Abstract
Objectives: The protective response against Treponema pallidum (Tp) infection of a DNA vaccine enhanced by an adjuvant CpG ODN was investigated.
Results: The mucosal adjuvant CpG ODN enhanced the production of higher levels of anti-TpGpd antibodies induced by pcD/Gpd-IL-2 in rabbits. It also resulted in higher levels of secretion of IL-2 and IFN-γ, and facilitated T cell proliferation and differentiation (p < 0.05). No significant difference about testing index above-mentioned was found in the intranasal immunization group of pcD/Gpd-IL-2 vaccine adjuvanted by CpG ODN when compared with the immunization by pcD/Gpd-IL-2 vaccine intramuscular injection alone (p > 0.05). Furthermore, CpG ODN stimulated the production of mucosa-specific anti-sIgA antibodies and resulted in the lowest Tp-positive rate (6.7%) for Tp-infection of skin lesions and the lowest rates (8.3%) of ulceration lesions, thus achieving better protective effects.
Methods: New Zealand rabbits were immunized with the eukaryotic vector encoding recombinant pcD/Gpd-IL-2 using intramuscular multi-injection or together with mucosal enhancement via a nasal route. The effect of the mucosal adjuvant CpG ODN was examined.
Conclusions:The CpG ODN adjuvant significantly enhances the humoral and cellular immune effects of the immunization by pcD/Gpd-IL-2 with mucosal enhancement via nasal route. It also stimulates strong mucosal immune effects, thus initiating more efficient immune-protective effects.
Keywords: Treponema pallidum, membrane protein, Tp Gpd, CpG ODN, DNA vaccine, mucosal immune, mucosal adjuvant
Introduction
Treponema pallidum (Tp) infects humans through mucous membranes or injured skin. Local mucosal specific sIgA is of great importance for the early anti-Tp infection. Systemic humoral and cellular immune responses are the main factors to prevent Tp proliferation and ultimately eliminate Tp.1 Therefore, ideal anti-Tp vaccine should stimulate systemic immunity and mucosal immunity. However, conventional intramuscular injection of DNA vaccine usually induces strong systemic humoral and cellular immune responses, but low levels of local mucosal immunity, which makes it difficult to achieve the desired immune protection. However, DNA vaccines enhanced by adjuvants may resolve this problem.
Tp usually invades genital mucosa. Due to the inconvenient operation and low efficacy, rectal and vaginal immunization requires large doses of antigen and adjuvant. The common mucosal immune system (CMIS) in the body induces distant mucosal surface to secrete antigen-specific sIgA after part of mucosal surface is immunized.2,3 In recent years, the nasal mucosal immunity gets more and more attention. It has been shown4,5 that intranasal vaccination is the most effective way to obtain mucosal immunity. The intranasal vaccination induces relatively high levels of IgA and IgG antibodies not only in the proximal nasal, bronchia and lung, but also in the rectum, vagina and other remote mucosa, resulting in various mucosal protections.
Because of the special physical and chemical condition of mucosal surface, the immune responses to vaccine immunogen are weakened. Therefore appropriate mucosal immune vector is required to effectively stimulate the mucosal immune system. Some material from bacterium have been used for mucosal vaccine adjuvants and vectors, such as cholera toxin (CT) and heat-labile entertoxin (LT) of Escherichia coli.6-12 Although CT and LT are good mucosal adjuvants, animal experiments have shown considerable toxicity. Therefore, clinical application of CT and LT is limited for their enterotoxin. Synthetic unmethylated CpG oligodeoxynucleotides (CpG ODN) are a kind of mucosal adjuvants with worldwide attention for its broad application prospects in the recent years. McCluskie et al.13 performed rat experiments with hepatitis B surface antigen nuclear TT as the antigen and CpG as an adjuvant through oral and nasogastric immunization, respectively. Compared with CT adjuvant group, CpG was better both for the mucosal immunity induction and immune response, which shows CpG is an effective mucosal immune adjuvant.
Our previous studies have shown the enhanced humoral and cellular immune response and protective efficacy of Tp92 or TpGpd membrane protein DNA vaccine, adjuvanted with IL-2 and vectored with chitosan (CS) nanoparticles, but the intramuscular injection of DNA vaccines cannot induce complete immune protection in a Tp rabbit challenge model.14,15 Therefore, based on the previous research work,14 fusion expression of recombinant pcDNA3.1/Gpd-IL-2 was successfully constructed by genetic engineering techniques. The immune response was enhanced and induced by IL-2 as an adjuvant in this study. In the meantime, intramuscular injection of primary CpG ODN DNA vaccine enhanced by nasogastric mucosal vaccination was adopted in order to fully strengthen immunization, especially mucosal immunity and eventually establish active anti-Tp immunity in animal models.
Results
CpG ODN significantly enhances levels of the serum-specific IgG antibody induced by DNA vaccine
New Zealand white rabbits were randomly divided into six groups, with each group consisting of 18 rabbits. Primary immunization of the pcD/Gpd-IL-2 DNA vaccine and empty plasmid were intramuscularly multi-inoculated into the quadriceps of left leg in rabbits. The vaccination strategy and experimental grouping were given in Table 1. To compare the pcD/Gpd-IL-2 DNA vaccine-induced humoral immune effects among groups, indirect enzyme linked immunosorbent assay (ELISA) method was used to measure antigen-specific antibody levels in the serum in each group.
