Abstract
Despite recent advances in vaccine design and strategies, latent infection with herpes simplex virus (HSV) remains a formidable challenge. Approaches involving live-attenuated viruses and inactivated viral preparations were popular throughout the twentieth century. In the past ten years, many vaccine types, both prophylactic or therapeutic, have contained a replication-defective HSV, viral DNA or glycoproteins. New research focused on the mechanism of immune evasion by the virus has involved developing vaccines with various gene deletions and manipulations combined with the use of new and more specific adjuvants. In addition, new “prime-boost” methods of strengthening the vaccine efficacy have proven effective, but there have also been flaws with some recent strategies that appear to have compromised vaccine efficacy in humans. Given the complicated lifecycle of HSV and its unique way of spreading from cell-to-cell, it can be concluded that the development of an ideal vaccine needs new focus on cell-mediated immunity, better understanding of the latent viral genome and serious consideration of gender-based differences in immunity development among humans. This review summarizes recent developments made in the field and sheds light on some potentially new ways to conquer the problem including development of dual-action prophylactic microbicides that prohibit viral entry and, in addition, induce a strong antigen response.
Keywords: symptomatic, asymptomatic, T cells, humoral, adjuvant, seronegative, seropositive, immunity
Introduction
Vaccine research for Herpes Simplex Virus types I (HSV I) and II (HSV II) has seen many more failures than victories in the past several years, principally because the immune evasion mechanisms utilized by the two highly-related viruses are simply too complicated for a conventional vaccine to produce long-term benefits.1,2 While the vaccine candidates have mostly failed, a clear understanding has come to light that the induction of cellular immunity is essential for strong protection.1 We can also now appreciate it better that the vaccines that only “mask” the symptoms of HSV-1 are inherently flawed because people who acquire the disease can still shed the virus asymptomatically and spread it to their uninfected partners.3,4
The lifelong HSV infection is usually acquired through physical contacts, affecting the epithelial cells of oral and genital tissues.5 The infected cells are normally non-keratinized epithelial cells when the infection is oral or vaginal and keratinized on other body tissues. The replication occurs very quickly after the entry of the virions into the host cells. Entry occurs through fusion of viral envelop with plasma membrane of the target cell. It requires concerted action from HSV glycoproteins gB, gC, gD, gH and gL. Participation of host cell co-receptor heparan sulfate and gD receptors, nectin-1, HVEM, or 3-O sulfated heparan sulfate is required for successful penetration of the nucleocapsid and the release of tegument proteins into the cytoplasm of the cell.6,7 Demonstrating the complexity of HSV infection from the very beginning of its lifecycle in the host, alternate routes of entry for HSV including manipulation of a phagocytosis-like pathway have also been shown.7
Tissues infected with HSV become the areas of inflammatory cell recruitment which can give rise to one or more discrete ulcers. While a primary infection can remain mostly asymptomatic, it quietly allows the infection to spread to local neurites of sensory neurons that supply the infected tissues.8 Virions travel along the axons to ultimately reach the sensory ganglia, where they become latent within the neuronal nuclei and re-activate periodically. Many factors can cause viral reactivation, including physical and emotional stress.9 During reactivation, the virus spreads back along the axons to skin or mucosal regions that are supplied by the infected neuron, which results in viral replication in the epithelial cells, human to human shedding and a lifetime of sporadic mucocutaneous lesion formation for many infected individuals.9 Even during latency, hosts are still able to spread infection by shedding virions asymptomatically to other humans.8
