Abstract
Background: Since influenza predisposes to bacterial pneumonia caused by Streptococcus pneumoniae, studies have suggested that pneumococcal vaccination might reduce its occurrence during pandemics. We assessed the effectiveness of pneumococcal polysaccharide vaccination alone and in combination with influenza vaccination in preventing influenza hospitalization during the 2009–2010 pandemic wave and 2010–2011 influenza epidemic.
Methods: We conducted a multicenter case-control study in 36 Spanish hospitals. We selected patients aged ≥ 18 y hospitalized with confirmed influenza and two hospitalized controls per case, matched according to age, date of hospitalization and province of residence. Multivariate analysis was performed using conditional logistic regression. Subjects were considered vaccinated if they had received the pneumococcal or seasonal influenza vaccine > 14 d (or > 7 d for pandemic influenza vaccine) before the onset of symptoms (cases) or the onset of symptoms in matched cases (controls).
Results: 1187 cases and 2328 controls were included. The adjusted estimate of effectiveness of pneumococcal vaccination in preventing influenza hospitalization was 41% (95% CI 8–62) in all patients and 43% (95% CI 2–78) in patients aged ≥ 65 y. The adjusted effectiveness of dual PPV23 and influenza vaccination was 81% (95% CI 65–90) in all patients and 76% (95% CI 46–90) in patients aged ≥ 65 y. The adjusted effectiveness of influenza vaccination alone was 58% (95% CI 38–72).
Conclusions: In elderly people and adults with chronic illness, pneumococcal vaccination may reduce hospitalizations during the influenza season. In people vaccinated with both the influenza and pneumococcal vaccines, the benefit in hospitalizations avoided was greater than in those vaccinated only against influenza.
Keywords: effectiveness, hospitalization, influenza vaccine, laboratory-confirmed influenza, pneumococcal polysaccharide vaccine
Introduction
In April 2009, the first cases of illness caused by a new subtype of the influenza A virus [A/California/04/2009 (H1N1)] were reported,1 and in Spain, a pandemic vaccine containing the A/California/04/2009 (H1N1) strain was recommended for persons with medical conditions that increased the risk of complications after November 16, 2009. During the subsequent influenza season (2010–2011), the vaccine strain and the predominant circulating influenza subtype remained the same.2 The 2010–11 trivalent vaccines contained A/California/7/2009 (H1N1)-like, A/Perth/16/2009 (H3N2)-like and B/Brisbane/60/2008-like antigens. The A/California/7/2009 (H1N1)-like antigen is derived from a pandemic 2009 influenza A (H1N1) virus and was the same vaccine antigen used in the influenza A (H1N1) 2009 monovalent vaccines.3
Since influenza predisposes to bacterial pneumonia caused by Streptococcus pneumoniae,4-6 several studies have suggested that pneumococcal vaccination might reduce the occurrence of S. pneumoniae pneumonia during the pandemic.6-8 The effectiveness of the 23-valent polysaccharide pneumococcal vaccine (PPV23) in preventing pneumonia, the most frequent complication of influenza, has been reported in several studies9-11 although other studies found that PPV23 vaccination was not effective.12,13 Concomitant use of pneumococcal and influenza vaccines has provided added protection against hospitalization for several diseases, including respiratory illness and death.14-16 After the 2009 influenza pandemic was declared, it was suggested that coadministration of the PPV23 and influenza vaccines might have a greater protective effect in the elderly and high risk groups than programs aimed at just one of these vaccines.17 To our knowledge, no study has reported on whether PPV23 vaccination helped reduce hospitalizations related to influenza A(H1N1)2009 infection.
The objective of this study was to estimate, by means of a case-control study, the effectiveness of PPV23 vaccination alone and in combination with influenza vaccination in preventing influenza hospitalization during the 2009–2010 pandemic wave and the 2010–2011 influenza epidemic in Spain.
Results
A total of 1385 possible eligible cases and 2611 possible eligible controls were considered for the study. Twenty-nine patients were excluded because influenza was acquired after hospital admission and 169 were excluded because they did not give consent to participate; 283 potential controls were excluded, all of whom did not give consent. Therefore, 1187 cases and 2328 controls were finally included in the study. In the 2009–2010 season, all cases were due to influenza A(H1N1) 2009. In the 2010–11 season, 547 cases (96.1%) were due to influenza A(H1N1) 2009, 17 cases (3%) to influenza B and 5 cases (0.9%) to influenza A(H3N2). The reason for admission of cases was pulmonary decompensation (54.5%), worsened general health status (19.7%), presence of risk conditions (17.0%) and pregnancy (0.2%); the reason was not determined in 8.6% of cases. The distribution of demographic variables and medical conditions of cases and controls are shown in Table 1.
