Abstract
Interactions between costimulatory molecules and their receptors are vital for Ag-presenting dendritic cells (DCs) to initiate T cells activation, expansion and their antitumor immune responses. Augmentation of costimulatory signal due to the interaction of DCs and T cells may amplify, sustain and drive diversity of cytotoxic T lymphocytes (CTLs) and consequently enhance the antitumor response. 4-1BBL/4-1BB is such a pair of costimulatory ligand and receptor, playing an important role in the co-stimulation of CTLs. Previously, we demonstrated that DCs transduced with recombinant adenovirus encoding truncated PSMA (tPSMA) and m4-1BBL could induce prostate cancer regression in mouse models. In the present study, we further explored the adjuvant role of 4-1BBL in modulating CTLs activation induced by tPSMA gene-pulsed DCs. The apoptosis and cytotoxicity against tPSMA expressing RM-1 cells of CTLs were determined. Results showed that tPSMA gene-pulsed DCs effectively induced T lymphocyte activation and cytotoxicity, which was enhanced by upregulated expression of 4-1BBL, displaying better cell viability, lower CTLs apoptosis, higher expression anti-apoptotic protein of Bcl-xL and phosphorylation of P38, enhanced NF-κB activation, as well as more IFN-γ production. These results demonstrated that 4-1BBL may play a significant role in the co-stimulation pathway for Ag-presenting DCs-mediated CTLs activity, which might be a beneficial adjuvant factor for DCs-based cancer immunotherapy.
Keywords: dendritic cells, cytotoxic T lymphocytes, prostate cancer, 4-1BBL, costimulatory molecule
Introduction
Dendritic cells (DCs) are the most potent professional antigen-presenting cells (APCs), which display a powerful capacity to induce, sustain and regulate T-cell-mediated immune responses providing the opportunity of DCs-based vaccination for cancer immunotherapy.1 DCs express high levels of MHC class I and class II antigens along with several different costimulatory molecules, which are crucial to break peripheral tolerance and thus induce tumor immune responses. Several reports demonstrated that systemic immunotherapy with DCs-based vaccines are capable of inducing potent antitumor responses.2,3 Prostate cancer (PCa) are the most frequently diagnosed cancer in old men and also the second leading cause of male cancer death in the Western countries.4 More than 400 prostate cancer patients have been treated with DCs-based immunotherapy to date and immune responses have been reported in two-thirds of these, resulting in clinical responses in almost half of the patients treated.5 Definitely, DCs-based cancer immunotherapy aims at eliciting a CTLs response directed against tumor antigens to eliminate residual tumor cells and thereby improve survival and quality of life of cancer patients.
CTLs are among the most powerful immune cells in controlling both viral infections and cancer regression. However, despite their potent activity, CTLs-mediated anti-tumor responses in cancer patient are rare.6 Even among responses in prostate cancer patients accepted DCs-based immunotherapy, most were modest and transient.3 It is due to a variety of reasons, such as difficulties in stimulating CTLs response to cancer antigens and the resistance of solid tumors to CTLs-mediated killing. It has been proven that DCs are more or less inhibited by cytokines secreted from tumor cells and fail to activate lymphocytes effectively in cancer patients due to less costimulatory expression.7 Furthermore, tumor cells usually express insufficient activating signals resulting in immune ignorance.8,9 These deficiencies weaken the antitumor response mediated by DCs-induced CTLs. Therefore, approaches to augment he insufficient co-stimulation are meaningful for facilitating CTLs activation and such a strategy has been proven to be practicable.10
Effective CTLs activation requires signals derived from the T-cell receptor (TCR) after being triggered by the antigenic peptides presented by MHC molecules on the surface of DCs and co-stimulation signals mediated by the interaction between costimulatory receptors and ligands.11 More importantly, a strong co-stimulation signal could help induce high-avidity CD8+ T cells that killed target cells more efficiently. In the absence of a strong MHC/peptide signal, co-stimulation played a crucial role.12 Therefore, appropriate activation of dendritic cells to express a variety of costimulatory molecules is a vital component of a cancer vaccine. However, without appropriate antigen presentation, stimulation of CTLs will not be achieved. Previously, we demonstrated that DCs transduced with recombinant adenovirus encoding truncated PSMA and m4-1BBL could induce prostate cancer regression in mouse models.13 PSMA is an overexpressed membrane-bound cell surface protein on prostate cancer cells and is an ideal target for prostate cancer immunotherapy.14 4-1BBL (CD137L), the counter receptor for 4-1BB, is a member of the TNF (ligand) superfamily and serves as a secondary signal to activated T cells. 4-1BB signaling can induce cytokine production, expansion and functional maturation of CD4+ and CD8+ T cells.15-17 A soluble 4-1BBL has also been shown to overcome immunological ignorance, allowing immunization with tumor-derived peptide to induce a protective CTLs response.18
In this study, we further investigated how DCs modified with truncated PSMA and 4-1BBL to enhanced function of CTLs to kill prostate cancer, including lymphocyte proliferation, IFN-γ secretion, cell viability and cytotoxicity for exploring the recognition of 4-1BBL as an immunoadjuvant for DCs-based anti-prostate cancer strategy.