It is mostly due to the asymptomatic shedding that people who have the virus can unknowingly transmit it to others through physical contact. In prospective studies of partners where one had HSV, most transmission events were not associated with prodromes or discovered during clinically recognized recurrences. Obviously, many of the transmissions occurred when the symptoms were not present. Thus, given the unpredictable nature of the virus shedding, it is hard to regulate horizontal transmission. Vertical transmission can also occur, in which the mother passes HSV-2 to her neonate. This only occurs, however, if she acquired HSV-2 late in her pregnancy.8 Asymptomatic shedding by a vast majority of infected individuals underscores the urgent need for a therapeutic vaccine that can stop the virus release. Hence, the field of vaccine research for HSV has two major challenges to consider: prevent future transmission to uninfected people and boost the immune system to new levels so that the infected individuals do not develop recurrent symptoms and also do not asymptomatically shed the virus.10
There are two major vaccine types under which vaccine strategies controlling HSV 1 and HSV 2 fall: prophylactic and therapeutic. Prophylactic vaccines are designed to prevent infection in those who are seronegative. Therapeutic vaccines ameliorate symptoms and infectivity for those who are seropositive. In the past, prophylactic vaccines have struggled to prevent latency even in animal models including mice, although strong protection may have been reported in terms of relieving the symptoms of the disease.1,2 In this regard it is worth mentioning that mice are not considered good models to study reactivation since mice do not suffer recurrent episodes.11 The vaccines were also expected to provide strong protection in humans but many such attempts have failed for some of the reasons discussed in this article. Likewise, therapeutic vaccines also needed to reduce the risk of transmission, but none showed consistent success in reducing symptoms or viral shedding. Over the years it has become very clear that the strategies for preventing initial infection and those for controlling recurrence must differ because these two pathways have different mechanisms.11
A Brief and Recent History of Vaccines used for HSV-I and HSV-2
Since the 1920s, HSV-I and HSV-2 vaccines have been tested in laboratory environments, but none have been proven very effective.1 In the past 30 y, great strides have been made in the fields of immunology and cellular biology, enough so that new and creative ways to find vaccines have evolved over the years. Every few years, we stand on the shoulders of previous researchers to determine why vaccines have failed in the past, and to answer some fundamental questions about the HSV virus itself in order to find an effective vaccine to address it. For example, some investigators have put their efforts into identifying the differences in recognition of T cell epitopes between asymptomatic and symptomatic individuals that cause the latter to exhibit frequent outbreaks from the virus. This is an emerging area for research; it is not yet well established that the differences in the recognition of epitopes alone can be responsible for the outbreaks.10
Some of the first vaccine strategies included a virus prepared from formalin treated tissues of HSV-infected animals and UV-inactivated HSV grown in embryonic eggs.1 None of the original strategies gave significant long-term protection, and the failures, in fact, slowed down the herpes vaccine research. Another factor for the slowdown was the lack of new vaccine strategies. Starting in the 1980s, an approach developed that fostered development of vaccines consisting of inactivated viral preparations containing multiple strains.12 The virus particle vaccine F. HSV-2V(PRK), prepared from sonicated lysates of rabbit cells infected with five clinical strains of HSV-2, was investigated in Bulgaria. 55 patients were given 2–4 vaccinations and monitored for six years. It was shown to be antigenic for HSV-1, and showed 5.4% contractions for HSV-2.13 However, this trial was not performed as a double blind, randomized clinical trial, hence the results are harder to interpret. Since then, multiple live-attenuated vaccines have gone through trials, as it was believed that they would elicit a much broader immune response. For example, the vaccine ICP10DPK was found to be protective in guinea pigs, and the results of the initial trials suggested that the vaccine has some therapeutic effectiveness as well.14 This vaccine is based on a deletion of the protein kinase domain of the large subunit of ribonucleotide reductase ICP10, and this domain has polarizing Th2 activity and is required for viral replication and latency reactivation.15