Table 1. Distribution of cases and controls according to demographic variables, medical conditions and history of influenza vaccination.
| Characteristics | Cases (n = 1187) | Controls (n = 2328) | p-value |
|---|---|---|---|
| Age (in years, median ± SD) | 50.6 ± 16.1 | 50.7 ± 16.2 | 0.07 |
| Female | 552 (46.5%) | 1122 (48.2%) | 0.29 |
| Ethnicity | |||
| Caucasian | 1054 (90.1%) | 2142 (92.4%) | |
| Gypsy | 24 (2.1%) | 28 (1.2%) | 0.03 |
| Hispanic | 52 (4.4%) | 77 (3.3%) | 0.29 |
| Arab or North African | 19 (1.6%) | 28 (1.2%) | 0.47 |
| Other | 21 (1.8%) | 43 (1.9%) | 0.91 |
| Educational level | |||
| Secondary or higher | 704 (62.5%) | 1442 (62.3%) | 0.92 |
| Smoker | 640 (54.7%) | 1151 (49.6%) | 0.002 |
| Alcoholism | 125 (10.8%) | 239 (10.3%) | 0.49 |
| Pneumonia last 2 y | 121 (10.2%) | 97 (4.2%) | < 0.001 |
| COPD | 153 (12.9%) | 137 (5.9%) | < 0.001 |
| Asthma | 155 (13.1%) | 144 (6.2%) | < 0.001 |
| Cardiovascular disease | 147 (12.4%) | 228 (9.8%) | 0.005 |
| Renal failure | 99 (8.3%) | 204 (8.8%) | 0.79 |
| Diabetes | 193 (16.3%) | 348 (14.9%) | 0.25 |
| HIV Infection | 41 (3.5%) | 43 (1.8%) | 0.003 |
| Neurological disease | 38 (3.2%) | 47 (2.0%) | 0.02 |
| Neoplasia | 155 (13.1%) | 309 (13.3%) | 0.93 |
| Transplantation | 82 (6.9%) | 62 (2.7%) | < 0.001 |
| Previous antibiotics | 334 (28.1%) | 388 (16.7%) | < 0.001 |
| Corticosteroids | 345 (29.1%) | 292 (12.5%) | < 0.001 |
| Pandemic influenza vaccinea | 10 (1.7%) | 62 (5.6%) | 0.001 |
| Seasonal influenza vaccineb | 98 (17.3%) | 248 (22.6%) | 0.002 |
| PPV23 vaccine | 81 (7.5%) | 196 (9.4%) | 0.04 |
| PPV23 + Influenza vaccine | 23 (2.2%) | 118 (5.7%) | < 0.001 |
a Season 2009–10. b Season 2010–11.
Of the 93 cases with documented secondary bacterial pneumonia (7.8% of all cases), the etiological agent was determined in 31 (33.3%) and, of these, S. pneumoniae was detected in 22 (70.9%). Therefore, 22/93 cases of secondary bacterial pneumonia (23.7%) were due to S. pneumoniae. No patient with confirmed pneumococcal pneumonia had received the PPV23. No interactions were observed between PPV23 vaccination and influenza vaccination or between PPV23 and individual risk factors.
The crude and adjusted associations between PPV23 vaccination and influenza hospitalization in the different age groups (all patients and only those with risk conditions) and the adjusted vaccination effectiveness are shown in Table 2. PPV23 coverage was low in all patients with risk conditions (8.6% in cases and 11% in controls). In patients aged 18–64 y with risk conditions, PPV23 coverage was 5.2% in cases and 4.7% in controls, whereas, in those aged ≥ 65 y, it was 19.6% in cases and 27.4% in controls. The adjusted estimate of effectiveness for all patients was 41% (95% CI 8–62); in patients aged 18–64 y with risk conditions it was 31% (95% CI -18 to 68), but the statistical power was only 31.1%. In all patients aged ≥ 65 y, the effectiveness of PPV23 vaccination was 43% (95% CI 2–78).
Table 2. Association between a history of PPV23 vaccination and influenza hospitalization.
