Corticosteroid injection is frequently used for the control of inflammation of the joint, tendon, and ligament. Compared to systemic corticosteroid injection, the incidence of complications after local corticosteroid injection is extremely low with an estimated risk of less than 1% [1].
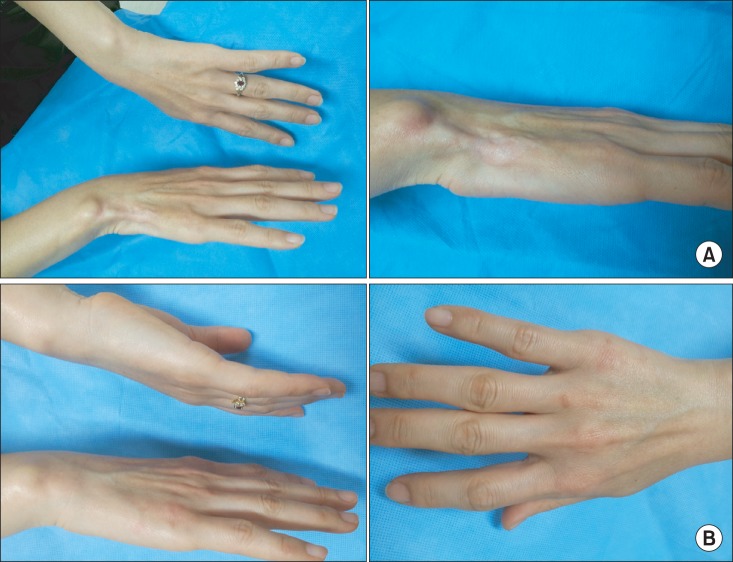
A previously 46-year-old woman, who had a splint for approximately one month due to right wrist pain caused by a car accident in April 2012, visited an orthopaedic clinic due to continuing pain after one month. A corticosteroid was injected (triamcinolone acetonide 20 mg) into the transverse carpal ligament once. The patient had numbness for 2 days after injection, and had pain, hypopigmentation, muscle atrophy one month after the corticosteroid injection (Fig. 1A). The patient visited our pain clinic in September 2012. She had pain of visual analogue scale (VAS) 2 in the medial region of the right wrist in the resting state, and had increased pain of VAS 4-5 during contact on the right wrist or wrist motion. No sensory deficit or hyperalgesia was observed in the right wrist.
Fig. 1.
(A) Hypopigmentation and subcutaneous atrophy and muscle atrophy in the wrist after local corticosteroid injection. (B) Resolved symptoms in the wrist at one year after local corticosteroid injection.
As she was suspected to have nerve injury due to corticosteroid injection, she underwent three phase bone scan, electromyography, and the nerve conduction test. The results showed normal findings. Her muscle atrophy was severe. Accordingly, the patient underwent electromyography and the nerve conduction test specifically on the atrophied muscle after discussion with the department of rehabilitation and the department of neurology. As a result, nerve injury was observed in the focal deep branch of the ulnar nerve that governs the opponens digiti minimi muscle.
Gabapentin (Neurontin®, Pfizer Inc., New York, NY, USA) 100 mg, tramadol 37.5 mg/acetaminophen 325 mg 1 tablet, and clonazepam 0.5 mg 0.5 tablet were administered to the patient twice a day. After drug administration, pain was reduced, but discomfort persisted during contact. Thus, she was referred to the department of plastic surgery for autologous fat injection. Approximately one year after the onset of her symptom, she visited our clinic and showed spontaneous recovery of hypopigmentation and fat and muscle atrophy and decrease in pain to VAS 0-1 during contact (Fig. 1B). Thus, we decided to observe residual symptom without any procedure.