More recently, there has been a shift toward a search for an HSV vaccine that contains replication-impaired HSV. For example, D106, a multiple IE gene deleted, replication-defective HSV-1 recombinant, was shown in 2006 to effectively express foreign genes and create a strong humoral response in mice. It was appealing as a vaccine vector, with strong expression of transgenes, limited cytopathogenicity, and an ability to transfer dendritic cells and bring about their maturation to lymph nodes. A recombinant expressing E. coli β-galactosidase brought about durable β -gal-specific IgC and CD8+ T cell responses in naive and HSV-immune mice.16 In 2007, a project was underway in which the replication-defective mutant strain of the HSV-2 virus, dl5–29, was evaluated to determine if it would protect against HSV-1 corneal infection. It was discovered that in mice, there was a strong correlation between dosage at higher levels of the strain and reductions in facial swelling and lesions. The mice with the greatest levels of dl5–29, at 106 PFUs, also showed a 10-fold reduction in latent viral load.17 In continuation with research on dl5–29, the UL41 gene was disrupted, resulting in the dl5–29–41L candidate, which increased immunogenicity for the HSV-1 virus in a murine model, protecting mice for at least 7 mo.18 Further studies were done manipulating this gene with various deletions, including a 2011 study in which a modified version of dl5–29 was created by replacing the HSV virion host shut-off (vhs) gene with an HSV-1 vhs gene. The immunogenicity, unfortunately, was not found to be too much greater.19
The most recent study involving dl5–29 was also done with a murine model. This was focused on HSV-2, but since HSV-2 is strongly linked with human immunodeficiency virus (HIV), dl5–29 was expected to play an important role as a weapon against HIV as well. A total of six strains were used, strains G, 89–390 and 186 from the United States, and a panel of 3 HSV-2 strains originating in South Africa, strains SD15, SD66 and SD90. The results indicated that it was more difficult to induce protection from the African disease than it was from the United States one: the challenge with the African virus caused less reduction in overall disease (p < 0.001), viral shedding (p = 0.051), or paralysis (p = 0.016), than with the US WT viruses.20
Subunit Vaccines
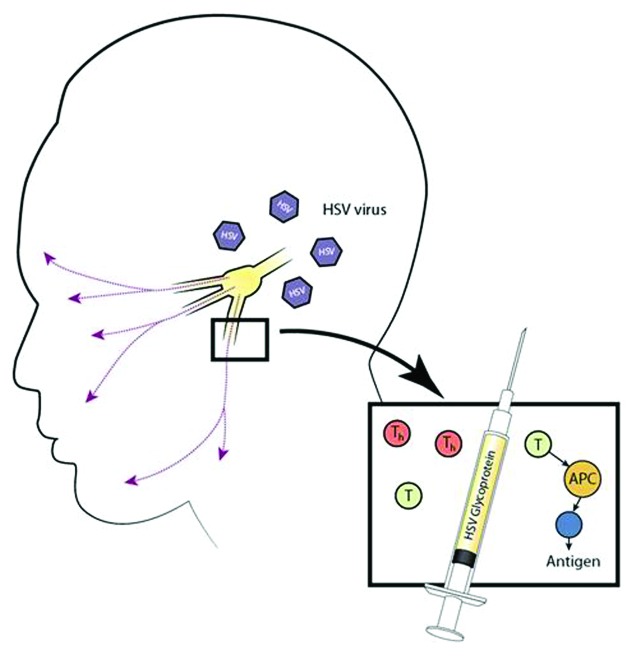
Vaccines with recombinant HSV glycoproteins, primarily gB and gD, have also been developed (Fig. 1). An example of this is a vaccine developed by Chiron, which consists of gD and gB from HSV-2 with the adjuvant MF59.21 This vaccine was successful in inducing high antibody titers and demonstrated about 26% efficacy in women. However, protection among women was effective for only six months and male volunteers were not protected at all. Continuing down this line, GlaxoSmithKline (GSK) developed a sub-unit vaccine that was in phase III clinical trials in 2007, and BioVex also had a vaccine enter phase I clinical trials in 2007 in the UK. The GSK vaccine used glycoprotein-D, which was considered the antigen of choice because, as a protein essential for viral entry, it creates a strong antibody response. The GSK gD-2 subunit vaccine was about 73% protective in women who were seronegative for both HSV-1 and HSV-2 at the time of vaccination. However, it was not protective for women who were seropositive for HSV-1. The vaccine was also not protective in men or HSV-2 seropositive women as far as disease and seroconversion are concerned.22 It has been theorized that the reason that it protects women better than men is due to inherent differences in genital anatomy; vaginal tissues contain components of the mucosal immune system that are likely to be more effective at preventing infection than the ones found in the epidermal tissues of men. As far as why the vaccine was not protective in seropositive women, the answer probably lies in the fact that the gD-2 vaccine may not be powered to adequately boost the immunity in individuals previously infected with HSV-1. Thus, the current vaccine trial is aimed at affirming protection in only seronegative women, which also underscores the need for better understanding of the gender-based differences in immunity development.2
Figure 1. Subunit vaccine. This illustration shows a glycoprotein subunit vaccine administered through a syringe into the oro-facial pathway inducing an accessory cell to elicit a T cell response. The antigen presentation will cause these cells to become either CD4+ cells or CD8+ cells. These will then act against HSV, which has established latency in the trigeminal or cervical ganglia.