| PPV23 | Casesa vaccinated/N (%) | Controls vaccinated/N (%) | Crude OR (95% CI) | Adjusted ORb (95% CI) | Adjusted Vaccination Effectiveness (95% CI) |
|---|---|---|---|---|---|
| All patients | |||||
| With Risk Conditions | 72/833 (8.6%) | 178/1625 (11.0%) | 0.72 (0.52–0.99) | 0.58 (0.32–0.85) | 42% (15–68)f |
| All | 81/1073 (7.5%) | 196/2085 (9.4%) | 0.70 (0.50–0.97) | 0.59 (0.38–0.92) | 41% (8–62)g |
| 18–64 y | |||||
| With Risk Conditions | 33/632 (5.2%) | 57/1201 (4.7%) | 0.94 (0.58–1.53) | 0.69 (0.32–1.18)c | 31% (-18–68)h |
| All | 37/849 (4.4%) | 65/1609 (4.0%) | 0.93 (0.59–1.46) | 0.70 (0.36–1.32)d | 30% (-32–64)i |
| ≥ 65 y | |||||
| With Risk Conditions | 39/201 (19.4%) | 121/424 (28.5%) | 0.62 (0.38–1.02) | 0.58 (0.25–1.32)e | 42% (-32–75)j |
| All | 44/224 (19.6%) | 131/476 (27.4%) | 0.63 (0.39–1.00) | 0.57 (0.28–0.98) | 43% (2–78)k |
Abbreviations: OR, odds ratio; CI, confidence interval. aLaboratory-confirmed by RT-PCR. bAdjusted for: With Risk Conditions: Age, seasonal influenza vaccine in 2010–11 (patients recruited in 10–11), pandemic influenza vaccine in 2009–10, sex (only for all patients), educational level (only for ≥ 65y), smoker, pneumonia, COPD (only for all patients and 18–64y), asthma (only for all patients and 18–64y), cardiovascular disease (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), neoplasia (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. All: Age, seasonal influenza vaccine in 2010–11 (patients recruited in 10–11), pandemic influenza vaccine in 2009–10, sex (only for all patients and ≥ 65y), ethnicity (only for all patients and 18–64y), smoker (only for all patients and ≥ 65y), pneumonia (only for all patients and 18–64y), COPD (only for all patients and 18–64y), asthma (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. Statistical power: c31.1%; d33.0%; e75.7%. Hosmer-Lemeshow test: fp = 0.42; gp = 0.32; hp = 0.87; ip = 0.21; jp = 0.39; kp = 0.52
The crude and adjusted association between influenza vaccination and hospitalization and the estimate of adjusted vaccination effectiveness are shown in Table 3. The crude and adjusted associations between previous vaccination with both PPV23 and influenza vaccine and hospitalization are shown in Table 4. The lowest adjusted OR corresponded to all patients aged 18–64 y (0.16; 95% CI 0.05–0.52), but the adjusted ORs were under 0.25 in all categories. The adjusted effectiveness of dual PPV23 and influenza vaccination in preventing influenza hospitalization was 81% (95% CI 65–90) for all patients and 81% (95% CI 35–94) in patients aged 18–64 y with risk conditions. In all patients aged ≥ 65 y, the effectiveness of dual vaccination was 76% (95% CI 46–90).
Table 3. Association between a history of influenza vaccination and influenza hospitalization.
| Influenza vaccine | Casesa vaccinated/N (%) | Controls vaccinated/N (%) | Crude OR (95% CI) | Adjusted ORb (95% CI) | Adjusted Vaccination Effectiveness (95% CI) |
|---|---|---|---|---|---|
| All patients | |||||
| With Risk Conditions | 102/883 (11.6%) | 268/1731 (15.5%) | 0.62 (0.47–0.83) | 0.45 (0.29–0.69) | 55% (31–71)d |
| All | 108/1138 (9.5%) | 310/2210 (14.0%) | 0.55 (0.24–0.51) | 0.42 (0.28–0.62) | 58% (38–72)e |
| 18–64 y | |||||
| With Risk Conditions | 51/677 (7.5%) | 105/1302 (8.1%) | 0.87 (0.60–1.28) | 0.63 (0.36–1.12)c | 37% (-12–64)f |
| All | 54/909 (5.9%) | 124/1729 (7.2%) | 0.75 (0.52–1.07) | 0.60 (0.36–0.99) | 40% (1–64)g |
| ≥ 65 y | |||||
| With Risk Conditions | 51/206 (24.8%) | 163/429 (38.0%) | 0.37 (0.22–0.62) | 0.31 (0.15–0.65) | 69% (35–85)h |
| All | 54/229 (23.6%) | 186/481 (38.7%) | 0.36 (0.22–0.58) | 0.32 (0.16–0.65) | 68% (35–84)i |
| Season (all patients) | |||||
| 2009–10 | 10/572 (1.7%) | 62/1112 (5.6%) | 0.30 (0.15–0.61) | 0.21 (0.08–0.50) | 79% (50–92)j |
| 2010–11 | 98/566 (17.3%) | 248/1098 (22.6%) | 0.62 (0.46–0.84) | 0.49 (0.30–0.79) | 51% (21–70)k |
Abbreviations: OR, odds ratio; CI, confidence interval. aLaboratory-confirmed by RT-PCR. b Adjusted for: With Risk Conditions: Age, PPV23 vaccine, education level (only for all patients and ≥ 65y), smoker, pneumonia, COPD (only for all patients and 18–64y), asthma (only for all patients and 18–64y), cardiovascular disease (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), neoplasia (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. All: Age, PPV23 vaccine, ethnicity (only for all patients and 18–64y), smoker (only for all patients and ≥ 65y), pneumonia, COPD (only for all patients and 18–64y), asthma (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. Season 2009–10: Age, PPV23 vaccine, ethnicity, alcoholism, pneumonia, COPD, asthma, HIV infection, neoplasia, transplantation, previous antibiotics, corticosteroids. Season 2010–11: Age, PPV23 vaccine, sex, smoker, alcoholism, pneumonia, COPD, diabetes, neurological disease, transplantation, previous antibiotics, corticosteroids. c Statistical power: 67.9%. Hosmer-Lemeshow test: dp = 0.24; ep = 0.44; fp = 0.40; gp = 0.65; hp = 0.57; ip = 0.63; jp = 0.78; kp = 0.42.