Results
tPSMA expression
Recombinant adenovirus Ad-tPSMA was constructed, and identified by immunoblot detecting the expression of tPSMA in RM-1 cells infected with recombinant adenovirus Ad-tPSMA for 48 h. tPSMA protein was detected in cells infected with Ad-tPSMA, but nothing in the control cells (Fig. 1). B-actin protein was detected at similar levels in all samples.
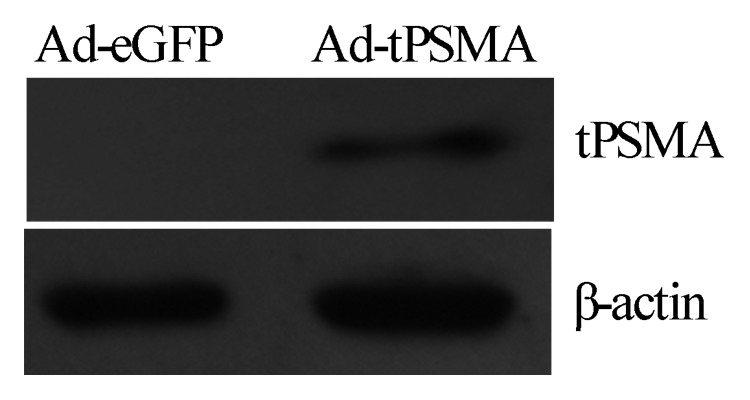
Figure 1. Detection of tPSMA expression. Recombinant adenovirus Ad-tPSMA was constructed and identified by immunoblot detecting the expression of tPSMA in RM-1 cells. Total cell lysates were harvested and presence of tPSMA protein was detected by anti-PSMA polyclonal antibody. A specific band was identified in RM-1 cells transfected with Ad-tPSMA but not in none-transfected RM-1 cells. β-actin was used as reference.
IL-4, IL-10 and IFN-γ production
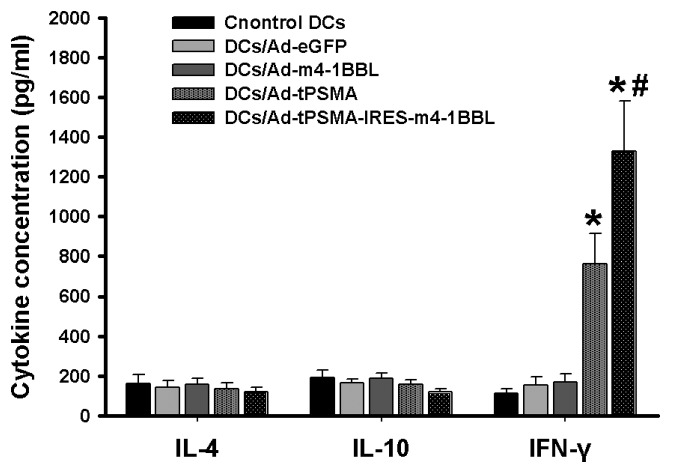
DCs transduced with Ad-eGFP, Ad-m4-1BBL, Ad-tPSMA, Ad-tPSMA-IRES-m4-1BBL or none were coculture with T cells. Supernatants of co-cultures were harvested and tested for IL-4, IL-10 and IFN-γ by ELISA. The data was normalized to viable cell numbers at 48 h after re-stimulation. The secretion of IFN-γ by T lymphocytes were significantly enhanced by stimulation with DCs transduced with Ad-tPSMA or Ad-tPSMA-IRES-m4-1BBL when compared with that with DCs transduced with Ad-eGFP or Ad-m4-1BBL or none (Fig. 2) (p < 0.05). The highest level of IFN-γ were produced when stimulated with Ad-tPSMA-IRES-m4-1BBL-transduced DCs. T lymphocytes did not exhibit any increase in IFN-γ secretion when co-cultured with Ad-eGFP-transduced DCs, or Ad-m-4-1BBL-transduced DCs, or neither. However, the production of IL-4 and IL-10 did not show significant difference (Fig. 2). It demonstrated that tPSMA gene-pulsed DCs could effectively induce IFN-γ production by T lymphocyte, which was enhanced by upregulated expression of 4-1BBL.
Figure 2. IL-4, IL-10 and IFN-γ production. DCs transduced with Ad-eGFP, Ad-m4-1BBL, Ad-tPSMA, Ad-tPSMA-IRES-m4-1BBL or none were co-culture with T cells. Supernatants were harvested for IL-4, IL-10 and IFN-γ production assay by ELISA. Data were shown as mean ± SD. Similar results were obtained from three independent experiments. *p < 0.05.
The expression of IκB-α, NF-κB, P38 and Bcl-xL
CTLs were induced by DCs transduced with Ad-eGFP, Ad-m4-1BBL, Ad-tPSMA, Ad-tPSMA-IRES-m4-1BBL or none, then total cellular proteins and nuclear extracts were collected for detecting IκB-α, P38, Bcl-xL and NF-κB p65 by Immunoblot. Immunoblot analysis showed decreased expression of IκB-α, but NF-κB p65 and phosphorylation of P38 were enhanced in CTLs induced by Ad-tPSMA-IRES-m4-1BBL-transduced DCs compared with CTLs induced by Ad-tPSMA-transduced DCs, total expression of P38 did not display any significant difference in all groups (Fig. 3). Ad-tPSMA-IRES-m4-1BBL-transduced DCs induced higher expression of Bcl-xL in CTLs than Ad-tPSMA-transduced DCs, however, both of which were upregulated in CTLs compared with DCs transduced with Ad-eGFP or Ad-m4-1BBL or none (Fig. 3). It demonstrated that upregulation of 4-1BBL could enhance the expression of Bcl-xL, phosphorylation of P38 and NF-κB activation in CTLs induced by tPSMA gene-pulsed DCs.