One possible way to overcome gender-based differences is to focus on viral immune evasion strategies. Newer subunit vaccines are also focusing on viral glycoproteins that directly participate in immune evasion. In this regard, a recently described candidate involves a combined immunization with gC and gD.23 The driving hypothesis is that the latter is a strong immunogen while the former binds complement component C3b and inhibits complement-mediated immunity. The authors found that the IgG purified from mice immunized with the combination vaccine blocked C3b binding to gC-1 and greatly increased neutralization by gD-1 IgG in the presence of complement. They also noted better protection than gD-1 alone in preventing zosteriform disease and infection of dorsal root ganglia. The authors concluded that gC-1 immunization prevents HSV-1 immune evasion and enhances the protection provided by gD-1 immunization.23
Viral Vaccines
Viral vaccines have been the most focused on since the 1980s. They work partly by inducing antibodies intended to neutralize the virus, which allows for later immune resistance against the virus. However, the methods within which strategies function using this principle are significant.24 For example, live virus vaccines were found to induce greater cellular immunity than inactivated vaccines.25 Neutralizing antibodies in HSV-2 infected persons are mainly directed against gB and gD. It has come to light in later years that the body can react with a CD8 and CD4 T cell response, which certain vaccines can elevate. A research study done at the University of Washington in 2006 was the first to evaluate CD8+ T cell responses to a significant range of the predicted HSV-2 proteome, and it discovered 48 ORFs that CD8+ T cells responded to. Using peptide sets based on the complete genome of a laboratory strain of HSV-2, the study indicated that persons with allele HLA A*02 had a lower response to HSV-2 than those who did not have that allele, and the persons with HLA B*07 showed a greater frequency of responses to the peptides than those without. Before this, the range of ORFs that were thought to be responded to by HSV-2 was relatively narrow.26
A live virus vaccine developed by BioVex, called ImmunoVex, aims at addressing the problem of HSV immune evasion by removing the genes involved. This is because in addition to HSV glycoprotein C binding to C3b to prevent the activation of the complement cascade, HSV also encodes proteins that block interferon (IFN) responses and antigen presentation to CD8+ T-cells. The genes that were deleted in this vaccine normally downregulate major histocompatibility complex (MHC) presentation, dendritic cell activation and IFN responses. Two doses of the vaccine showed efficacy in guinea pigs, and this vaccine is now under Phase 1 clinical study using human volunteers.4 Results of the study are expected soon and hope is that apart from providing prophylactic efficacy it will also be helpful in treating preexisting infections. A similar live attenuated HSV-2 vaccine based on a replication competent ICP10 mutant is also currently Phase II clinical study by AuRix Biotech.27
Many other vaccine strategies are also taking into account immune evasion aspects of HSV. The HSV immediate early protein ICP47 can inhibit CD8+ T cell recognition of infected cells and has a predilection to bind with transporter protein TAP and thus inhibit the transport of peptides into the endoplasmic reticulum, where they otherwise would form a complex with MHC class I molecules and be transported to the cell surface to be recognized by CD8+ CTLs. This contributes to viral virulence.28 Also, HSV infects dendritic cells (DCs) in their immature state, bringing about downregulation of adhesion and costimulatory molecules.29 It has been discovered that HSV-2 infection of human monocyte-derived dendritic cells causes apoptosis, but uninfected dendritic cells can phagocytose cell fragments from the dying cells and cross-present the antigens. Thus, the host may counter the viral effects on dendritic cells by priming T cells in the lymph nodes through cross-presentations of the antigens by uninfected dendritic cells.30 Replication incompetent live viruses can provide another alternative for a safe therapeutic vaccine development. Recently, a randomized multicenter placebo-controlled trial was performed using a replication incompetent HSV-2 lacking the essential glycoprotein H gene. The live virus was tested as a disabled infectious single cycle (DISC) virus therapeutic vaccine for reducing reactivation and clinical disease among immunocompetent persons with recurrent genital HSV-2 infection.31 Healthy volunteers with six or more recurrences of genital herpes per year received injections of vaccine at 0 and 8 or 0, 4 and 8 or 0, 2, 4 and 8 weeks or placebo and were followed for subsequent recurrences for 1 y. While this replication incompetent HSV-2 vaccine lacking the glycoprotein H gene did not show any safety problems, the median times to first recurrence of genital herpes, mean number of recurrences, and time to lesion healing of the first recurrence were statistically very similar for all treatment or control groups. It was therefore concluded that it had no clinical or virological benefits in the amelioration of the genital disease among healthy men and women. Current efforts on the development of the DISC vaccine are now refocused on prophylactic usages.
DNA Vaccines
DNA vaccines, such as ones that encode cytokines, like interleukin-8, have been shown to cause enhanced cell-mediated immune responses. Encoding various molecules and modifying the vector for high level gene expression are two of many methods that were started to increase the potency of DNA vaccines. In 2000, a study found higher survival rates by co-injecting chemokines IL-8 and RANTES cDNA than by just using a gD vaccine alone.32 In a study done in 2004 by Johns Hopkins University, the number of antigen-expressing DCs was characterized and the question as to whether the linkage of VP22 to antigen would influence the ability of antigen-expressing DCs to activate antigen-specific CD8+ T cells in vivo was posed. Also, linkage of VP22 to E7 improved the MHC class I presentation of E7 in transfected DCs and led to enhanced activation of E7-specific CD8+ T cells.33 This was one of many studies in which an adjuvant combined with a DNA vaccine approach appeared to be a plausible method of boosting immune responses.