Table 1. Vaccination strategy and experimental grouping.
| Group | No. of rabbits | pcDvaccine inoculation /each rabbit | pcD/Gpd-IL-2 vaccine inoculation /each rabbit | CpG ODN adjuvant inoculation /each rabbit | Immunization strategy immunization phase: vaccine types (inoculation method) intramuscular injection(im) Nasal mucosal immunization(Nasal) |
|---|---|---|---|---|---|
| A1 | 18 | 100 μg | Primary: pcD control(im), Second:pcD control(im) | ||
| A2 | 18 | 100 μg | Primary: pcD/Gpd-IL-2 (im), Second:pcD/Gpd-IL-2 (im) | ||
| B1 | 18 | 100 μg | Primary: pcD/Gpd-IL-2 (im), Second:pcD/Gpd-IL-2(Nasal) | ||
| B2 | 18 | 100 μg | 10 μg | Primary: pcD/Gpd-IL-2 (im), Second:pcD/Gpd-IL-2+CpGODN(Nasal) | |
| C1 | 18 | 100 μg | Primary: pcD (im), Second:pcD(Nasal) | ||
| C2 | 18 | 100 μg | 10 μg | Primary: pcD (im), Second:pcD+CpGODN(Nasal) |
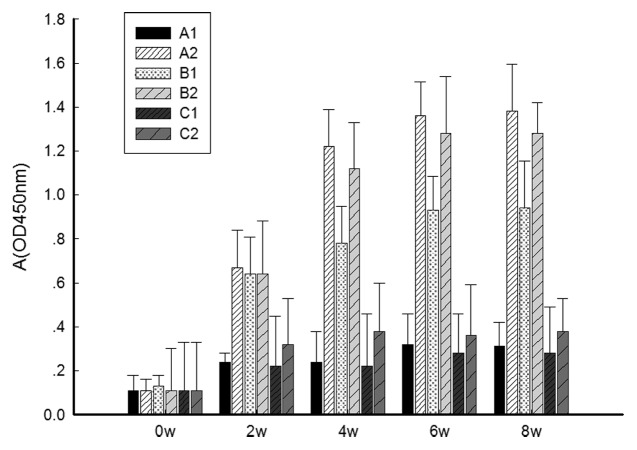
As shown in Figure 1, the specific anti-Gpd IgG antibody levels in all experimental groups (A2, B1 and B2) were significantly (p < 0.001) higher than the levels in the control groups 4 weeks, 6 weeks and 8 weeks after primary immunization, respectively (A1, C1 and C2). Anti-Gpd IgG antibody levels were significantly (p < 0.05) higher at each point after immunization (4 weeks, 6 weeks, 8 weeks) between group B2 and B1, group A2 and B1. However, there were no significantly difference (p > 0.05) between group A2 and B2, group A1 and C1, C2 and control group. The above results suggest that the mucosal adjuvant CpG ODN significantly enhances serum specific IgG antibody levels of the pcD/Gpd-IL-2 DNA vaccine.
Figure 1. Changes in absorbance values of IgG antibodies in the sera of experimental rabbits after immunization. At 0, 2, 4, 6 and 8 weeks post-immunization, blood was drawn from rabbit ear veins. The anti-TpGpd antibody levels from sera determined by the indirect ELISA method. OD 450, optical density at 450 nm. Groups: A1, 2× intramuscular immunization of pcD (100 μg); A2, 2× intramuscular immunization of pcD/Gpd-IL-2(100 μg); B1, 1× intramuscular immunization pcD/Gpd-IL-2 (100 μg) and 1× intranasal immunization of pcD/Gpd-IL-2(100 μg); B2, 1× intramuscular immunization pcD/Gpd-IL-2(100 μg) and 1× intranasal immunization of pcD/Gpd-IL-2 (100 μg) and CpGODN (10 μg); C1, 1× intramuscular immunization pcD (100 μg) and 1× intranasal immunization of pcD (100 μg); C2, 1× intramuscular immunization pcD (100 μg) and 1× intranasal immunization of pcD (100 μg) and CpGODN (10 μg).
CpG ODN significantly enhances the secretion levels of DNA vaccine-induced IFN-γ and IL-2 cytokines
In the above inoculation experiments, the rabbit spleen cells were collected in week 8 and cultured. The IL-2 and IFN-γ levels in the supernatants harvested from the cultured cells were determined by ELISA to evaluate and compare cytokine stimulation of each vaccine. As given in Table 2, IL-2 and IFN-γ secretion in rabbit spleen lymphocyte culture supernatant of vaccine groups (A2, B1, B2) were significantly (p < 0.001) higher than those in the control group (A1, C1 and C2). The IL-2 secretion level of rabbit spleen lymphocyte culture supernatant was not significantly different between group B1 and B2 (p > 0.05), but there was significantly difference between B1 and A2 (p < 0.001). There was significant difference (p < 0.05) in IFN-γ secretion levels between B1 and A2 or B2. There was significantly difference in IL-2 and IFN-γ secretion levels between C2 and A1 or C1 (p < 0.05). There was no significantly difference between A1 and C1 (p > 0.05). The above results suggest that mucosal adjuvant CpG ODN significantly enhances pcD/Gpd-IL-2 DNA vaccines to stimulate IFN-γ cytokine secretion levels in spleen lymphocytes.