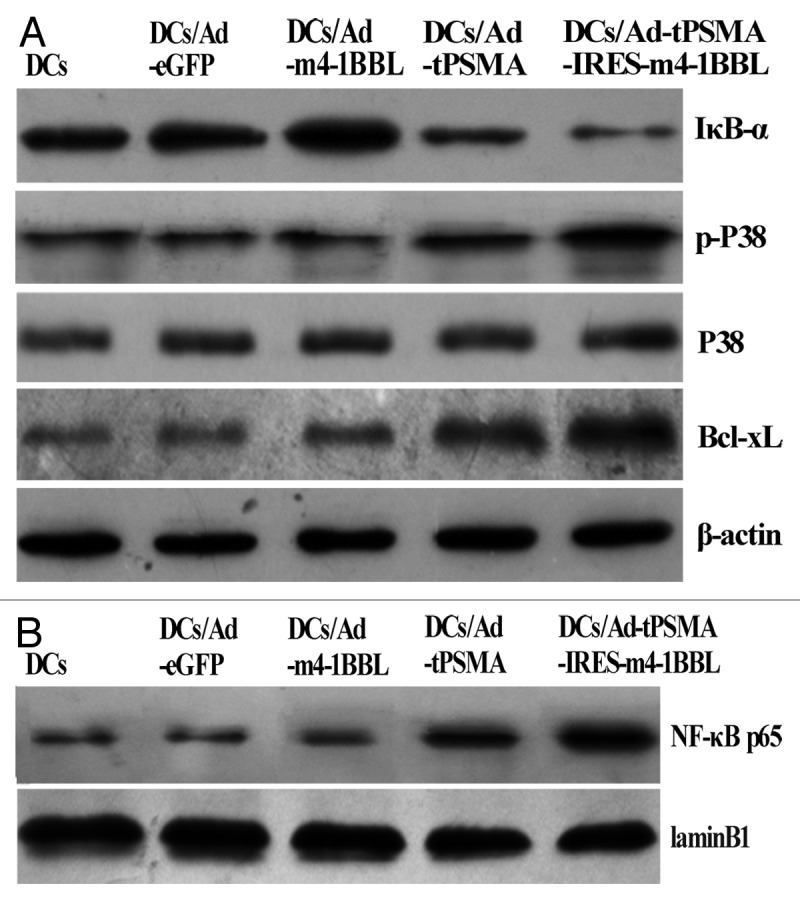
Figure 3. The expression of IκB-α, NF-κB, P38 and Bcl-xL. CTLs were induced by DCs transduced with Ad-eGFP, Ad-m4-1BBL, Ad-tPSMA, Ad-tPSMA-IRES-m4-1BBL or none, then total cellular proteins and nuclear extracts were collected for detecting IκB-α, P38, Bcl-xL and NF-κB p65 by immunoblot. (A) Ad-tPSMA-IRES-m4-1BBL-transduced DCs induced lower IκB-α, but enhanced phosphorylation of P38 and Bcl-xL expression, with on change in total expression of P38. (B) Ad-tPSMA-IRES-m4-1BBL-transduced DCs induced higher expression of NF-κB p65 in nuclear of CTLs.
Apoptosis of CTLs
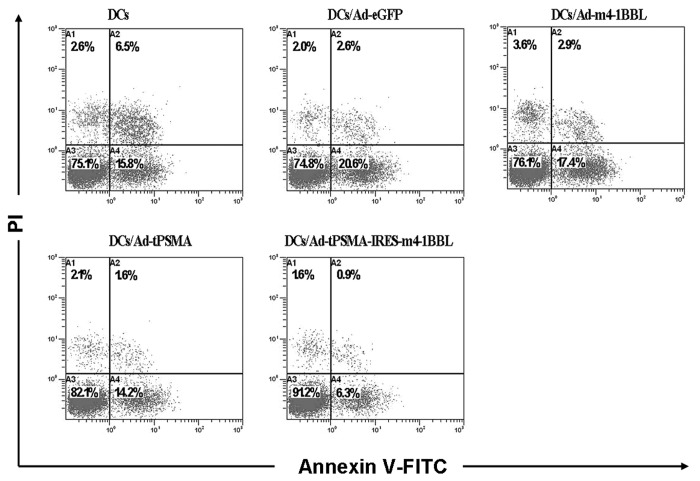
CTLs induced by DCs were collected for detecting apoptosis by staining with FITC-conjugated annexin V and PI. The data of flow cytometry displayed that the apoptosis rate of CTLs stimulated by DCs transduced with Ad-tPSMA or Ad-tPSMA-IRES-m4-1BBL was reduced compared with that stimulated by DCs transduced with Ad-eGFP or Ad-m4-1BBL or none (Fig. 4). However, Ad-tPSMA-IRES-m4-1BBL-transduced DCs induced more apoptosis of CTLs than DCs transduced with Ad-tPSMA (Fig. 4). It demonstrated that upregulation of 4-1BBL could enhanced anti-apoptosis of CTLs induced by DCs.