Another study involving adjuvants done more recently occurred in 2006 at Göteburg University in Sweden. The study was initiated to investigate the potential of CpG-containing oligodeoxynucleotide (CpG ODN) for induction of chemokine responses in the vaginal mucosa as well as its potential as a vaginal adjuvant in combination with gD of HSV-2. Both results demonstrated potential: a single intravaginal administration of CpG ODN in mice was found to induce a quick and potent response of CC chemokines macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta and chemokine RANTES as well as of CXC chemokines MIP-2 and IP-10 in the vagina and/or in the genital lymph nodes. In addition, the adjuvant, when acting with gD, was found to bring about an antigen-specific Th1-like immune response in the genital lymph nodes as well as the spleens of the vaccinated mice.34
Most DNA vaccine research has shifted into prime-boost immunization strategies, which seem to be necessary to elicit an effective immune response in humans. In an older study, a plasmid vector that expressed gD and a recombinant modified vaccinia virus Ankara vector that expressed gD (MVA-gD2) were combined. The IgG antibody response to gD and the HSV-2 neutralizing antibody response were greatest when the MVA-gD2 vector was used as the priming immunization and then was boosted with either pgD2 or MVA-gD2. When examining the isotype profile, a much broader distribution was found than that seen after the DNA vaccination. It was concluded that the prime boost strategy that used the MVA and plasmid DNA vector enhanced the humoral and cellular immune response to HSV-2 gD.35 A follow-up study done by the same researchers four years later evaluated the use of a needle-free delivery platform (Biojector) for delivery of plasmid and MVA gD-expressing vectors in a prime-boost immunization strategy. They found similar results in that the delivery induced a more potent immune response and broader isotype response than that found by gene gun delivered plasmid DNA. It resulted in a diverse antibody isotype distribution, much like the previous study using the MVA.36 Increases in T cell number and response have been observed in mice across prime-boost strategy studies, and both CD4+ and CD8+ T responses were observed in humans following the delivery of the antigen by pDNA followed by that of the recombinant vaccinia virus.37
Recent Progress in Viral and DNA Vaccines
A unique and recent research study conducted by the Hutchinson Cancer Research Center in 2012 recognized that the main reason for the failure of a therapeutic herpes simplex virus vaccine is that the current vaccine strategies have mostly focused on replicating viruses, their proteins or DNA and do not put any emphasis on eradicating latently integrated or nonreplicating episomal viral genomes. This study concerned endonucleases, which can cleave viral genomes that are latently integrated within the body. These enzymes are being engineered with a high level of accuracy so that there will not be any errors in critical site binding. A critical region of interest is one within a latent viral genome. Also, imprecise non-homologous end joining (NHEJ) repair, which when occurring during repeated cleavage at the same site, can disrupt the entry of crucial viral proteins. The delivery vectors and considerations of different DNA cleavage enzymes, zinc-finger endonucleases, transcription activator-like (TAL) endonucleases (TALENs) and homing endonucleases, are now under investigation.38 Another study that also paid a close attention to the latent viral genome was done recently with a cationic lipid-based antigen called Vaxfectin expressing gD2, in a murine model: 40% of the mice vaccinated with this Vaxfectin-gD2 pDNA had no detectable HSV-2 viral genomes.39
Another new focus of emerging strategies includes cell-mediated responses to viral proteins. Much of new research has continued with the focus on the envelope glycoproteins of HSV-1 and HSV-2 and resulting cell-mediated responses. A recent study conducted by the University of Pisa used mice to test the gB1 vaccine by way of a feline immunodeficiency virus (FIV) vector. The vaccine induced cross-neutralizing antibodies to activate and cell-mediated responses that protected 100% and 75% of the mice from HSV-1 and HSV-2 infection.40 A study done recently in Sweden showed glycoprotein G, in combination with adjuvant CpG, might be a promising vaccine antigen. The sera from these mice showed macrophage-mediated, antibody-dependent cellular cytotoxicity and antibody-dependent complement-mediated cytolysis. The protection was caused by a gamma interferon (IFN-γ) response by splenic CD4(+) T cells associated with antigen restimulation in vitro.41 Also showing this gamma interferon response, i.d. immunization from IC31(®)-adjuvanted gD showed 