Table 2. Levels of cytokines from splenocytes after TpGpd stimulation in vitro.
| Group | IL-2 (pg/ml) | P-value* | IFN-γ (pg/ml) | P-value* |
|---|---|---|---|---|
| A1 | 36.6 ± 6.2 | p < 0.001(A1-A2,B1,B2), p = 0.724(A1-C1), p = 0.085(A1-C2) | 31.7 ± 5.4 | p < 0.001(A1-A2,B1,B2), p = 0.823(A1-C1), p = 0.111(A1-C2) |
| A2 | 158.3 ± 14.6 | p = 0.004 (A2-B2,), p < 0.001(A2-B1, C1,C2) | 449.8 ± 22.5 | p = 0.379 (A2-B2), p < 0.001(A2-B1, C1,C2) |
| B1 | 107.4 ± 23.8 | p = 0.217 (B1-B2), p < 0.001(B1-C1,C2) | 363.3 ± 34.4 | p = 0.002(B1-B2), p < 0.001(B1-C1,C2) |
| B2 | 122.2 ± 19.4 | p < 0.001(B2-C1,C2) | 433.4 ± 32.8 | p < 0.001(B2-C1,C2) |
| C1 | 32.5 ± 4.7 | p = 0.045(C1-C2) | 27.6 ± 4.6 | p = 0.075(C1-C2) |
| C2 | 57.9 ± 8.8 | 62.6 ± 9.2 |
The groups are as given in legend of Figure 1. With a one-way analysis of variance with a Bonferroni post hoc test (one-way ANOVA),IL-2 (A1, A2, B1, B2, C1, C2): f = 42.598, p < 0.001; IFN-γ (A1, A2, B1, B2, C1, C2): f = 267.779, p < 0.001. Pairwise comparison was tested in groups (A1-C2). For example, “A1-A2” indicates that pairwise comparison was detected between the A1 control group and the A2 vaccine experimental group.
CpG ODN significantly enhances the pcD/Gpd-IL-2 DNA vaccine-induced rabbit spleen cell stimulation index (SI)
Rabbit spleen cells were collected at week 8 and proliferation of T cells were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT) method. As shown in Table 3, 8 weeks after immunization, rabbit spleen cell SI in vaccine groups (A2, B1, B2) was significantly (p < 0.001) higher than those in the control groups (A1, C1, C2). The SI was significantly (p < 0.05) higher in group B1 than groups A2 and B2. There was no significant difference between group A2 and group B2, or between C2 and C1 (p > 0.05). The above results suggest that CpG ODN significantly enhances the pcD/Gpd-IL-2 DNA vaccine-induced rabbit spleen cell SI.
Table 3. Stimulation index (SI) of spleen cells.
| Group | n | SI(week 8) | P-value* |
|---|---|---|---|
| A1 | 3 | a 0.87 ± 0.23 | p < 0.001(A1-A2,B1,B2), p = 0.418(A1-C1), p = 0.820(A1-C2) |
| A2 | 3 | 4.52 ± 0.34 | p = 0.418(A2-B2), p < 0.001(A2- B1,C1,C2) |
| B1 | 3 | 3.42 ± 0.24 | p = 0.001(B1-B2), p < 0.001(B1-C1,C2) |
| B2 | 3 | 4.34 ± 0.32 | p < 0.001(B2-C1,C2) |
| C1 | 3 | 0.69 ± 0.17 | p = 0.305 (C1-C2) |
| C2 | 3 | 0.92 ± 0.24 |
Note: a, mean ± SD *The groups are as given in the legend of Figure 1. By a one-way ANOVA,SI (A1, A2, B1, B2, C1, C2): f = 145.147, p < 0.001. Pairwise comparison was tested in groups (A1−C2). For example, “A1−A2” indicates that pairwise comparison was detected between the A1 control group and the A2 vaccine experimental group.
CpG ODN significantly enhances pcD/Gpd-IL-2 DNA vaccine-induced mucosal specific sIgA antibody
ELISA was performed to evaluate and compare mucosal immunity, using specific SIgA secretion levels in nasopharynx and vaginal washing fluid 8 weeks after immunization. As shown in Table 4, the average sIgA levels of nasopharynx and the vaginal washing fluid were significantly (p < 0.005) higher in the vaccine group (B1, B2) than in the control group (A1, C1, C2) 8 weeks after immunization. There was significant difference (p < 0.05) between group A2 and B1 or B2. There was significant difference (p < 0.05) between groups B1 and B2. But there was no significant difference (p > 0.05) between A1, A2 and C1 or C2. The above results suggeste that CpG ODN significantly enhances the pcD/Gpd-IL-2 DNA vaccine-induced specific mucosal SIgA antibody.
Table 4. Levels of SIgA antibody from immuned rabbits.
| Group | The SIgA antibody level of Nasopharyngeal fluid (A492) | P-value* | The SIgA antibody level of Vaginal fluid(A492) | P-value* |
|---|---|---|---|---|
| A1 | 0.082 ± 0.010 | p = 0.078 (A1-A2), p < 0.001(A1-B1,B2), p = 0.808(A1-C1), p = 0.508(A1-C2) |
0.05 ± 0.008 | p = 0.087 (A1-A2) p < 0.001(A1-B1,B2) p = 0.503(A1-C1) p = 0.154(A1-C2) |
| A2 | 0.113 ± 0.016 | p = 0.002 (A2-B1) p < 0.001(A2-B2) p = 0.120(A2-C1) p = 0.238(A2-C2) |
0.077 ± 0.007 | p = 0.005 (A2-B1) p < 0.005(A2-B2) p = 0.263(A2-C1) p = 0.736(A2-C2) |
| B1 | 0.175 ± 0.026 | p < 0.001(B1- B2, C1,C2) | 0.127 ± 0.024 | p = 0.001 (B1-B2, C1) p = 0.003(B1-C2) |
| B2 | 0.253 ± 0.026 | p < 0.001(B2- C1,C2) | 0.195 ± 0.032 | p < 0.001(B2- C1,C2) |
| C1 | 0.086 ± 0.012 | p = 0.672 (C1-C2) | 0.060 ± 0.007 | p = 0.423(C1-C2) |
| C2 | 0.093 ± 0.022 | 0.072 ± 0.011 |
The groups are as given in the legend of Figure 1. By a one-way ANOVA, the SIgA antibody level of Nasopharyngeal fluid (A492) (A1, A2, B1, B2, C1, C2): f = 35.401, p < 0.001. The SIgA antibody level of Vaginal fluid(A492)(A1, A2, B1, B2, C1, C2): f = 28.882, p < 0.001. ※pairwise comparison was tested in groups (A1-C2). For example, “A1−A2” indicates that pairwise comparison was detected between the A1 control group and the A2 vaccine experimental group.