Table 4. Association between a history of PPV23 and influenza vaccination and influenza hospitalization.
| Influenza vaccine + PPV23 | Casesa vaccinated/N (%) | Controls vaccinated/N (%) | Crude OR (95% CI) | Adjusted ORb (95% CI) | Adjusted Vaccination Effectiveness (95% CI) |
|---|---|---|---|---|---|
| All patients | |||||
| With Risk Conditions | 23/813 (2.8%) | 105/1610 (6.5%) | 0.30 (0.18–0.52) | 0.19 (0.09–0.38) | 81% (62–91)c |
| All | 23/1051 (2.2%) | 118/2068 (5.7%) | 0.26 (0.15–0.45) | 0.19 (0.10–0.35) | 81% (65–90)d |
| 18–64 y | |||||
| With Risk Conditions | 5/618 (0.8%) | 26/1196 (2.2%) | 0.27 (0.09–0.80) | 0.19 (0.06–0.65) | 81% (35–94)e |
| All | 5/834 (0.6%) | 31/1602 (1.9%) | 0.23 (0.08–0.66) | 0.16 (0.05–0.52) | 84% (48–95)f |
| ≥ 65 y | |||||
| With Risk Conditions | 18/195 (8.3%) | 79/414 (19.1%) | 0.38 (0.20–0.74) | 0.24 (0.10–0.60) | 76% (40–90)g |
| All | 18/217 (8.3%) | 87/466 (18.7%) | 0.33 (0.17–0.63) | 0.24 (0.10–0.54) | 76% (46–90)h |
Abbreviations: OR, odds ratio; CI, confidence interval. aLaboratory-confirmed by RT-PCR. bAdjusted for: With Risk Conditions: Age, sex (only for ≥ 65y), smoker, alcoholism (only for ≥ 65y), pneumonia, COPD (only for all patients and 18–64y), asthma (only for all patients and 18–64y), cardiovascular disease (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), neoplasia (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. All: Age, ethnicity (only for all patients and 18–64y), smoker (only for all patients and ≥ 65y), pneumonia, COPD, asthma (only for all patients and 18–64y), diabetes (only for all patients and 18–64y), HIV infection (only for all patients and 18–64y), neurological disease (only for all patients and 18–64y), transplantation, previous antibiotics, corticosteroids. Hosmer-Lemeshow test: cp = 0.89; dp = 0.39; ep = 0.49; fp = 0.36; gp = 0.52; hp = 0.43
The conditional logistic regression analysis with the observed variable and with the estimate variable (i.e., propensity score) for each vaccine studied, both in terms of the estimates and also of their statistical significance, were very similar, and therefore the results shown consider only the observed variables.
The association between a history of PPV23 vaccination alone and hospitalization, considering only patients who received the vaccine during the past 9 y as vaccinated, was slightly lower for all ages (adjusted OR 0.55; 95% CI 0.28 to 0.75) and also for patients aged ≥ 65 y (adjusted OR 0.53; 95% CI 0.17 to 0.99) (Table S1). When this criterion was used to assess the association between a history of vaccination with both vaccines (PPV23 and influenza) (Table S2), the results were very similar to those shown in Table 4. When the criterion for considering a patient as vaccinated was receiving the PPV23 vaccine during the past 5 y, the values of the adjusted OR were 0.52 (95% CI 0.31 to 0.88) for all patients and 0.62 (95% CI 0.29 to 1.81) for patients aged ≥ 65 y although, in the latter group, the statistical power was only 0.54. However, the adjusted OR values were very low when the association between PPV23 and influenza vaccination was assessed (Tables S3 and 4).
Discussion
The results of this study show that vaccination with both the influenza and PPV23 vaccines reduced influenza hospitalization (VE: 81%; 95% CI 65–90), and that PPV23 vaccination alone contributed to this reduction in hospital admissions (VE 41; 95% CI 8–62).
Secondary bacterial pneumonia was recorded in 7.8% of cases, and in the cases where the etiology was determined, more than 70% were caused by S. pneumoniae. This result is similar to that found by Viasus et al., who detected secondary pneumonia in 6.6% of patients hospitalized for influenza A (H1N1) 2009 and S. pneumoniae in 72.2% of these cases.18 In this study, as in ours, no patient who had received PPV23 vaccination developed confirmed pneumococcal pneumonia. In a study based on autopsy specimens, Shieh et al. identified bacterial coinfection in more than 25% of samples and of those in which a specific agent was identified, 46% were S. pneumoniae.19 Koon et al. found S. pneumoniae in 10.2% of respiratory specimens from patients with influenza A (H1N1) 2009.20 Palacios et al.21 suggested that nasopharyngeal colonization by S. pneumoniae was more frequent in severe cases (56.4%) than in patients with mild forms of pandemic influenza (25%). As colonization by S. pneumoniae is a precursor of pneumonia, this suggests that the agent plays an important role in the evolution of influenza infection to severe forms requiring hospitalization. Other authors found no bacterial co-infection in pneumonia, but difficulties in detecting S. pneumoniae cannot be ruled out, since blood cultures were obtained in few patients.22 Cases of bacterial pneumonia are probably also underdetected in the present study.
