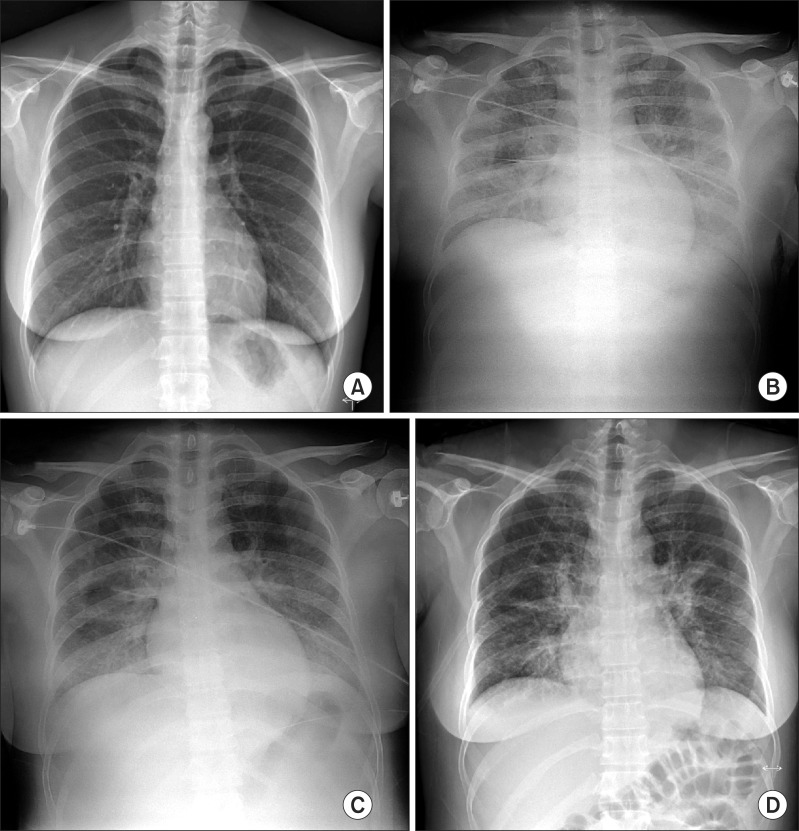
A 32-year-old healthy female patient (height, 162 cm; weight, 60 kg) was admitted for hysteroscopic myomectomy and uterine septal adhesiolysis under general anesthesia. She had no history of asthma or any other pulmonary disease. Preoperative chest X-ray showed no definite abnormality (Fig. 1A).
Fig. 1.
(A) Preoperative chest roentgenogram showing no definite abnormality. (B) Chest roentgenogram showing diffuse increased opacity in both lung fields, suggesting pulmonary edema. (C) Pulmonary edema is slightly improved. (D) Pulmonary edema is markedly improved.
On preoperative airway examination, she was expected to be difficult to intubate because of a mildly receding mandible, short thyromental distance, a small mouth, and Mallampati classification class III. We decided to use an I-gel laryngeal mask airway (Intersurgical Ltd, Berkshire, UK), because the operation duration was expected to be short and was scheduled as day surgery. Anesthesia was induced with 150 mg propofol, 35 mg rocuronium, 100 µg fentanyl, and 40 mg lidocaine. An I-gel laryngeal mask airway #3 was inserted. No significant problems occurred with the insertion, and no airway leakage sign was detected. Anesthesia was maintained with 50% N2O and 4-6 vol% of desflurane. During surgery, 4 ml/kg/h of Normosol-R pH 7.4 (Plasma solution A; CJ Pharma, Eumseong, Korea) was infused.