Figure 4. Viability of CTLs. CTLs were induced by DCs and collected for detecting apoptosis by staining with FITC-conjugated annexin V and PI. The apoptosis of CTLs induced by Ad-tPSMA-IRES-m4-1BBL-transduced DCs was lowest. The results were representative of three independent experiments.
Tumor-specific CTLs activity
T cells isolated from C57BL/6 mice were co-cultured with mitomycin C (MMC) treated-five types of mature DCs (Ad-tPSMA-IRES-m4-1BBL-tranduced DCs, Ad-tPSMA-transduced DCs, Ad-1BBL-tranduced DCs, Ad-eGFP-transduced DCs and none-transduced DCs, respectively). After being stimulated twice with DCs, CTLs were induced cytotoxic activities against the RM-1-tPSMA. The cytotoxic activity was enhanced with increased ratio of effector-to-target cells (Fig. 5). CTLs pulsed with Ad-tPSMA-IRES-m4-1BBL-transducted DCs displayed highest cytotoxic activity, and Ad-tPSMA-transduced DCs induced higher cytotoxic activity than DCs transduced with Ad-eGFP or Ad-m4-1BBL or none (p < 0.05). This cytotoxic activity was antigen-specific, in that none of these cells showed detectable cytotoxic activities against the RM-1 tumor cells (Fig. 5). It demonstrated that 4-1BBL modified DCs pulsed with tPSMA gene could induce enhanced prostate cancer-specific CTLs activity.
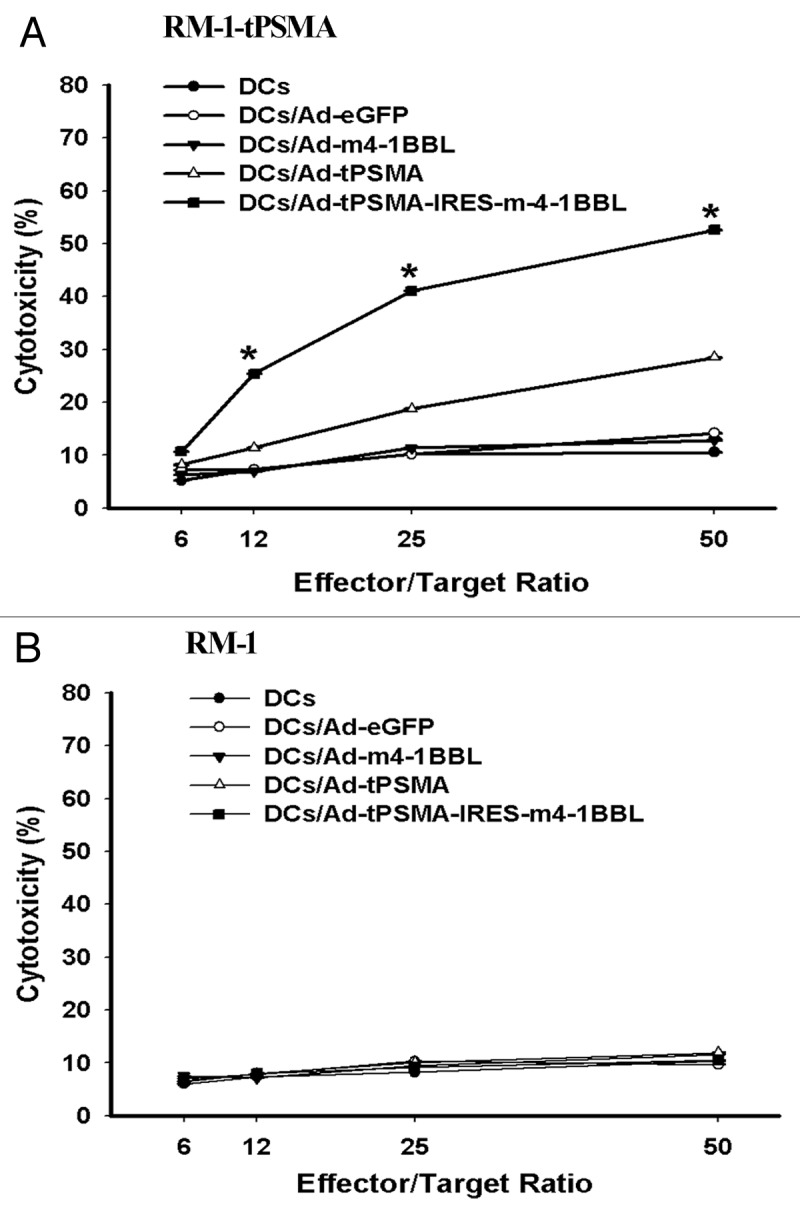
Figure 5. Cytotoxicity against RM-1. T cells isolated from C57BL/6 mice were co-cultivated with mitomycin C (MMC) treated with five types of mature DCs (Ad-tPSMA-IRES-m4-1BBL-tranduced DCs, Ad-tPSMA-transduced DCs, Ad-1BBL-tranduced DCs, Ad-eGFP-transduced DCs and none-transduced DCs, respectively) for 7 d, then treated again for another 7 d. Then T cells were harvested and used for effector cells for detecting specific cytotoxicity against target cells. The results are expressed as means ± SD of three replicates. (A) Ad-tPSMA-IRES-m4-1BBL-tranduced DCs-primed T cells showed high cytotoxicity against RM-1-PSMA cells compared with other four types of DCs-primed T cells (*p < 0.05). (B) Five types of DCs-primed T cells do not demonstrate detectable cytotoxic activities against the RM-1 cells used as a target control. All experiments were done in triplicate.