All of these studies confirm that bacterial pneumonia complicates the disease course of many patients with influenza. Acute exacerbations of COPD are often triggered by bacterial or viral infection in the airways, and the influenza virus is a frequent contributor.23 In our study, 12.9% of cases and 5.9% of controls had COPD, and therefore this variable was included to control for confounding.
In all patients studied, the effectiveness of pneumococcal vaccination in preventing influenza hospitalizations was 41% (95% CI 8–62). For the pandemic influenza vaccine it was 79% (95% CI 50–92) and for the seasonal influenza vaccine it was 51% (95% CI 21–70), but the benefit was higher (81%; 95% CI 65–90) when vaccination with both the PPV23 and influenza vaccine (pandemic or seasonal) was considered. Few studies have evaluated the additional benefit of administering both vaccines to the elderly. In 1992 and 1993, Nichol et al. studied the association between vaccination with these two vaccines and pneumonia and influenza (P&I) hospitalizations and observed, as we did, that the effects were additive.24 Their estimate of the effectiveness of pneumococcal vaccination alone in preventing P&I hospitalization in persons aged ≥ 65 y (43%; 95% CI 16–62) was similar to the result we obtained in the same age group (43%; 95% CI 2–78). For the combination of both vaccines, effectiveness was 82% (95% CI 42–86) in the study by Nichol et al. and 76% (95% CI 46–90) in the present study. In a study performed in 1999, Christenson et al. observed that the effectiveness of dual vaccination in preventing influenza hospitalization was 37% (95% CI 9–50) in patients who received both vaccines and only 26% (95% CI -3 to 46) in patients who received only the influenza vaccine.16 Hung et al. evaluated the impact of dual PPV23 and influenza vaccination vs. PPV23 vaccination alone during the 2007–2008 influenza season15 and found that the incidence of hospital admissions due to influenza-like illness in people vaccinated with both vaccines was 10 per 1000 persons-year, while in those vaccinated with influenza vaccine or PPV23 alone, the rates were 15 and 14 hospitalizations per 1000 persons-year, respectively; a significant difference (p = 0.005) was observed between patients who had received both vaccines and those who had received only influenza vaccine. Considered together, the results of these studies and the present one indicate that programs for immunizing all persons aged ≥ 65 y with both vaccines should be strongly encouraged.
Another finding of the present study was the low PPV23 coverage in hospitalized patients. High coverage rates in adults should not be expected unless vaccination is indicated according to the presence of specific risk factors.25-27 The PPV23 coverage was very low in controls with risk conditions aged ≥ 18 y (11%) and in those aged ≥ 65 (28.5%). Although PPV23 is recommended in Spain for people aged ≥ 65 y and other risk groups, and vaccination is administered free of charge, our data suggest that coverage levels are suboptimal (27.4% in all controls aged ≥ 65 y). Controls attending these hospitals may have had a lower rate of vaccination than the whole population with risk conditions (including people aged ≥ 65 y) and therefore there may have been a selection bias. However, because the cases and controls were matched by hospital and province of residence we believe that our results show real differences between cases and controls and the estimate for the PPV23 is valid. We collected data on vaccination in both cases and controls from hospital medical records, vaccination cards and primary care centers. Therefore, there may have been an underestimation of vaccine coverage, but if this occurred, the underestimation would be similar in cases and controls.
Low PPV23 coverage is not unique to Spain. In most European countries, PPV23 coverage rates are low, ranging between 20 and 30%.27 In Canada, in a telephone-based survey of Toronto residents conducted in 1999–2002, only 14% of high-risk persons aged < 65 y had received PPV23, and coverage was 33% in high-risk persons aged > 65 y.28 In Australia, PPV23 coverage was 9.7% in 2000, before a publicly-funded program began, but increased to 57.9% after its implementation.29 In contrast, a high coverage rate (over 70%) has been observed in the United Kingdom.30 In the United States, in a study performed in 2006, 73% of all persons aged > 65 y had received PPV23, and those with chronic illness tended to have a higher coverage.31
As other authors have shown,27 our results confirm that a universal age-based vaccination strategy was more effective than a risk-based vaccination strategy, because the latter needs to identify each person who should be vaccinated. Most studies show the main problem in increasing PPV23 coverage in developed countries is not cost or concern about side effects, but physicians’ behavior. Some physicians have doubts about the effectiveness of PPV23 and do not recommend vaccination.32 Johnson et al. found that this factor explained why 57% of people in whom PPV23 was indicated had not been vaccinated.33
Observational studies of vaccination effectiveness are prone to several biases, among them selection bias. The protocol used in all Spanish National Health System hospitals included obtaining nasopharyngeal swabs for laboratory testing from all patients admitted with influenza-like illness or acute respiratory infection. Although the possibility of unknown selection bias cannot be excluded, the participating hospitals followed the protocol of swabbing patients with suspected influenza very closely during the two study periods and therefore we do not believe that selection bias was a prominent feature of our study. On the other hand, the results obtained using propensity scores suggest that it is unlikely that the protection observed in cases compared with controls could be attributed to factors studied other than PPV23 and influenza vaccination.