Tp positive rates and ulceration numbers were reduced in the experimental groups
To evaluate skin anti-Tp protection in each group, Tp positive rates and ulceration in infection lesions were recorded. As shown in Table 5, Tp positive rates and ulceration numbers on the rabbit back were reduced significantly (p < 0.001) in vaccine groups (A2, B1, B2) than in the control groups (A1, C1, C2). There were significant (p < 0.05) difference in Tp positive rates and ulcerations between A2 (Number of DF+ lesions/total, 18.3%; Number of ulcerative lesions/total, 15%) and B2 (Number of DF+ lesions/total, 6.7%; Number of ulcerative lesions/total, 8.3%). The differences in the number of DF+ lesions/total or the number of ulcerative lesions/total between the B1 and B2 groups were also significant (p < 0.05). But there was no significant (p > 0.05) difference between group A2 and B1 (Number of DF+ lesions/total, 20%; Number of ulcerative lesions/total, 18.3%). There was also no significant (p > 0.05) difference between groups C1 and C2. The above results showed that Tp positive rate and ulceration were reduced significantly in the vaccine-inoculated groups regardless of vaccination strategy, indicating strong immune protections. Intramuscular injection of primary DNA vaccine-CpG ODN-nasogastric mucosal vaccination (in group B2) enhanced pcD/TpGpd-IL-2 vaccine immune protection significantly.
Table 5. Challenge results for immunized rabbits with Tp (Nichols) spirochetes.
| Group | No. of rabbits | No. of DF+ lesions/total (%)a | P-valueb | No. of ulcerative lesions/total (%) | P-valueb |
|---|---|---|---|---|---|
| A1 | 15 | 110/120 (91.7) | p < 0.001 (A1-A2,B1,B2,) |
106/120 (88.3) | p < 0.001 (A1-A2,B1,B2,) |
| A2 | 15 | 22/120 (18.3) | p > 0.05(A2-B1) p < 0.05(A2-B2,C1,C2) |
18/120 (15.0) | p > 0.05(A2-B1) p < 0.05(A2-B2,C1,C2) |
| B1 | 15 | 24/120(20.0) | p < 0.05(B1-B2,) p < 0.001(B1- C1,C2) |
22/120(18.3) | p < 0.05(B1-B2) p < 0.001(B1- C1,C2) |
| B2 | 15 | 8/120 (6.7) | p < 0.001(B2- C1,C2) | 10/120 (8.3) | p < 0.001(B2- C1,C2) |
| C1 | 15 | 104/120 (86.7) | P > 0.05(C1-C2) | 102/120 (85.0) | P > 0.05(C1-C2) |
| C2 | 15 | 98/120 (81.7) | 94/120 (78.3) |
Note: aAspirates were taken from lesions and examined for the presence of treponemes under a DF (dark-field) microscope; bχ2 Analysis comparing test values with nonimmunized control values (A1-C2). The groups are as given in the legend of Figure 1. p < 0.05 was considered significant.
The intranasal immunization of pcD/Gpd-IL-2 vaccine adjuvanted by CpG ODN significantly reduces the prolonged healing time and swelling diameter of infection lesions casued by Tp
For further evaluation of Tp infection lesion healing, this study recorded the various infection sites, swelling and ulcer sizes from the infection day to 60 d after infection. As shown in Figure 2, eight lesions on each rabbit back were examined 21 d after infection. The swelling diameter was the largest and the ulceration was the most severe in the A1 group (Fig. 2A). The swelling diameter was mediate in the B1 group (Fig. 2B). The swelling diameter was smallest in the A2 group (Fig. 2C) and the B2 group (Fig. 2D). There was no significant difference between the groups A2 (Fig. 2C) and B2 (Fig. 2D).
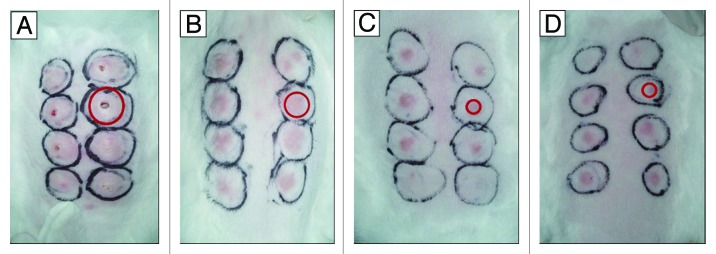
Figure 2. Representative photos of rabbits taken 21 d post intradermal challenges with Tp Nichols strain at eight locations on their backs. At week 10 after the first time of DNA vaccine immunization, 15 of the 18 rabbits in each group were challenged with Tp Nichols strain. Skin lesions were observed and measured at the challenged sites every 3 d. The red circle indicated one of the lesions in each panel. (A) Ulcerative and indurated lesions (a rabbit from the A1 group immunized with pcD); (B) Intermediate erythematous lesions (a rabbit from the B1group immunized with pcD/Gpd-IL-2); (C) The smallest, least indurated erythematous lesions(a rabbit from A2 group immunized with pGpd-IL-2); (D) The smallest, least indurated erythematous lesions(a rabbit from B2 group immunized with pGpd-IL-2).