Discussion
DCs are the most potent APCs currently known for induction of antitumor immune responses and have been recognized as potentially important tools for cancer vaccine strategies.19 It is well reported that BM-derived DCs stimulated successfully with cytokines in vitro has been employed in both animal models and clinical trials.20,21 Previously, we demonstrated that DCs transduced with recombinant adenovirus encoding tPSMA and m4-1BBL could induce obvious prostate cancer regression in mouse models.13 It is widely acknowledged that a potent anti-tumor response requires an effective CTLs response against cancer through recognizing tumor associated antigen (TAA). However, antigen-specific cellular immunity primarily relies on the interaction between APCs and T lymphocyte. APCs serves to present antigen peptide and co-stimulatory signals for T cell activation; then, the subsequent CTLs can damage the tumor cells specifically with means of the MHC class I-restricted specific peptide. Therefore, in this study, we intended to explore the role of DCs modified with tPSMA and 4-1BBL in regulating CTLs induction in killing prostate cancer.
PSMA is a TAA, and may serves as an ideal target for DCs-based immunotherapy against prostate cancer. Many methods for priming the DCs with antigens were used to induce anti-tumor immune responses. Adenoviral vectors mediated-TAA gene modification of DCs has been considered more efficient for cell surface presentation than exogenous loading of synthetic TAA peptides. Recombinant adenovirus mediated-tPSMA gene expression in DCs could cause endogenous processing and presentation of multiple and/or undefined antigenic peptides independent of MHC alleles. Furthermore, specific CTLs-mediated immunity may be stimulated by vaccine-involved DCs without prior knowledge of responder MHC haplotypes or of relevant MHC class I- or class II-restricted peptide epitopes. However, CTLs activity is decided not only by the antigen signal but also the costimulatory molecules on DCs, such as CD80/86, CD40, RANK and so on. Upregulation of costimulatory molecule signaling for T cell activating during interaction of a peptide-bound MHC complex on DCs with cognate TCR may amplify, sustain and drive diversity in the ensuing T cell immune response.6 4-1BBL is such a costimulatory molecule expressed on DCs and its ligand, 4-1BB, is a costimulatory molecule induced on naive T cells following TCR-MHC/peptide and CD80/86/CD28 binding following interaction of cognate T cells with DCs.22 Therefore, we hypothesized that exogenous expression of 4-1BBL on DCs may play an important role in modulating CTLs activation during an Ag-presenting DCs encounters a cognate T cell.
4-1BB signal has been proven to be superior to CD28 co-stimulation for generating CD8+ CTLs for adoptive immunotherapy.23 In this study, our data demonstrated that none-pulsed DCs, even with upregulation of 4-1BBL, failed to activate T lymphocytes. However, tPSMA gene-pulsed DCs effectively induced T lymphocyte activation, which was further enhanced by upregulated expression of 4-1BBL, showing better cell viability, lower CTLs apoptosis and higher expression of Bcl-xL. Activated CTLs tend to undergo apoptosis, dysfunction, or induced anergy in the tumor microenvironment.24,25 Therefore, effective antitumor immune response depends on not only “activation” but also “maintenance” of CTLs numerically and functionally. Bcl-xL, a well-known anti-apoptotic protein of a Bcl-2 family, is able to suppress apoptosis in multiple cell types26 and plays a significant role in the regulation of cells survival of immune system.27,28 It has been proven that Bcl-xL could protect cells from Fas/FasL-mediated killing29 and remarkably inhibit caspase activation.30 It is reported that FasL expressed in a quite number of types of tumor cells including prostate cancer and their capacity to cause apoptosis of Fas-expressing T cells has been proposed as a crucial mechanism of tumor escaping from immune surveillance.31,32 Eaton D et al.33 showed that CTLs introduced with BcL-xL gene could modulate resistance to apoptosis and prolonged the survival of tumor-specific CTLs. Furthermore, our data indicate that it is 4-1BB-mediated NF-κB activation prompted by upregulation of 4-1BBL that provides CTLs with prolonged survival via upregulation of Bcl-xL. It is likely that CD28-mediated NF-κB activation is essential for Bcl-xL induction and anti-apoptotic effects in primary human CD4+ T lymphocytes.34 In this study, we demonstrated tPSMA and 4-1BBL modified DCs induced higher level of phosphorylation of P38 and more IFN-γ production in T cell activation. p38 activation is required for the development of both Th1 and Th2 cells in response to either 4-1BB or CD28-dependent co-stimulation. Th1 cells require co-stimulation and p38 activation for the continued secretion of the Th1 cytokines, IL-2 and IFN-γ. In contrast, Th2 cells did not require p38 activation.35 Thereby, these enhanced CTLs functions in turn may promote the antitumor efficacy of CTLs. However, how does 4-1BBL transduction act on the observed phenomena merit further investigation, including whether 4-1BBL affect the intrinsic properties of DCs, as showed in our previous data that CD80 and CD86 are upregulated in DCs by Ad-tPMSA-IRES-4-1BBL, and/or act via engagement of 4-1BB on T cells.13 Our results are consistent with the published data that agonistic 4-1BB mAbs promoted the proliferation and the cytokine secretion of the activated lymphocyte.36 Moreover, we demonstrated that the tumor killing was antigenic-specific, as the antitumor cytotoxicity occurred only on tPSMA expressing cells, whereas not in the specific antigen negative tumor cell line, RM-1.
