Abstract
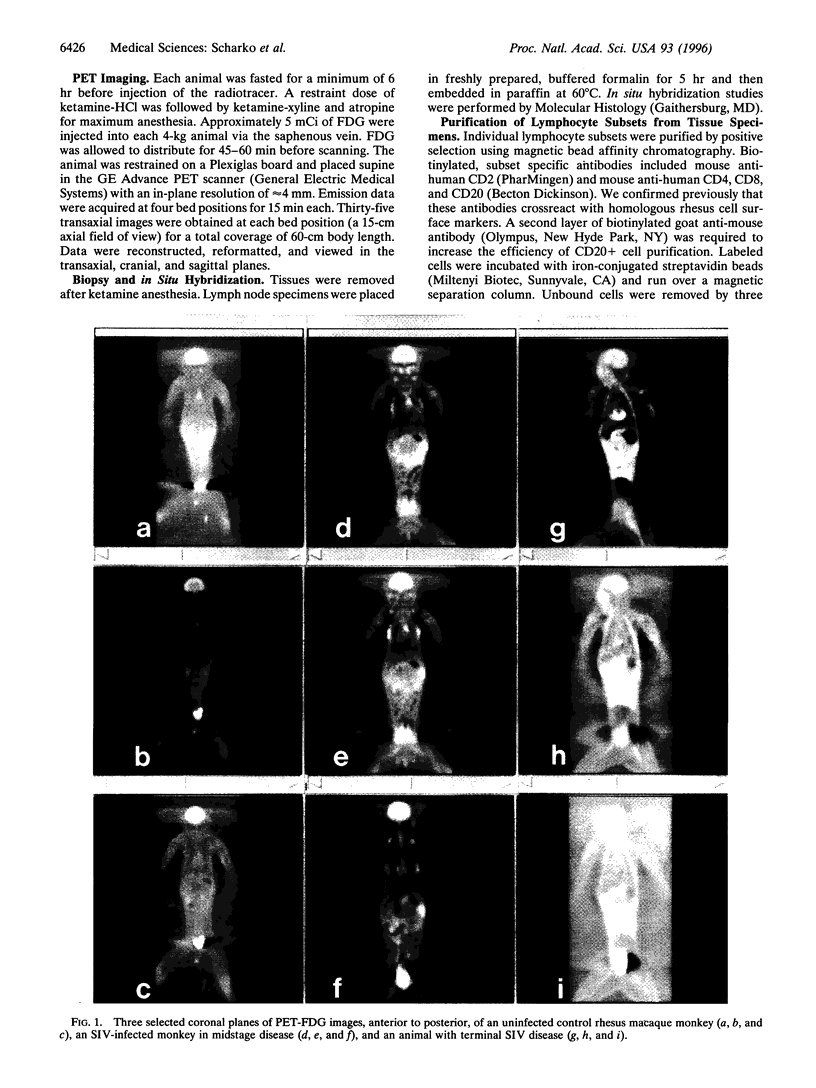
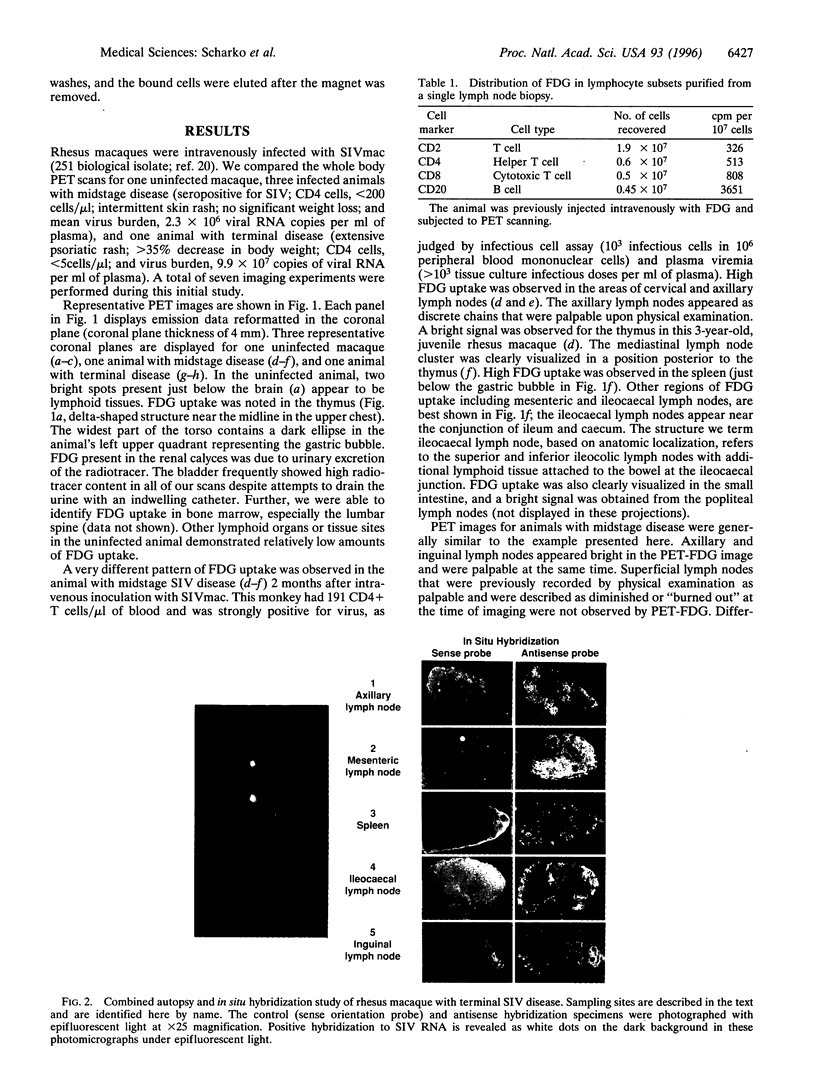
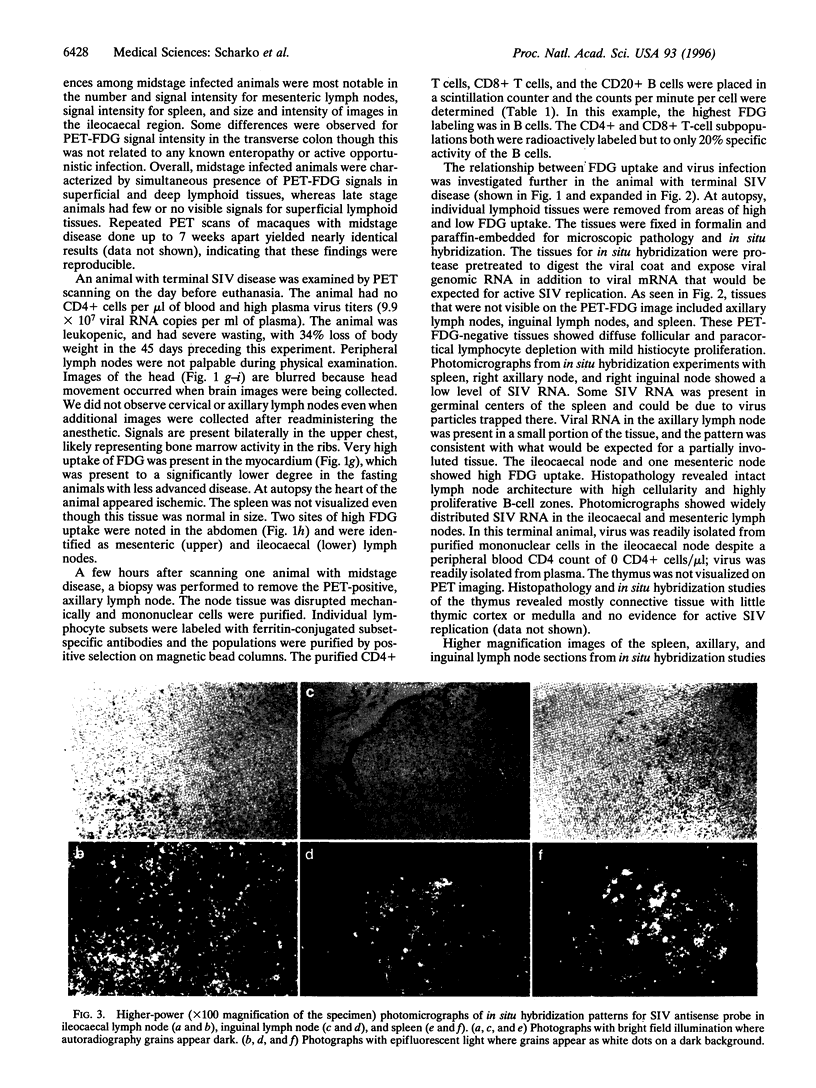
Pathogenesis of simian immunodeficiency virus (SIV) infection in rhesus macaques begins with acute viremia and then progresses to a distributed infection in the solid lymphoid tissues, which is followed by a process of cellular destruction leading to terminal disease and death. Blood and tissue specimens show the progress of infection at the cellular level but do not reveal the pattern of infection and host responses occurring throughout the body. The purpose of this investigation was to determine whether positron emission tomography (PET) imaging with intravenous 2-18F-2-deoxyglucose (FDG) could identify activated lymphoid tissues in a living animal and whether this pattern would reflect the extent of SIV infection. PET images from SIV-infected animals were distinguishable from uninfected controls and revealed a pattern consistent with widespread lymphoid tissue activation. Significant FDG accumulation in colon along with mesenteric and ileocaecal lymph nodes was found in SIV infection, especially during terminal disease stages. Areas of elevated FDG uptake in the PET images were correlated with productive SIV infection using in situ hybridization as a test for virus replication. PET-FDG images of SIV-infected animals correlated sites of virus replication with high FDG accumulation. These data show that the method can be used to evaluate the distribution and activity of infected tissues in a living animal without biopsy. Fewer tissues had high FDG uptake in terminal animals than midstage animals, and both were clearly distinguishable from uninfected animal scans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castillo M. Brain infections in human immunodeficiency virus positive patients. Top Magn Reson Imaging. 