At week10 after Tp Gpd DNA vaccine immunization, 15 of 18 rabbits in each group were challenged intradermally at eight sites on their shaved backs with 105 T. pallidum (Nichols) spirochetes. Diameters of the erythematous lesions from all groups were determined at 3-d intervals (in 0 to 60 d). As shown in Figure 3, each bar represents the mean ± SE of diameters of erythematous lesions from 15 rabbits in each group. The swellings in all experimental groups appeared in the first 3 d (about 3 - 5 mm in diameter). In the group A2, a swelling peak appeared at 12th day with a mean swelling diameter up to 9.50 mm. The swellings in the A2 group disappeared completely at the 45th day. In the group B2, a swelling peak appeared at 21st day with a mean swelling diameter of 9.50 mm. The swellings in the B2 group disappeared at the 48th day. In the group B1, a swelling peak appeared at 21st day with a mean swelling diameter of 12.0 mm. The swellings in the B1 group disappeared at the 54th day. In the groups A1 and C1, the swelling appeared relatively late (at 6th day) and the mean size was smaller (2.5 mm). However, the swelling increased rapidly and reached its peaks at 15th day (14.35 mm), then reduced slowly and remained at 4 mm at the 60th day. The results above suggest that vaccine groups significantly reduce Tp positive rate and ulceration regardless of the vaccination strategy, indicating strong immune protections. However,mucosal adjuvant CpG ODN (the B2 group) could not significantly reduce the prolonged healing times and swelling diameters of infection lesions casued by Tp, when compared with the immunization by pcD/Gpd-IL-2 vaccine intramuscular injection alone (the A2 group).
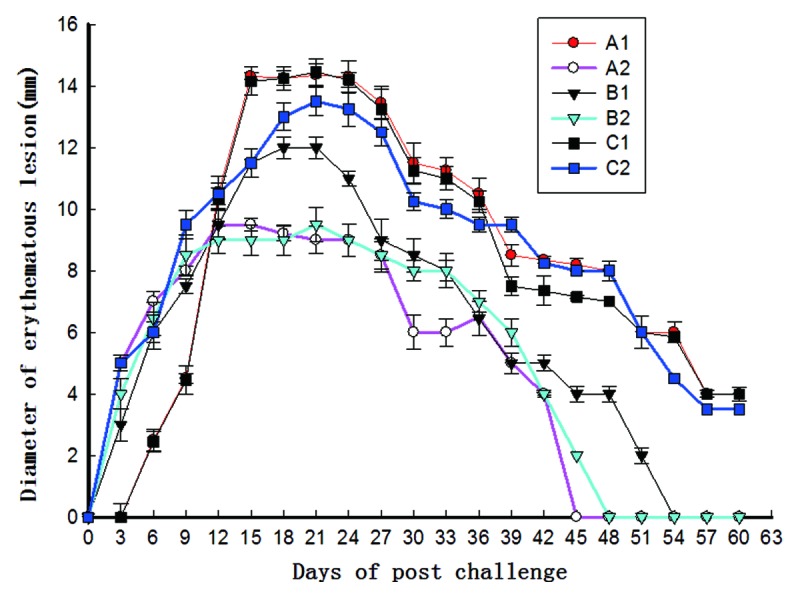
Figure 3. Diameter measurements of erythematous lesions from groups of 15 rabbits infected with T. pallidum after the challenge (week 10) at 3-d intervals (from 0 to 60 d). The groups are as given in Figure 1. At week10 after Tp Gpd DNA vaccine immunization, 15 of 18 rabbits in each group were challenged intradermally at eight sites on their shaved backs with 105T. pallidum (Nichols) spirochetes. Each bar represents the mean ± SE of diameters of erythematous lesions from 15 rabbits in each group.
Discussion
Although development of Tp vaccines includes four stages, the inactivated vaccine, live attenuated vaccines, recombinant protein vaccines and DNA vaccines, no effective vaccine against Tp infection is currently available. One reason is that Tp cannot be cultured in vitro so far, which makes it difficult to prepare enough Tp antigens. Although recombination technology can be used for production of the antigen protein, the recombinant protein in E. coli cannot accurately reflect structures of Tp proteins, thus affecting the efficacy of vaccines in studies using the rabbit model. Compared with the other kinds of vaccines, DNA vaccines have relatively better and longer protective effects.