Taken together, 4-1BBL modified DCs pulsed with tPSMA gene could induce enhanced function of CTLs, which may overcome the side effects from a whole agonistic 4-1BB mAb due to its immunogenicity. This mechanism could account for obvious prostate cancer regression in mouse models induced by tPSMA and 4-1BBL-engineered DCs vaccine in our previous publication.
Materials and methods
Animals, cell lines and recombinant adenovirus
Female C57BL/6 (H-2 Kb) mice, 6–8 week old, were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. Animals were maintained at the Central Animal Facility of Wuhan University according to standard guidelines and experiments were conducted according to the guidelines of the China Council for Animal Care. RM-1, a murine prostate cancer cell line, is obtained from Chinese Academy of Sciences. HEK 293, a human embryonic kidney 293 cell line, is kindly provided by the Ministry of Education Key Laboratory of Virology. All cells were cultured in RPMI-1640 medium with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Stable expressed tPSMA of RM-1-tPSMA cell, recombinant adenoviruses Ad-tPSMA-IRES-m4-1BBL and Ad-m4-1BBL have been described previously.13,37
Recombinant adenovirus Ad-tPSMA
The E1 and E3 regions deficient serotype 5 recombinant adenovirus vector was used to construct Ad-tPSMA using pAdEasy-1 system gifted by the Ministry of Education Key Laboratory of Virology. Briefly, the cDNA for extracellular domain of human prostate-specific membrane antigen (tPSMA) was amplified from plasmid pCR3.1®-Uni-hPSMA plasmid, a kind gift from Dr. Xiangzhong Yu (Department of Biological Sciences, Clemson University) and inserted into the shuttle vector pAdTrack-CMV between BglII and Hind III-restriction sites. The resultant plasmid was linearized by PmeІ and subsequently co-transformed into E. coli BJ5183 with an adenoviral backbone plasmid (pAdEasy-1). Then the recombinant adenoviral plasmid (pAd-tPSMA) was transfected into HEK 293 cells with Lipofectamine™ 2000 (Invitrogen) for amplification. Adenovirus was purified by centrifugation in a cesium chloride gradient. The Ad-eGFP was constructed similarly serves as control Adenovirus.
Dendritic cells preparation
Mouse DCs generated from bone-marrow suspensions harvested of 6–8 week old C57BL/6 mice has been described previously.13 Brieñy, bone-marrow cells were harvested from femurs and tibias depleted of red blood cells and washed twice in phosphate-buffered saline (PBS). Then cells were resuspended in RPMI 1,640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco), 10 ng/mL GM-CSF (PeproTech), 10 ng/mL IL-4 (PeproTech) and 50 mM 2-mercaptoethanol, 100 IU/ml penicillin and 100 µg/ml streptomycin and cultured (37°C, 5% CO2) in 6-well plates at 1 × 106cells/3ml/well. On day 3 and 5 of culture, floating cells were gently removed, and fresh GM-CSF/IL-4-contained medium was added. On day 7, non-adherent cells and loosely adherent proliferating DCs aggregates were collected as immature DCs (iDCs) or were activated with lipopolysaccharide (LPS, 1 µg/ml, Sigma) for 24 h to obtain mature DCs (mDCs).
CTLs generation
iDCs transduced with four types of adenovirus (Ad-tPSMA-IRES-m4-1BBL, Ad-tPSMA, Ad-m4-1BBL and Ad-eGFP) separately at MOI 300 according to our previous publication13 or no iCDs were used as stimulator cells. Nylon wool-purified splenic T cells were used as responder cells. Stimulator cells were matured with LPS and were incubated with Mitomycin C (MMC) at 50 ng/ml at 37°C for 30 min and then washed with PBS twice. Responder cells (2 × 106) were co-cultured with stimulator cells (1 × 105) in a 24-well tissue culture plate in 1ml complete medium. IL-2 was added to a final concentration of 20 IU/ml all wells and every 3 d thereafter. Responder cells were re-stimulated weekly for 2 weeks with transfected DCs at a responder cells-to-stimulator DCs ratio of 20:1. The CTLs were then collected.
ELISA for measuring cytokines in supernatants
48h after last re-stimulation in generation of CTLs, culture supernatants were harvested and analyzed for IL-4, IL-10 and IFN-γ production by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems), following the manufacturer’s instructions.