80–100% protection in mice against a lethal vaginal HSV-2 challenge; it also lessened disease severity and viral replication.42 gB2466–473, gC2216–223, gE2483–491, gG2572–579 and gI2286–295, immunodominant epitope peptides from glycoproteins B, C, E, G and I of HSV-2, respectively, were shown to stimulate mice to create antibodies that could neutralize HSV-2 in vitro at Nanjing Medical University.43 CJ2-gD2, a new class of a replication-defective viral vaccine expressing gD2, demonstrated preclinical immunogenicity, eliciting strong HSV-2-specific memory CD4(+) and CD8(+) T-cell responses. gD2 is expressed as efficiently as wild-type HSV-2 infection and can lead to a 150-fold reduction in wild-type HSV-2 viral replication in cells co-infected with CJ2-gD2 and wild-type HSV-2 at the same multiplicity of infection.44-46
A DNA prime-formalin-inactivated-HSV-2 (FI-HSV2) boost vaccine approach has recently been tested in the guinea pig model of acute and recurrent HSV-2 genital disease. Two groups were primed with plasmid DNAs encoding the secreted form of glycoprotein D2 (gD2t) and either the helicase (UL5) and DNA polymerase (UL30) genes or the single-stranded DNA binding protein (UL29) and primase (UL52) genes. Both DNA-primed groups were boosted with FI-HSV2 formulated with monophosphoryl lipid A (MPL) and alum adjuvants. All mock controls developed recurrent lesions. However, the UL5, UL30, gD2t DNA-FI-HSV2 group demonstrated a 97% loss of recurrent lesions, and contained the lowest mean HSV-2 DNA load in the dorsal root ganglia. Thus, this combined prime-boost approach may hold new promise for protection against HSV-2.47,48
Another newer approach involves a candidate vaccine (called HerpV) consisting of 32 synthetic 35mer HSV-2 peptides non-covalently complexed with recombinant human Hsc70 protein. These peptides represent 22 HSV-2 proteins associated with viral replication. A recently concluded Phase 1 study had four randomized groups: HerpV+QS-21 (saponin adjuvant), HerpV, QS-21, or vehicle. In all cased HerpV was safe and well tolerated. The HSV-2 positive participants who were administered HerpV+QS-21 demonstrated strong CD4(+) and CD8(+) T cell responses to HSV-2 antigens. The findings are very encouraging that a broad CD4(+) and CD8(+) T cell response in HSV-2(+) participants can be obtained via a peptide-based vaccine.49,50
What Have We Learned
It is essential to have a vaccine that elevates T cell based immune responses. It is not that neutralizing antibody response is not helpful, but mostly due to the clever immune evasion strategies adapted by the virus, the best protection would require stronger cell-mediated immunity as well as better humoral responses against the virus. This conclusion has been clearly supported by the shortcomings of the above-mentioned examples of recent vaccine strategies.1,2 Also, besides the endonucleases and Vaxfectin studies discussed previously, recent studies are still too focused on ameliorating viral replication rather than annihilating the latent viral genomes.
In the most recent history of developing vaccine strategies against HSV-1 and HSV-2, as well as in all history, vaccines have either not reached phase II of clinical trials or they haven’t been as effectual in humans as they were in the murine or other laboratory models. What the future seems to hold in store is based on what has been most recently discovered about T cells. T cells respond differently to HSV epitopes depending on whether the individual is “symptomatic” or “asymptomatic,” or having no recurrent clinical disease vs. having clinical, orofacial and or genital episodes, respectively.1,45 Vaccine research now needs to focus on identifying the entire spectrum of HSV-1 and HSV-2 epitopes that are recognized by T cells from both asymptomatic and symptomatic individuals. A new vaccine rich in asymptomatic epitopes can boost local mucosal and systemic protective immunity, which may permanently stop symptoms and virus shedding.9 In addition, new and bold ideas such as generating local memory T cells using a recently described 'prime and pull' approach to concentrate tissue-resident memory T cells at a site of potential viral exposure,46 using novel virally encoded co-stimulatory molecules51 or a microbivac, where a microbicide provides immediate protection by trapping virus particles and then presenting them for mucosal immunity development, can revolutionize the field and help eradicate the virus (Fig. 2).5
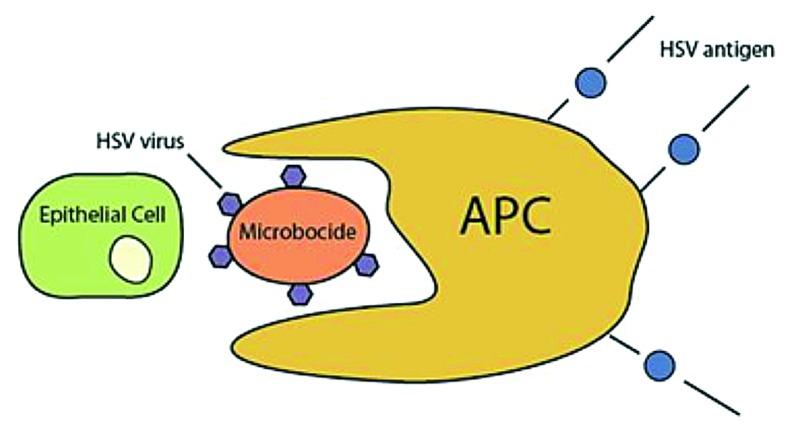
Figure 2. Microbivac model. This model shows a microbicide preventing viral entry into an epithelial cell while eliciting antigen presentation from an accessory cell, inducing long-term immunity. The microbivac concept is based on a hypothetical microbicide that traps virions and serves as an antigen-presenting vaccine platform.