Another potential limitation is information bias due to the fact that interviewers knew whether interviewees were cases or controls. The same protocol was followed for both cases and controls and information on the vaccination history was collected from medical records, vaccination cards or registers and was recorded before the study began. Thus it is unlikely that the results were affected by information bias. On the other hand, because the duration of effectiveness of the PPV23 vaccine is open to discussion,34 we repeated the analyses considering only patients who received the vaccine during the past 9 y or during the past 5 y35,36 as vaccinated and, although the statistical significance diminished, the results did not change significantly.
A history of risk factors and underlying conditions were more frequent in patients hospitalized for influenza, and these patients had lower rates of vaccination with both the pandemic and seasonal influenza vaccines than controls. Most of the potential confounding factors described in the literature, including influenza vaccination, were taken into account and their possible effects limited by adjustment. In addition, there may have been imperfect matching for age, and because this variable, together with the history of influenza vaccination, are very important variable for the outcome, we also adjusted for them to estimate the adjusted vaccination effectiveness. However, it is possible that vaccinated persons were more concerned about their health (or had different characteristics that were not studied) than unvaccinated people and therefore residual confounding cannot be ruled out.
In conclusion, the results of this study suggest that in elderly people and in adults with chronic illness, PPV23 vaccination reduces hospitalizations during the influenza season and that in those receiving both influenza and PPV23 vaccination, the benefit in hospitalizations avoided is greater than in those vaccinated only against influenza.
Materials and Methods
Design, setting and study population
A multicenter matched case-control study was performed in 36 hospitals located in seven Spanish regions (Andalusia, Basque Country, Catalonia, Castile and Leon, Madrid, Navarra and Valencian Community).37 Cases and controls were recruited between July 2009 and February 2010 during the pandemic wave and between December 2010 and April 2011 during the next influenza season.
Selection of cases and controls
During the study period, all patients admitted to participating hospitals with influenza-like illness, defined as the sudden onset of any general symptom (fever or feverishness, headache, myalgia) in addition to any respiratory symptom (cough, sore throat, shortness of breath) were systematically swabbed.38 A case was defined as a patient aged ≥ 18 y admitted to hospital for > 24 h with influenza virus infection confirmed by real-time reverse-transcription polymerase chain reaction (RT-PCR).39 We excluded patients who had nosocomial infection, defined as influenza virus infection appearing ≥ 48 h following admission for another identified cause and patients who did not give informed consent.
We selected two matched controls for each case from patients with unplanned hospital admission for reasons other than acute respiratory infection or influenza-like illness. Controls were patients aged ≥ 18 y matched with each case according to age (± 5 y), date of hospitalization (± 10 d) and province of residence. Controls were sought from among patients admitted to the internal medicine service through the emergency department (first option). If no suitable control was available, one was selected from patients admitted to the general surgery, otorhinolaryngology, ophthalmology, dermatology, or traumatology services. Exclusion criteria for controls were symptoms of either influenza or respiratory infection at admission and patients who did not give informed consent.
Data collection
Specifically-trained health professionals used a structured questionnaire to collect information on cases and controls through interview and review of medical records. The following demographic variables and pre-existing medical conditions were recorded: age, sex, ethnicity, educational level, smoking, alcoholism, history of pneumonia in the previous two years, chronic obstructive pulmonary disease (COPD), asthma, cardiovascular disease, renal failure, diabetes, HIV infection, disabling neurological disease, neoplasia, transplantation and treatment with corticosteroids or antibiotics in the 90 d before the date of hospitalization. The medical conditions retrieved from the patients’ medical records that were considered as risk conditions were: solid organ or hematological neoplasia, renal failure, transplant, asplenia, oral corticosteroid therapy (> 20 mg/day/15 d) in the last month, immunosuppressive therapy (chemotherapy or other treatment), autoimmune disease, nephrotic syndrome, disabling neurological disease or severe alteration of psychomotor development, AIDS, asymptomatic HIV infection, diabetes mellitus, congestive or hemodynamically unstable cardiomyopathy, COPD, asthma, chronic liver disease, hemoglobinopathy or anemia, alcoholism and smoking. Pneumonia was defined as new infiltrate on a chest radiograph plus fever and/or respiratory symptoms. Documented secondary bacterial pneumonia was defined as one or more positive cultures obtained from blood, normally sterile fluid, isolation of Legionella pneumophila in sputum, detection of S. pneumoniae or Legionella serogroup 1 antigen in urine or 4-fold increase in the antibody titer or seroconversion for atypical pathogens. Confirmed pneumococcal pneumonia was defined as S. pneumoniae detected in any normally sterile site or a positive S. pneumoniae antigen test in urine in a patient with pneumonia.