Preparation of total cell lysates and nuclear fraction and immunoblot analysis
For total cell lysates preparation, CTLs were collected and lysed in ice-cold lysis buffer (25 mmol/L Tris/HCl, pH 7.6, 150 mmol/L NaCl, 1 mmol/L Na3VO4, 5 mmol/L EDTA, 10 mmol/L NaF, 50 mmol/L b-glycerophosphate, 0.5 mmol/L phenylmethyl sulfonylfluoride and 1% Triton X-100) containing a protease inhibitor cocktail (Roche Diagnostics Ltd.) and then vortexed at 4°C for 10 min. Cell lysates were subjected to a centrifugation of 10,000 rpm for 10min at 4°C, and the insoluble pellet was discarded. Nuclear extracts were prepared using a modification of a previous publication.38 Briefly, CTLs were harvested and lysed with buffer A (10 mM HEPES, 10mM KC1, 1.5 mM MgCl2, 0.1 mM EDTA, 0.2% NP40, 1 min MDTT and 0.5 min M phenylmethylsulfonyl fluoride), followed by vortexing at 4°C for 10 min to shear the cytoplasmic membranes and nuclear pellets were collected by a centrifugation at 3,000 rpm for 5 min at 4°C. Nuclear proteins were extracted with high-salt buffer B (20 mM HEPES, 25% glycerol, 1.5 mM MgCl2, 0.1 mM EDTA, 420 mM NaCl, 1 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride). Protein concentration of total cell lysates and nuclear fraction were determined by Protein Assay (Bio-Rad laboratories). Total cellular proteins or nuclear extracts (50 μg) were subjected to SDS-PAGE, and transferred to nitrocellulose membranes (Amersham). Specific polyclonal antibodies against IκB-α, P38, phosphorylated of P38, Bcl-xL and NF-κB p65 diluted in TBS-T containing 5% nonfat milk were used to detect indicated proteins. The appropriate horseradish peroxidase (HRP) conjugated secondary antibodies were used for all primary antibodies. Antibodies on membrane were visualized by enhanced chemiluminescence (Pierce). Immunoblot for β-actin was used as an internal control. To detect tPSMA expression, RM-1 cells (1 × 106) were infected with the Ad-tPSMA [multiplicity of infection (MOI) 50]. 48hr later, cells were scraped and lysed and detected by immunoblot.
Flow cytometry
CTLs (2 × 106 cells) were collected by centrifugation and then washed twice with ice-cold PBS. Apoptotic cells were detected by flow cytometry using FITC-conjugated Annexin V and propidium iodide (PI) (Molecular Probes, Invitrogen).
Cytotoxicity assay
RM-1 cells or RM-1-tPSMA cells used as target cells were placed in 96-well tissue culture plates at 1 × 104 cells per well and co-cultured with CTLs used as effector cells at the ratio of 1:6, 1:12, 1:25 and 1:50 for 48 h at 37°C in 5% CO2. Target cells and effector cells incubated in medium alone served as target cells control and effector cells control, respectively. The cytotoxic activities were measured with Cell Counting Kit-8 assay. 10 µl CCK-8 resolution was added to each well in 100 µl medium. Absorbance was detected at 450 nm using automatic ELISA reader (TRITURUS). All determinations were performed in triplicate. The percentage of specific cytotoxicity was calculated as [target control-(experimental-effector control)/target control] × 100%.
Statistical analysis
All data were presented as mean ± standard deviation. Differences were considered statistically significant for p < 0.05 as determined by student's t-test using SPSS13.0. All means were calculated from at least three independent experiments.
Acknowledgment
A special thanks to Dr. Xiangzhong Yu (Department of Biological Sciences, Clemson University) for providing pCR3.1®-Uni-hPSMA vector, Dr. Tania Watts (Department of Immunology, University of Toronto) for providing pcDNA3-m4-1BBL, Dr. Jianguo Wu (Ministry of Education Key Laboratory of Virologyo, Wuhan University) and Vecter Gene Technology Company Ltd.
Glossary
Abbreviations:
- DCs
dendritic cells
- CTLs
cytotoxic T lymphocytes
- tPSMA
truncated Prostate Specific Membrane Antigen
- APCs
antigen-presenting cells
- PCa
prostate cancer
- TCR
T-cell receptor
- TNF
Tumour Necrosis Factor
- PBS
phosphate-buffered saline
- FCS
fetal calf serum
- MMC
Mitomycin C
- ELISA
enzyme-linked immunosorbent assay
- TAA
tumor associated antigen
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23116
References
- 1.Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010;2:847–62. doi: 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, et al. Dendritic cell-based vaccine in advanced melanoma: update of clinical outcome. Melanoma Res. 2011;21:524–9. doi: 10.1097/CMR.0b013e32834b58fa. [DOI] [PubMed] [Google Scholar]
- 3.Di Lorenzo G, Buonerba C, Kantoff PW. Immunotherapy for the treatment of prostate cancer. Nat Rev Clin Oncol. 2011;8:551–61. doi: 10.1038/nrclinonc.2011.72. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Thomas-Kaskel AK, Waller CF, Schultze-Seemann W, Veelken H. Immunotherapy with dendritic cells for prostate cancer. Int J Cancer. 2007;121:467–73. doi: 10.1002/ijc.22859. [DOI] [PubMed] [Google Scholar]
- 6.Durrant LG, Ramage JM. Development of cancer vaccines to activate cytotoxic T lymphocytes. Expert Opin Biol Ther. 2005;5:555–63. doi: 10.1517/14712598.5.4.555. [DOI] [PubMed] [Google Scholar]
- 7.Haile ST, Bosch JJ, Agu NI, Zeender AM, Somasundaram P, Srivastava MK, et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011;186:6822–9. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–92. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–40. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervais A, Toutirais O, Bouet-Toussaint F, De la Pintiere CT, Genetet N, Catros-Quemener V. In vitro antitumor lymphocyte generation using dendritic cells and innate immunity mechanisms as tumor cell treatments. Anticancer Res. 2007;27(4B):2385–92. [PubMed] [Google Scholar]