The surgery was conducted as a bipolar resectoscope hysteroscopy utilizing 0.9% saline as the distention/irrigation medium. However, after two or three submucosal myomas were excised, new lesions connected to an intramural myoma appeared; thus, the surgery was prolonged for > 100 min and 10 mg rocuronium was added. At that time, up to 12,000 ml of 0.9% saline had been used as distention/irrigation media. The suction bottle contained 8,000 ml of irrigation fluid. Peak inspiratory pressure (PIP) increased from 20 to 30 cmH2O, tidal volume (TV) decreased from 500 to 300 ml, and pulse oximetry (SpO2) dropped from 100 to 90%. No wheezing was heard. Other vital signs, including ETCO2, blood pressure, and pulse rate were relatively stable. FiO2 was increased to 0.6; however, SpO2 dropped to 83%, PIP rose to 40 cmH2O, TV decreased to below 200 ml, and ETCO2 increased to 45 mmHg within a few minutes. Laryngospasm, which could be a severe problem upon application of a supraglottic airway, were ruled out because no wheezing was heard, and manual ventilation raised the thoracic cage sufficiently against some resistance. Serous fluid leaked out from the I-gel mask when the patient was manually ventilated with 100% oxygen. Crackling sounds were heard at the lung base area. Respiratory failure due to acute pulmonary edema was suspected, and the operation was stopped. At that moment, SpO2 was 75%, blood pressure was 140/95 mmHg, and pulse rate was 110 beats per min. Fiber-optic or blind intubation through the I-gel mask was considered, but SpO2 continued to drop rapidly, which together with the possibility that the stimulation may cause a laryngospasm, forced us to remove the I-gel mask and to intubate endotracheally to ensure effective oxygenation/ventilation. It was a difficult intubation, as the laryngoscopic view showed Cormack and Lehane grade IV. We fit a mask and the anesthesiologist began bagging with the help of another anesthesiologist. We stopped administering anesthetics and started to wake the patient, as the surgeon decided to quit the operation, and restoring spontaneous ventilation is an effective strategy for improving oxygenation/ventilation. The SpO2 started to rise from its lowest value of 45%. After about 5 min, the patient recovered spontaneous ventilation, SpO2 increased to 70% as we assisted with manual bagging. After 20 min, the patient could breathe spontaneously and became fully alert. The total duration of surgery was 125 min, the volume of Normosol-R infused was 550 ml, and urine output was 400 ml. We decided to apply non-invasive positive pressure ventilation (NIPPV) at recovery room.
On arrival to the recovery room, the arterial blood gas analysis on oxygen mask with 10 L/min of O2 was as follows: pH, 7.26; PaO2, 52 mmHg; and PaCO2, 48 mmHg. Chloride concentration increased from preoperative value of 106 to 115 mEq/L. Chest X-ray findings revealed pulmonary edema (Fig. 1B); thus, we administered 40 mg furosemide. After applying NIPPV of 15 cmH2O with a FiO2 of 0.6 for 3 h, SpO2 recovered to 100% without any dyspnea or neurologic abnormality. Her chest X-ray showed improvement of the pulmonary edema (Fig. 1C). Urine output in the PACU for 3 h amounted to 1400 ml. The patient was transferred to the general ward where oxygen was applied at 6 L/min through a mask. A chest roentgenogram taken the next morning showed much improvement in the pulmonary edema (Fig. 1D). And SpO2 reached 98% on room air. The patient was discharged without any further complications.
The duration of operation is an important factor for the development of OHIA. Wegmüller et al. [3] experienced OHIA syndrome during an 80-min hysteroscopic myomectomy. They argued that limiting operative time might prevent OHIA syndrome. The surgery in this case was of three times longer duration than the average of 37.6 min [4].
The volume of deficit, which is the difference between the volume of distention/irrigation fluid infused and irrigated out of the surgical field, is another important factor in the development of OHIA syndrome. Pacini and Belloni [5] asserted that when the deficit is > 1,000 ml, it is safe to stop the operation. In our case, the volume of the deficit was estimated to be 3,000 ml.
Myomectomy and resection of uterine septa are associated with significantly higher rates of OHIA. In this case, hysteroscopic adhesiolysis and myomectomy, which caused significant fluid absorption, may have contributed to the development of OHIA.
The use of diuretics is advocated as in this case. Urine output must be closely monitored and judicious correction of an electrolyte imbalance will prevent morbidity.
References
- 1.Jackson S, Lampe G. Operative hysteroscopy intravascular absorption syndrome. West J Med. 1995;162:53–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Darwish AM, Hassan ZZ, Attia AM, Abdelraheem SS, Ahmed YM. Biological effects of distension media in bipolar versus monopolar resectoscopic myomectomy: a randomized trial. J Obstet Gynaecol Res. 2010;36:810–817. doi: 10.1111/j.1447-0756.2010.01244.x. [DOI] [PubMed] [Google Scholar]
- 3.Wegmüller B, Hug K, Meier Buenzli C, Yuen B, Maggiorini M, Rudiger A. Life-threatening laryngeal edema and hyponatremia during hysteroscopy. Crit Care Res Pract. 2011;2011:140381. doi: 10.1155/2011/140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wortman M, Daggett A, Ball C. Operative hysteroscopy in an office-based surgical setting: review of patient safety and satisfaction in 414 cases. J Minim Invasive Gynecol. 2013;20:56–63. doi: 10.1016/j.jmig.2012.08.778. [DOI] [PubMed] [Google Scholar]
- 5.Pasini A, Belloni C. Intraoperative complications of 697 consecutive operative hysteroscopies. Minerva Ginecol. 2001;53:13–20. [PubMed] [Google Scholar]