The common mucosal immunity includes oral immunization and intranasal immunization. Oral immunization requires high doses and repeated vaccinations to induce appropriate immune responses. However, its immune response duration is short, and sometimes causes immune tolerance. Meanwhile, intranasal immunization required less vaccination and adjuvant. Intranasal immunization targets natural sites used by the invasive pathogens. Furthermore, intranasal immunization avoids the damages of the acid environment and enzymes in gastrointestinal tract. Therefore, intranasal immunization is a kind of effective DNA vaccine inoculation. Intranasal immunizations not only induce high titers of mucosal antibodies in the proximal nasal, bronchia and lung, but also rectum, vagina and other remote mucosal tissues to obtain broad mucous membrane protection. It is also shown that intranasal immunization induced higher levels of systemic immune responses.16,17
For strong immunity with intramuscular immunization of DNA vaccine and effective stimulation of early mucosal immunity, intramuscular injection of primary DNA vaccine enhanced by CpG ODN via nasogastric mucosal vaccination was performed in this study to immunize New Zealand rabbits. It is shown that traditional intramuscular injection of pcD/Gpd-IL-2 immunity (in the A2 group) induced stronger antibody response, cytokine levels and immune protection than intramuscular injection of primary DNA vaccine-nasogastric mucosal vaccination (the B1 group). Though DNA vaccination-nasogastric mucosal vaccination induces a certain level of sIgA in the nasopharynx and vaginal mucosa, due to the intake of epithelial cells in the nasopharynx and upper respiratory tract mucosal, expression of DNA are weak and transient because of quick metabolic rate. Therefore, its ability of DNA vaccine to express exogenous genes is relatively weak. Moreover, this vaccination strategy causes weaker immune response compared with traditional multiple injection method. The sIgA secretion in nasopharynx and vagina was significantly higher than intramuscular multiple injection (the A2 group). However, there was no significant difference in Tp DF-positive rate and ulceration between these two groups, indicating that sIgA may not be the only early anti-Tp infection factor.
Recently it is shown18-21 that the diversified immune stimulation of the synthetic CpG ODN leads to a variety activation of immune cells, particularly B cells. While it induces cytokine secretions, such as IL-12 and IFN, and it activates NK cells, macrophages, T cell and innate immune responses, thereby enhancing the adaptive immune response, mainly as Th l-type cellular immune responses. It is shown that levels of antibodies increased 15-folds when compared with separately immune animals when hepatitis B vaccine (HBsAg) is adjuvanted with CpG ODN to immune gorillas and monkeys.22 It indicates that CpGODN is great adjuvant for hepatitis B vaccine.22 It is also shown23-25 that CpG ODN is of strong mucosal adjuvant activity and it has a synergistic effect with the mucosal adjuvant CT and LT with limited toxic side effects on the human body. Therefore it is a very promising mucosal adjuvant.
However, different CpG ODN molecules have different effects on immune activation. The same CpG ODN sequence from different animal species may trigger different biological activities.26 Previous report27 indicated that the GTCGTT sequence has good stimulation activity to human lymphocyte, which also has good stimulation activity to lymphatic cell proliferation of a variety of animals, including horses, pigs, dogs, cattle, sheep, goats, cats and chickens.28 However, the experimental animals used in this study are rabbits, whose CpG ODN sequence contains a GACGTT motif. The GACGTT motif is found to be sensitive only to rabbits and mice.29 Therefore, such specificity of ODN sequences may affect their application in future research of human vaccine candidates.
In this study, intramuscular injection of primary DNA vaccine CpG ODN via intranasal immunization (the B2 group) was applied to strengthen the immunization of rabbits. It is shown that because of the adjuvant effect of CpG ODN, specific IgG antibody levels and cytokine levels in the group B2 were higher than in the group B1. There is no significant difference between the group B2 and traditional intramuscular injection of pcD/Gpd-IL-2 (the A2 group). However, levels of sIgA in the nasopharynx and vaginal mucosal increased significantly compared with the group B1 and the group A2. Tp DF-positive rate and ulceration on the rabbit back were significantly reduced in group B2 compared with groups B1 and A2, but its healing time (48 d) was longer than the A2 group (45 d), further indicating that sIgA is not the only factor of early anti-Tp infection. In this study, Tp infection was performed on the back of rabbit, which may not perfectly reflect the effectiveness of the protection by the Tp mucosal infection. Therefore, better experimental methods are needed in the future research. In our previous study,14,15 induced humoral and cellular immunity is of great importance in protection against the early anti-Tp infection via multiple intramuscular immune model.
In conclusion, this study shows that intramuscular injection of primary DNA vaccine with CpG ODN via intranasal immunization could not enhance significantly the protective response against Tp infection, compared with multiple intramuscular injection groups. Although it induced strong humoral immune (especially mucosal immunity) and cellular immunity. Several points remain to be further studied in the future, such as how to further optimize the release system of Tp mucosal DNA vaccine for more efficiently body intake and how to target antigen presentation to submucosal immune cells.
Materials and methods
Bacterial strains, plasmids and animals
Standard Tp strains (Nichols strain) were donated by Weiming Gu, director of department of laboratory, Skin Diseases and STD Hospital, Shanghai and preserved by Pathogenic Biology Institute, University of South China, Hengyang City. Eukaryotic expression plasmid pcDNA3.1 (+) was purchased from Invitrogen Company. Eukaryotic recombinant plasmid pcD/Gpd-IL-2 was constructed and preserved by Pathogenic Biology Institute, University of South China, Hengyang City.14 The plasmid pcD/Gpd-IL-2 and the empty plasmid pcDNA3.1 (+) were extracted according to methods described previously.13
New Zealand female rabbits were provided by Department of Experimental Animal, University of South China. All animal experiments were approved by the governing Animal Welfare Committee and conducted in accordance with the regulations of the institution.
Reagents
Standard syphilis positive serum was preserved at Pathogenic Biology Institute. HRP-labeled goat anti-human/rabbit IgG secondary antibody was purchased from Invitrogen Company. Rabbit IFN-γ, IL-2 and rabbit IgA ELISA quantitative detection kit were purchased from Shanghai Jianglai Biotechnology Co., Ltd. Fetal bovine serum (FBS) and RPMI-1640 medium were purchased from HyClone Company. The CpG ODN sequence (TCC ATG ACG TTC CTG ACG TT) was phosphorylated and synthesized by Invitrogen Co., Ltd.