- 11.Bear AS, Cruz CR, Foster AE. T cells as vehicles for cancer vaccination. J Biomed Biotechnol. 2011;2011:417403. doi: 10.1155/2011/417403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh SK, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–30. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 13.Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Jiang B, et al. Anti-tumor immune response induced by dendritic cells transduced with truncated PSMA IRES 4-1BBL recombinant adenoviruses. Cancer Lett. 2010;293:254–62. doi: 10.1016/j.canlet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Bühler P, Wolf P, Gierschner D, Schaber I, Katzenwadel A, Schultze-Seemann W, et al. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol Immunother. 2008;57:43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol. 2002;14:1155–67. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 16.Lu ZY, Condomines M, Tarte K, Nadal L, Delteil MC, Rossi JF, et al. B7-1 and 4-1BB ligand expression on a myeloma cell line makes it possible to expand autologous tumor-specific cytotoxic T cells in vitro. Exp Hematol. 2007;35:443–53. doi: 10.1016/j.exphem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao H, Huang B, Yuan Y, Li D, Han LF, Liu Y, et al. Soluble PD-1 facilitates 4-1BBL-triggered antitumor immunity against murine H22 hepatocarcinoma in vivo. Clin Cancer Res. 2007;13:1823–30. doi: 10.1158/1078-0432.CCR-06-2154. [DOI] [PubMed] [Google Scholar]
- 18.Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–26. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nencioni A, Grünebach F, Schmidt SM, Müller MR, Boy D, Patrone F, et al. The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol. 2008;65:191–9. doi: 10.1016/j.critrevonc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Nagaraj S, Pisarev V, Kinarsky L, Sherman S, Muro-Cacho C, Altieri DC, et al. Dendritic cell-based full-length survivin vaccine in treatment of experimental tumors. J Immunother. 2007;30:169–79. doi: 10.1097/01.cji.0000211329.83890.ba. [DOI] [PubMed] [Google Scholar]
- 21.Nencioni A, Brossart P. Cellular immunotherapy with dendritic cells in cancer: current status. Stem Cells. 2004;22:501–13. doi: 10.1634/stemcells.22-4-501. [DOI] [PubMed] [Google Scholar]
- 22.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062–70. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 23.Powell DJ, Jr., Levine BL. Adoptive T-cell therapy for malignant disorders. Haematologica. 2008;93:1452–6. doi: 10.3324/haematol.13717. [DOI] [PubMed] [Google Scholar]
- 24.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 25.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, et al. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–62. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–13. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa T, Niwa T, Mizuguchi H, Okada N, Nakagawa S. Engineering of highly immunogenic long-lived DC vaccines by antiapoptotic protein gene transfer to enhance cancer vaccine potency. Gene Ther. 2008;15:1321–9. doi: 10.1038/gt.2008.85. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Chen J, Majumder N, Lin H, Falo LD, Jr., You Z. ‘Survival gene’ Bcl-xl potentiates DNA-raised antitumor immunity. Gene Ther. 2005;12:1517–25. doi: 10.1038/sj.gt.3302584. [DOI] [PubMed] [Google Scholar]
- 29.Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–6. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 30.Bai H, Chen K, Gao YX, Arzigian M, Xie YL, Malcosky C, et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F. Fas counter-attack--the best form of tumor defense? Nat Med. 1999;5:267–8. doi: 10.1038/6477. [DOI] [PubMed] [Google Scholar]
- 32.Pirtskhalaishvili G, Shurin GV, Gambotto A, Esche C, Wahl M, Yurkovetsky ZR, et al. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165:1956–64. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- 33.Eaton D, Gilham DE, O’Neill A, Hawkins RE. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9:527–35. doi: 10.1038/sj.gt.3301685. [DOI] [PubMed] [Google Scholar]
- 34.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-κ B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–54. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 35.Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol. 2000;165:6193–204. doi: 10.4049/jimmunol.165.11.6193. [DOI] [PubMed] [Google Scholar]
- 36.Ju SA, Cheon SH, Park SM, Tam NQ, Kim YM, An WG, et al. Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int J Cancer. 2008;122:2784–90. doi: 10.1002/ijc.23457. [DOI] [PubMed] [Google Scholar]
- 37.Youlin K, Xiaodong W, Xiuheng L, Zhiyuan C, Hengcheng Z, Hui C, et al. The change of immunoactivity of dendritic cells induced by mouse 4-1BBL recombinant adenovirus. Yonsei Med J. 2010;51:594–8. doi: 10.3349/ymj.2010.51.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh CB, Hsieh MJ, Hsieh YH, Chien MH, Chiou HL, Yang SF. Antimetastatic effects of norcantharidin on hepatocellular carcinoma by transcriptional inhibition of MMP-9 through modulation of NF-kB activity. PLoS ONE. 2012;7:e31055. doi: 10.1371/journal.pone.0031055. [DOI] [PMC free article] [PubMed] [Google Scholar]