1994 Winter;6(1):3–10. [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Garcia C. F., Lifson J. D., Engleman E. G., Schmidt D. M., Warnke R. A., Wood G. S. The immunohistology of the persistent generalized lymphadenopathy syndrome (PGL). Am J Clin Pathol. 1986 Dec;86(6):706–715. doi: 10.1093/ajcp/86.6.706. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Hoffman J. M., Waskin H. A., Schifter T., Hanson M. W., Gray L., Rosenfeld S., Coleman R. E. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med. 1993 Apr;34(4):567–575. [PubMed] [Google Scholar]
- Ioachim H. L., Lerner C. W., Tapper M. L. Lymphadenopathies in homosexual men. Relationships with the acquired immune deficiency syndrome. JAMA. 1983 Sep 9;250(10):1306–1309. doi: 10.1001/jama.250.10.1306. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Spira T. J., Fishbein D. B., Pinsky P. F., Schonberger L. B. Lymphadenopathy syndrome in homosexual men. Evidence for continuing risk of developing the acquired immunodeficiency syndrome. JAMA. 1987 Jan 16;257(3):335–337. doi: 10.1001/jama.257.3.335. [DOI] [PubMed] [Google Scholar]
- Katzenstein D. A., Holodniy M., Israelski D. M., Sengupta S., Mole L. A., Bubp J. L., Merigan T. C. Plasma viremia in human immunodeficiency virus infection: relationship to stage of disease and antiviral treatment. J Acquir Immune Defic Syndr. 1992;5(2):107–112. [PubMed] [Google Scholar]
- Lackner A. A., Vogel P., Ramos R. A., Kluge J. D., Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994 Aug;145(2):428–439. [PMC free article] [PubMed] [Google Scholar]