DNA vaccine immunization and infection
New Zealand white rabbits were randomly divided into six groups, with each group consisting of 18 rabbits. The complete regimen included two immunizations, which were administered once in every two weeks. Groups: A1, 2× intramuscular immunization of pcD (100 μg); A2, 2× intramuscular immunization of pcD/Gpd-IL-2 (100 μg); B1, 1× intramuscular immunization pcD/Gpd-IL-2 (100 μg) and 1× intranasal immunization of pcD/Gpd-IL-2(100 μg); B2, 1× intramuscular immunization pcD/Gpd-IL-2 (100 μg) and 1× intranasal immunizationof pcD/Gpd-IL-2 (100 μg) and CpGODN (10 μg); C1, 1× intramuscular immunization pcD (100 μg) and 1× intranasal immunization of pcD (100 μg); C2, 1× intramuscular immunization pcD (100 μg) and 1× intranasal immunization of pcD (100 μg) and CpGODN (10 μg). Primary immunization of the vaccine and empty plasmid were intramuscularly multi-inoculated into the quadriceps of left leg in rabbits as described in the previous study14 Two days before immunization, 100 μl of 0.25% bupivacaine hydrochloride was injected into the quadriceps of left leg in rabbits as pre-injection, as was shown in Table 1. Eight weeks after immunization, 3 rabbits from each group were chosen for the rabbit spleen cell proliferation assay, cytokine detection and nasopharynx, vaginal mucosal sIgA detection. The remaining 15 rabbits were used for syphilis infection and protection experiment. Ten weeks after primary immunization (0 d post-infection), 8 lesions were subcutaneously inoculated in all groups (n = 15) after shaving. Each site was inoculated with the Tp Nichols strain (105 bacteria /site). The swelling and ulceration of infection lesions, if any in each site, were recorded every 3 d in the following 60 d. Tp positive rates and ulceration rates of each site were examined by the dark vision and silver staining methods 21 d after infection.
ELISA
As described in our previous study,14 0 week (at the immunization day), 2 weeks, 4 weeks, 6 weeks, 8 weeks after primary vaccination, 1 ml of ear vein blood of rabbits in each group (n = 15) was taken and centrifuged to get rid of serum. Purified Tp Gpd recombinant protein (pET28a/Gpd) was used as the coating antigen. Horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (1:2000) was used as the secondary antibody. ELISA (Multiskan MK-3, Finland) examined A value at 450 nm wavelength. Each experiment was repeated for 3 times.
Eight weeks after immunization, trachea of the primary immunized rabbits was cut from the middle. Nasopharynx was washed from the cutting site to the upper end with 500 μl of phosophate buffered saline (PBS) solution. The outflow washing fluid was collected with three consecutive times of rinsing. A total of 1,500 μl of nasopharyngeal washing fluid was collected. Rabbit limbs were fixed, 500 μl of PBS solution was used to rinse inward from the vaginal opening. Rabbit hind legs were slightly raised to prevent liquid leakage. After three times of washing, vaginal washing fluid was collected. The above washing fluids were centrifuged and supernatants were detected by ELISA. The absorbance values (A value) of antibody at the 492 nm wavelength were determined.
Determination of cytokines
As described in our previous study,13 8 weeks after immunization, 3 rabbits were taken randomly from each group for sterile spleen cells. Purified Tp Gpd recombinant protein was used as the stimulating antigen (20 μg/ml). The Concanavalin A (ConA, 10 μg/ml, Sigma) stimulation condition served as the positive control. An non-antigen-stimulating condition served as the negative control. According to the manual of cytokine ELISA kit, concentration of IL-2 and IFN-γ in cell culture supernatant was analyzed.
MTT Method
As described in our previous study,14 8 weeks after vaccination, sterile spleen was taken from each group to get the spleen lymphocytes suspension. Purified Tp Gpd recombinant protein was used as the stimulating antigen (8 μg/ml). ConA (5 μg/ml) stimulation was used as the positive control. Non-antigen-stimulating well was used as the negative control. Sixty-eight hours after incubation, T-cell proliferation response of the New Zealand rabbit was determined by MTT assay and expressed as stimulation index (SI). The A value at a wavelength of 570 nm was measured by ELISA to calculate the SI value. SI value = A value of the experimental group /A value of the control group.
Statistical analysis
Experimental data were expressed as mean ± SD. Mean serum endpoint titer comparisons between groups were performed using a two-way analysis of variance with a Bonferroni post hoc test of specific antibody titers by SPSS13.0 statistical software. Arithmetic means comparisons between groups were performed using a one-way analysis of variance with a Bonferroni post hoc test of cytokine concentration, and SI values of spleen cells. The Tp positive rates and ulceration rates of early infection lesions on the back skin of rabbit were analyzed by χ2 test. p < 0.05 was considered statistically significant.