The microbivac concept uses a virus-trapping agent that also acts as an adjuvant, prohibiting entry while also inducing a strong antigen response against the virions trapped by the microbivac. The virus attachment to cells via heparan sulfate (HS) can be targeted for microbivac action since, as mentioned earlier, HS is crucial for the entry of the virus. Targeting attachment will be especially helpful since HS has a “broad spectrum effect,” as many other viruses and bacteria bind HS as their first step in pathogenesis. Hopefully, such a “microbivac” would exhibit a strong vaccine efficacy by itself. Alternatively, it may also be combined with additional vaccines to potentially generate even stronger protective benefits.3 In any case, the field still needs innovative ways to overcome the challenges posed by HSV to traditional vaccines strategies.
Acknowledgments
This work was supported by National Institutes of Health grant AI103754 to D.S. and EY01792, a core grant.
Glossary
Abbreviations:
- gB
gC, gD, gL, gH, glycoprotein-B/C/D/L/H
- Th2
T-helper 2
- GSK
GlaxoSmithKline
- IFN
interferon
- IFN-γ
gamma interferon
- TAL
transcription activator-like
- TALENS
transcription activator-like endonucleases
- MHC
major histocompatibility complex
- DCs
dendritic cells
- ORFs
open reading frames
- CpG ODN
CpG-containing oligodeoxynucleotide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23289
References
- 1.Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121:4600–9. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, et al. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol. 2012;7:371–8. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brittle EE, Friedman HM. Current herpes simplex virus vaccine approaches - a short review. US Infectious Diseases. 2006;1:13–6. [Google Scholar]
- 5.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–10. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla D. Unraveling the cell entry mechanisms of HSV: therapeutic potential? Future Virol. 2012;7:427–30. doi: 10.2217/fvl.12.33. [DOI] [Google Scholar]
- 7.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–36. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koelle DM, Wald A. Herpes simplex virus: the importance of asymptomatic shedding. J Antimicrob Chemother. 2000;45(Suppl T3):1–8. doi: 10.1093/jac/45.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 9.Salameh S, Sheth U, Shukla D. Early events in herpes simplex virus lifecycle with implications for an infection of lifetime. Open Virol J. 2012;6:1–6. doi: 10.2174/1874357901206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–9. doi: 10.1016/S0264-410X(98)00434-4. [DOI] [PubMed] [Google Scholar]
- 11.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, Benmohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dundarov S, Andonov P, Bakalov B. Characterization of herpes simplex virus strains isolated from patients with various diseases. Arch Virol. 1980;63:115–21. doi: 10.1007/BF01320768. [DOI] [PubMed] [Google Scholar]
- 13.Skinner GR, Davies JA, Dundarov S, Andonov P. Prevention of herpes genitalis by the ‘Bulgarian’ vaccine F.HSV-2V(PRK): preliminary clinical evidence. Croat Med J. 2000;41:378–83. [PubMed] [Google Scholar]
- 14.Wachsman M, Kulka M, Smith CC, Aurelian L. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine. 2001;19:1879–90. doi: 10.1016/S0264-410X(00)00446-1. [DOI] [PubMed] [Google Scholar]
- 15.Aurelian L. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin Diagn Lab Immunol. 2004;11:437–45. doi: 10.1128/CDLI.11.3.437-445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe D, Brockman MA, Ndung’u T, Mathews L, Lucas WT, Murphy CG, et al. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357:186–98. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 17.van Lint AL, Torres-Lopez E, Knipe DM. Immunization with a replication-defective herpes simplex virus 2 mutant reduces herpes simplex virus 1 infection and prevents ocular disease. Virology. 2007;368:227–31. doi: 10.1016/j.virol.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudek T, Mathews LC, Knipe DM. Disruption of the U(L)41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology. 2008;372:165–75. doi: 10.1016/j.virol.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reszka NJ, Dudek T, Knipe DM. Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein. Vaccine. 2010;28:2754–62. doi: 10.1016/j.vaccine.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011;203:1434–41. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003;187:542–9. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 22.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Herpevac Trial for Women Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awasthi S, Lubinski JM, Friedman HM. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine. 2009;27:6845–53. doi: 10.1016/j.vaccine.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola JR. Herpes simplex virus vaccines--why don’t antibodies protect? JAMA. 1999;282:379–80. doi: 10.1001/jama.282.4.379. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen LH, Knipe DM, Finberg RW. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–72. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006;80:5509–15. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gyotoku T, Ono F, Aurelian L. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10DeltaPK. Vaccine. 