Local corticosteroid injection has complications such as infection, sepsis, facial flushing, hypopigmentation, perilymphatic atrophy, bleeding, tendon rupture, steroid flare, soft tissue atrophy, and hypersensitivity reaction. In this case study, the patient had hypopigmentation, subcutaneous fat and muscle atrophy, and nerve injury. Skin hypopigmentation has been reported to occur in 1.3-4% of patients who underwent local corticosteroid injection [2]. Although the exact mechanism of hypopigmentation is unclear, steroids or biologically inactive components of steroids have been known to be involved in hypopigmentation [3]. In addition, dermal complications after corticosteroid injection are also explained by mechanical effects caused by edema, changes in ground substances, or vasoconstriction. Hypopigmentation occurs 1-4 months after corticosteroid injection, and then resolves 6-30 months after the injection. However, it can be prevented if intradermal and subcutaneous injections are avoided [4]. Subcutaneous fat atrophy and hypopigmentation may occur by injection of any type of steroid into the soft tissue. However, if steroids with suitable solubility and potency are used, the risk of subcutaneous fat atrophy and hypopigmentation can be reduced. Therefore, steroids with low solubility, such as triamcinolone acetonide, are preferably injected into the joint of deep structures such as the knee, elbow, and shoulder, whereas steroids with high solubility, such as betamethasone sodium and dexamethasone, are preferably injected into soft tissues such as the bursa, tendon sheath, metacarpophalangeal joint, proximal phalangeal joint, and carpal tunnel. Steroids cause fewer complications if their efficacy duration is shorter. In addition, for the prevention of subcutaneous fat atrophy, compressing the injection site with gauze is recommended after pulling out the needle to prevent steroid leakage along the needle track [1]. Subcutaneous fat atrophy has been known to last for 6-12 months after corticosteroid injection, and it is known to be reversible and resolved within one year. If subcutaneous fat atrophy lasts for more than one year, surgical treatments such as fat graft and fat injection can be considered [5]. Thus, in the present case study, the necessity of surgical treatment was explained to the patient, followed by treatment under collaboration with the department of plastic surgery.
Few studies have been conducted to investigate the incidence of nerve injury after corticosteroid injection. Via microneuronal circulation studies and histological studies, steroids were reported to have neurotoxicity. Injection site is one of the important factors in the occurrence of nerve injury. In particular, intrafacicular injection causes nerve injury. Besides, the severity of nerve injury may vary depending on the drug used. Hydrocortisone and triancinolone acetonide cause more extensive axon and myelin degeneration compared to dexamethasone.
Muscle atrophy mainly occurs during systemic corticosteroid injection. However, its frequency or prognosis is unclear. In this case, muscular atrophy after local steroid injection occurred and resolved. Studies about the mechanism and cause of muscular atrophy will be required in the future. In conclusion, although subcutaneous fat atrophy, depigmentation, nerve injury and muscular atrophy after local corticosteroid injection are rare, physicians should be aware of potential adverse side effects. It is reported in the majority of cases that these side effects are generally resolved in 6 to 30 months. In the case discussed here, when a complication occurs, the physician can explain to the patients that close observation is demanded up to one year after local corticosteroid injection. The surgical option (ex. fat injection) can be considered when the symptom persists over year after local corticocteroid injection.
References
- 1.Papadopoulos PJ, Edison JD. The Clinical Picture - Soft tissue atrophy after corticosteroid injection. Cleve Clin J Med. 2009;76:373–374. doi: 10.3949/ccjm.76a.08096. [DOI] [PubMed] [Google Scholar]
- 2.Newman RJ. Local skin depigmentation due to corticosteroid injection. Br Med J (Clin Res Ed) 1984;288:1725–1726. doi: 10.1136/bmj.288.6432.1725-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogojan C, Hetland ML. Depigmentation--a rare side effect to intra-articular glucocorticoid treatment. Clin Rheumatol. 2004;23:373–375. doi: 10.1007/s10067-004-0905-8. [DOI] [PubMed] [Google Scholar]
- 4.Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206. doi: 10.1186/1471-2474-11-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imagawa K, Ohkuma S. A case of fat injection for treating subcutaneous atrophy caused by local administration of corticosteroid. Tokai J Exp Clin Med. 2010;35:66–69. [PubMed] [Google Scholar]