Acknowledgment
This work is supported by National Natural Science Foundation (30800996), projects of Science and Technology Department of Hunan Province (2011TT2014, 2012TT2006), Provincial Joint Funding of Nature and Science in Hunan Province (11JJ0923), and Natural Science Foundation in Hunan Province (11JJ4076).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23064
References
- 1.Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer L. Mucosal immunity. Pediatrics. 2003;111:1595–600. [PubMed] [Google Scholar]
- 3.Chen HM. Recent advances in mucosal vaccine development. J Control Release. 2000;67:117–28. doi: 10.1016/S0168-3659(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 4.McCluskie MJ, Weeratna RD, Clements JD, Davis HL. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine. 2001;19:3759–68. doi: 10.1016/S0264-410X(01)00088-3. [DOI] [PubMed] [Google Scholar]
- 5.Bakke H, Samdal HH, Holst J, Oftung F, Haugen IL, Kristoffersen AC, et al. Oral spray immunization may be an alternative to intranasal vaccine delivery to induce systemic antibodies but not nasal mucosal or cellular immunity. Scand J Immunol. 2006;63:223–31. doi: 10.1111/j.1365-3083.2006.01730.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamrick TS, Dempsey JA, Cohen MS, Cannon JG. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology. 2001;147:839–49. doi: 10.1099/00221287-147-4-839. [DOI] [PubMed] [Google Scholar]
- 7.Maier M, Seabrook TJ, Lemere CA. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G) Vaccine. 2005;23:5149–59. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Apostolaki M, Williams NA. Nasal delivery of antigen with the B subunit of Escherichia coli heat-labile enterotoxin augments antigen-specific T-cell clonal expansion and differentiation. Infect Immun. 2004;72:4072–80. doi: 10.1128/IAI.72.7.4072-4080.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Q, Yang J, Luo P, Zhang WJ, Zou QMLT. LT(K63/R72), a new mutant of Escherichia coli heat-labile enterotoxin, exhibits characteristics more similar to LT(K63) than LT(R72) Acta Biochim Biophys Sin (Shanghai) 2005;37:126–32. doi: 10.1093/abbs/37.2.126. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Sasaki K, Kato M, Arimitsu H, Ochi S, Yano T, et al. A mutant of Escherichia coli enterotoxin inducing a specific Thl-type of T cells to varicella-zoster vaccine enhances the production of IL-12 by IFNgamma-stimulated macrophages. Vaccine. 2006;24:3719–26. doi: 10.1016/j.vaccine.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Zhao CA, Wang K, Zheng J, Wang YL, Si LS. Enhanced immunization after intranasal coadministration of Escherichia coli heat-labile enterotoxin B subunit and human papillomavirus 16-L1 DNA vaccine. Chin Med J (Engl) 2006;119:408–11. [PubMed] [Google Scholar]
- 12.Yamanaka H, Ishibashi D, Yamaguchi N, Yoshikawa D, Nakamura R, Okimura N, et al. Enhanced mucosal immunogenicity of prion protein following fusion with B subunit of Escherichia coli heat-labile enterotoxin. Vaccine. 2006;24:2815–23. doi: 10.1016/j.vaccine.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 13.McCluskie MJ, Weeratna RD, Krieg AM, Davis HL. CpG DNA is an effective oral adjuvant to protein antigens in mice. Vaccine. 2000;19:950–7. doi: 10.1016/S0264-410X(00)00215-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Wang S, Zhang X, Gu W, Yu J, Liu S, et al. Protective efficacy of a Treponema pallidum Gpd DNA vaccine vectored by chitosan nanoparticles and fused with interleukin-2. Can J Microbiol. 2012;58:117–23. doi: 10.1139/w11-115. [DOI] [PubMed] [Google Scholar]
- 15.Zhao F, Wu Y, Zhang X, Yu J, Gu W, Liu S, et al. Enhanced immune response and protective efficacy of a Treponema pallidum Tp92 DNA vaccine vectored by chitosan nanoparticles and adjuvanted with IL-2. Hum Vaccin. 2011;7:1083–9. doi: 10.4161/hv.7.10.16541. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Christopher ME, Nagata LP, Zabielski MA, Li H, Wong JP, et al. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J Clin Virol. 2004;31(Suppl 1):S99–106. doi: 10.1016/j.jcv.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Vajdy M, O’Hagan DT. Microparticles for intranasal immunization. Adv Drug Deliv Rev. 2001;51:127–41. doi: 10.1016/S0169-409X(01)00167-3. [DOI] [PubMed] [Google Scholar]
- 18.Weiner GJ. CpG oligodeoxynucleotide-based therapy of lymphoid malignancies. Adv Drug Deliv Rev. 2009;61:263–7. doi: 10.1016/j.addr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Salem AK, Weiner GJ. CpG oligonucleotides as immunotherapeutic adjuvants: innovative applications and delivery strategies. Adv Drug Deliv Rev. 2009;61:193–4. doi: 10.1016/j.addr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211–7. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Easton A, Haque A, Chu K, Patel N, Lukaszewski RA, Krieg AM, et al. Combining vaccination and postexposure CpG therapy provides optimal protection against lethal sepsis in a biodefense model of human melioidosis. J Infect Dis. 2011;204:636–44. doi: 10.1093/infdis/jir301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 23.Cao D, Li H, Jiang Z, Cheng Q, Yang Z, Xu C, et al. CpG oligodeoxynucleotide synergizes innate defense regulator peptide for enhancing the systemic and mucosal immune responses to pseudorabies attenuated virus vaccine in piglets in vivo. Int Immunopharmacol. 2011;11:748–54. doi: 10.1016/j.intimp.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Giddings OK, Eickhoff CS, Sullivan NL, Hoft DF. Intranasal vaccinations with the trans-sialidase antigen plus CpG Adjuvant induce mucosal immunity protective against conjunctival Trypanosoma cruzi challenges. Infect Immun. 2010;78:1333–8. doi: 10.1128/IAI.00278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pun PB, Bhat AA, Mohan T, Kulkarni S, Paranjape R, Rao DN. Intranasal administration of peptide antigens of HIV with mucosal adjuvant CpG ODN coentrapped in microparticles enhances the mucosal and systemic immune responses. Int Immunopharmacol. 2009;9:468–77. doi: 10.1016/j.intimp.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 27.Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19:618–22. doi: 10.1016/S0264-410X(00)00249-8. [DOI] [PubMed] [Google Scholar]
- 28.Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001;11:333–40. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]