2002;20:2796–807. doi: 10.1016/S0264-410X(02)00199-8. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith K, Chen W, Johnson DC, Hendricks RL. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–8. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–7. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 30.Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, et al. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis. 2003;187:165–78. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- 31.de Bruyn G, Vargas-Cortez M, Warren T, Tyring SK, Fife KH, Lalezari J, et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine. 2006;24:914–20. doi: 10.1016/j.vaccine.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 32.Sin J, Kim JJ, Pachuk C, Satishchandran C, Weiner DB. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4(+) T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J Virol. 2000;74:11173–80. doi: 10.1128/JVI.74.23.11173-11180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TW, Hung CF, Kim JW, Juang J, Chen PJ, He L, et al. Vaccination with a DNA vaccine encoding herpes simplex virus type 1 VP22 linked to antigen generates long-term antigen-specific CD8-positive memory T cells and protective immunity. Hum Gene Ther. 2004;15:167–77. doi: 10.1089/104303404772679977. [DOI] [PubMed] [Google Scholar]
- 34.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J Virol. 2006;80:5283–91. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meseda CA, Elkins KL, Merchlinsky MJ, Weir JP. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J Infect Dis. 2002;186:1065–73. doi: 10.1086/344234. [DOI] [PubMed] [Google Scholar]
- 36.Meseda CA, Stout RR, Weir JP. Evaluation of a needle-free delivery platform for prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus 2 glycoprotein D. Viral Immunol. 2006;19:250–9. doi: 10.1089/vim.2006.19.250. [DOI] [PubMed] [Google Scholar]
- 37.Zhao HP, Sun JF, Li N, Sun Y, Wang Y, Qiu HJ. Prime-boost immunization using alphavirus replicon and adenovirus vectored vaccines induces enhanced immune responses against classical swine fever virus in mice. Vet Immunol Immunopathol. 2009;131:158–66. doi: 10.1016/j.vetimm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Schiffer JT, Aubert M, Weber ND, Mintzer E, Stone D, Jerome KR. Targeted DNA mutagenesis for the cure of chronic viral infections. J Virol. 2012;86:8920–36. doi: 10.1128/JVI.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlapobersky M, Marshak JO, Dong L, Huang ML, Wei Q, Chu A, et al. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J Gen Virol. 2012;93:1305–15. doi: 10.1099/vir.0.040055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiuppesi F, Vannucci L, De Luca A, Lai M, Matteoli B, Freer G, et al. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J Virol. 2012;86:6563–74. doi: 10.1128/JVI.00302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Görander S, Harandi AM, Lindqvist M, Bergström T, Liljeqvist JÅ. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol. 2012;86:7544–53. doi: 10.1128/JVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wizel B, Persson J, Thörn K, Nagy E, Harandi AM. Nasal and skin delivery of IC31(®)-adjuvanted recombinant HSV-2 gD protein confers protection against genital herpes. Vaccine. 2012;30:4361–8. doi: 10.1016/j.vaccine.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Pan M, Wang X, Liao J, Yin D, Li S, Pan Y, et al. Prediction and identification of potential immunodominant epitopes in glycoproteins B, C, E, G, and I of herpes simplex virus type 2. Clin Dev Immunol. 2012;2012:205313. doi: 10.1155/2012/205313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhrameyeva NV, Zhang P, Sugiyama N, Behar SM, Yao F. Development of a glycoprotein D-expressing dominant-negative and replication-defective herpes simplex virus 2 (HSV-2) recombinant viral vaccine against HSV-2 infection in mice. J Virol. 2011;85:5036–47. doi: 10.1128/JVI.02548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, et al. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol. 2012;86:4358–69. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–7. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morello CS, Kraynyak KA, Levinson MS, Chen Z, Lee KF, Spector DH. Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge. Vaccine. 2012;30:6541–50. doi: 10.1016/j.vaccine.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morello CS, Levinson MS, Kraynyak KA, Spector DH. Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease. J Virol. 2011;85:3461–72. doi: 10.1128/JVI.02521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wald A, Koelle DM, Fife K, Warren T, Leclair K, Chicz RM, et al. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine. 2011;29:8520–9. doi: 10.1016/j.vaccine.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 50.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, et al. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A. 2003;100:12899–904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vagvala SP, Thebeau LG, Wilson SR, Morrison LA. Virus-encoded b7-2 costimulation molecules enhance the protective capacity of a replication-defective herpes simplex virus type 2 vaccine in immunocompetent mice. J Virol. 2009;83:953–60. doi: 10.1128/JVI.02022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]