Information on the vaccination status was obtained from hospital medical records or vaccination cards. If neither was available, primary healthcare center registers were consulted. Cases were considered vaccinated with the seasonal influenza vaccine and PPV23 if they had received a dose of the vaccine at least 14 d before the onset of symptoms. Controls were considered vaccinated if they had received a dose at least 14 d before the onset of symptoms of the matched case. As there is evidence that the immune response induced by the adjuvanted pandemic vaccine is more rapid than that of seasonal influenza vaccines,40-42 patients were considered vaccinated if they had received the pandemic influenza vaccine at least 7 d before the onset of symptoms (cases) or the onset of symptoms of the matched case (controls).
Statistical analysis
A bivariate comparison of demographic variables and medical conditions between cases and controls was made using the McNemar test for categorical variables and the paired t-test for continuous variables. A two-tailed distribution was assumed for all p-values. To estimate vaccination effectiveness (VE), a multivariate analysis was performed using conditional logistic regression with backward selection of variables and a cut-off point of p < 0.2. The variables considered for adjustment are shown in Table 1. We also included the variables of age and influenza vaccination (pandemic vaccine in 2009–2010 and seasonal vaccine in 2010–2011) in the model. Interactions between PPV23 vaccination and the risk factors studied and influenza vaccination were independently analyzed by conditional logistic regression.
Crude and adjusted odds ratios (OR) were calculated to estimate the association between PPV23 vaccination, influenza vaccination or dual vaccination and the risk of hospitalization due to influenza. The goodness of fit was measured using the Hosmer-Lemeshow test.43 The effectiveness of PPV23 vaccination in preventing hospitalization during influenza periods was estimated using the formula: (1-OR) x 100. As there is a concern about the duration of effectiveness of the PPV23 vaccine, we repeated the analysis considering that cases and controls were vaccinated only if they had received the vaccine during the past 9 y or during the past 5 y.
To determine whether the results were confounded by indication bias, we used the propensity score, defined as the conditional probability of receiving a specific treatment given a vector of measured covariates.44 The propensity scores were constructed using the fitted values of a logistic regression model with the response variable, vaccination (yes/no) and the same explanatory variables used in the main model (Table 1).The analysis was performed using the SPSS v18 statistical package and the R v2.14.1 statistical software.
Ethics
All data collected were treated as confidential, in strict observance of legislation on observational studies. The study was approved by the Ethics Committees of the hospitals involved. Written informed consent was obtained from all patients included in the study. (Author, please add Fig. 1 citation in the appropriate place in the main text)
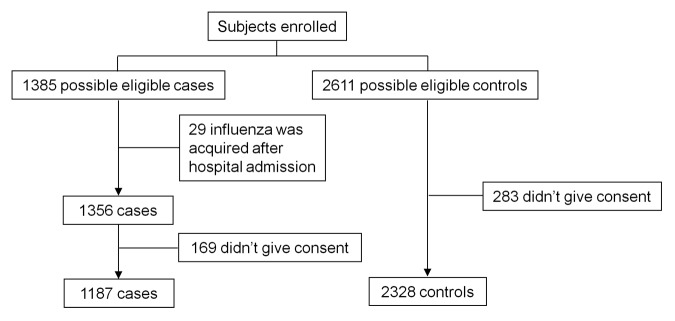
Figure 1. Flowchart of patients included in the study.