- LeBlang S. D., Whiteman M. L., Post M. J., Uttamchandani R. B., Bell M. D., Smirniotopolous J. G. CNS Nocardia in AIDS patients: CT and MRI with pathologic correlation. J Comput Assist Tomogr. 1995 Jan-Feb;19(1):15–22. doi: 10.1097/00004728-199501000-00003. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Sheppard H. W., Reis M., Dondero D., Osmond D., Busch M. P. Circulating HIV-1-infected cell burden from seroconversion to AIDS: importance of postseroconversion viral load on disease course. J Acquir Immune Defic Syndr. 1994 Apr;7(4):381–388. [PubMed] [Google Scholar]
- Letvin N. L., King N. W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3(11):1023–1040. [PubMed] [Google Scholar]
- McConnell J. R., Swindells S., Ong C. S., Gmeiner W. H., Chu W. K., Brown D. K., Gendelman H. E. Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS Res Hum Retroviruses. 1994 Aug;10(8):977–982. doi: 10.1089/aid.1994.10.977. [DOI] [PubMed] [Google Scholar]
- Mechtler L. L., Kinkel P. R. Magnetic resonance imaging in demyelinating, infectious, metabolic, and congenital diseases. Curr Opin Neurol. 1993 Dec;6(6):912–918. doi: 10.1097/00019052-199312000-00014. [DOI] [PubMed] [Google Scholar]
- Memel D. S., DeRogatis A. J., William D. C. Ga-67 citrate myocardial uptake in a patient with AIDS, toxoplasmosis, and myocarditis. Clin Nucl Med. 1991 May;16(5):315–317. doi: 10.1097/00003072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S., Okabayashi S., Dai S., Morimitsu Y., Murakami T., Matsuishi T., Motizuki M., Kato H. Neuroimaging and neuropathologic findings in AIDS patient with cytomegalovirus infection. Intern Med. 1994 Mar;33(3):158–162. doi: 10.2169/internalmedicine.33.158. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Cohen O. J., Vaccarezza M., Gantt K., Muro-Cacho C., Fauci A. S. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994 Aug;140:105–130. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Emau P., Salvato M. S., Trivedi P., MacKenzie D., Malkovsky M., Uno H., Schultz K. T. Pathogenesis of SIVmac251 after atraumatic inoculation of the rectal mucosa in rhesus monkeys. J Med Primatol. 1993 Feb-May;22(2-3):154–161. [PubMed] [Google Scholar]
- Reimann K. A., Tenner-Racz K., Racz P., Montefiori D. C., Yasutomi Y., Lin W., Ransil B. J., Letvin N. L. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994 Apr;68(4):2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. S., Joffe S. A., Itescu S. Spectrum of salivary gland disease in HIV-infected patients: characterization with Ga-67 citrate imaging. Radiology. 1992 Sep;184(3):761–764. doi: 10.1148/radiology.184.3.1509064. [DOI] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Kahl C., Feller A. C., Kern P., Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog Allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Crain M. J., Decker W. D., Campbell-Hill S., Robinson S., Brown W. E., Leuther M., Whitley R. J., Hahn B. H., Shaw G. M. High-level viremia in adults and children infected with human immunodeficiency virus: relation to disease stage and CD4+ lymphocyte levels. J Infect Dis. 1991 Jul;164(1):72–80. doi: 10.1093/infdis/164.1.72. [DOI] [PubMed] [Google Scholar]
- Slizofski W. J., Brown S. J., Dadparvar S., Glab L. B. Diagnostic significance of apparent abnormal bowel activity on Ga-67 scans in AIDS patients. Clin Nucl Med. 1991 Jul;16(7):473–477. doi: 10.1097/00003072-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]