The other members of the CIBERESP Cases and Controls in Pandemic Influenza Working Group
Andalusia: MA Bueno, ML Gómez, M Mariscal, B Martínez, JP Quesada, M Sillero, (Compl. Hosp. Jaén), M Carnero, J Fernández-Crehuet, J del Diego Salas (Hosp. Virgen de la Victoria), V Fuentes (Hosp. Costa del Sol), V Gallardo, E Pérez (Servicio de Epidemiología), R López (Hosp. Infanta Elena de Huelva), JR Maldonado (Hosp. Torrecárdenas), A Morillo (Hosp. Virgen del Rocío), I Pedrosa Corral, MF Bautista, JM Navarro, M Pérez (Lab. Referencia Gripe), S Oña (Hosp. Carlos Haya), MJ Pérez (Hosp. Virgen de Valme), MC Ubago (Hosp. Virgen de las Nieves), M Zarzuela (Hosp. Puerta del Mar). Castile and Leon: P Sanz (Universidad de León), D Carriedo, F Díez, I Fernández, S Fernández, MP Sanz (Compl. Asist. Universitario, León), JJ Castrodeza, A Pérez, (Dir. General de Salud Pública e Investigación, Desarrollo e Innovación), R Ortiz de Lejarazu (Centro Nacional de Gripe, Valladolid), J Ortiz (Hosp. El Bierzo), A Pueyo, JL Viejo, A Seco (Compl. Asist. Burgos), P Redondo (Serv. Territorial de Sanidad y Bienestar Social, León), A Molina (Inst. Biomedicina, Universidad de León). Catalonia: A Agustí, ATorres, ATrilla, A Vilella (Hosp. Clínic); F Barbé (Hosp. Arnau de Vilanova); L Blanch, G Navarro (Hosp. Sabadell); X Bonfill, J López-Contreras, V Pomar, MT Puig (Hosp. Sant Pau); E Borràs, A Martínez, N Torner (Dir. General de Salud Pública); C Bravo, F Moraga (Hosp. Vall d’Hebrón); F Calafell (Universitat Pompeu Fabra); J Caylà, C Tortajada (Agencia de Salud Publica de Barcelona); I Garcia, J Ruiz (Hosp. Germans Trias i Pujol); JJ García (Hosp. Sant Joan de Deu); J Alonso (IMIM- Hosp. del Mar), J Gea, JP Horcajada (Universitat Pompeu Fabra _CIBER Enfermedades Respiratorias); N Hayes (Hosp. Clínic_CRESIB); A Rosell, J Dorca (Hosp. de Bellvitge). Madrid: C Álvarez, M Enríquez, F Pozo (Hosp. Twelve de Octubre), F Baquero, R Cantón, A Robustillo, M Valdeón (Hosp. Universitario Ramón y Cajal); E Córdoba, F Domínguez, J García, R Génova, E Gil, S Jiménez, MA Lopaz, J López, F Martín, ML Martínez, M Ordobás, E Rodriguez, S Sánchez, C Valdés (Área de Epidemiología, Comunidad de Madrid), JR Paño, M Romero (Hosp. Universitario La Paz). Navarre: A Martínez, L Martínez (Inst. de Salud Pública), M Ruiz, P Fanlo, F Gil, V Martínez-Artola (Compl. Hosp. Navarra). The Basque Country: U Aguirre, A Caspelastegui, PP España, S García (Hosp. Galdakao), JM Antoñana, I Astigarraga, JI Pijoan, I Pocheville, M Santiago, JI Villate (Hosp. Cruces), J Arístegui, A Escobar, MI Garrote (Hosp. Basurto), A Bilbao, C Garaizar (Fundación Vasca de Innovación e Investigación Sanitarias), G Cilla, J Korta, E Pérez-Trallero, C Sarasqueta (Hosp. Donostia), F Aizpuru, JL Lobo, C Salado (Hosp. Txagorritxu), J Alustiza (Hosp. Mendaro), F J Troya (Hosp. de Santiago). Valencia Community: J Blanquer (Hosp. Clínico), M Morales (Hosp. Doctor Peset).
Supplementary Material
Acknowledgments
The authors thank D.S. Fedson (Sergy Haut, France) for his valuable comments on the manuscript.
Glossary
Abbreviations:
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- OR
odds ratio
- PPV23
23-valent polysaccharide pneumococcal vaccine
- VE
vaccination effectiveness
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Ministry of Science and Innovation, Institute of Health Carlos III, Programme of Research on Influenza A/H1N1 (Grant GR09/0030) and the Catalan Agency for the Management of Grants for University Research (AGAUR Grant number 2009/ SGR 42). The funders had no role in the study design, data collection, analysis, the decision to publish or the preparation of the manuscript.
Supplemental Materials
Supplemental materials may be downloaded here: www.landesbioscience.com/journals/vaccines/article/23090
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23090
References
- 1.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–2. [PubMed] [Google Scholar]
- 2.Red Nacional de Vigilancia Epidemiológica. Vigilancia de la gripe en España. Temporada 2010-2011. Available at: http://vgripe.isciii.es/gripe Accessed on September 15, 2012.
- 3.WHO Recommended viruses for influenza vaccines for use in the 2010-2011 northern hemisphere influenza season. Wkly Epidemiol Rec. 2010;85:81–92. [PubMed] [Google Scholar]
- 4.Walter ND, Taylor TH, Shay DK, Thompson WW, Brammer L, Dowell SF, et al. Active Bacterial Core Surveillance Team Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–83. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 5.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RK, George R, Nguyen-Van-Tam JS. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14:1187–92. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–12. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al. EVAN Study Group Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43:860–8. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez A, Izquierdo C, Salleras L, Ruiz L, Sousa D, Bayas JM, et al. Working Group for the Study of Prevention of CAP in the Elderly Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly. Eur Respir J. 2010;36:608–14. doi: 10.1183/09031936.00171309. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 13.Skull SA, Andrews RM, Byrnes GB, Kelly HA, Nolan TM, Brown GV, et al. Prevention of community-acquired pneumonia among a cohort of hospitalized elderly: benefit due to influenza and pneumococcal vaccination not demonstrated. Vaccine. 2007;25:4631–40. doi: 10.1016/j.vaccine.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine. 1999;17(Suppl 1):S91–3. doi: 10.1016/S0264-410X(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 15.Hung IFN, Leung AYM, Chu DWS, Leung D, Cheung T, Chan CK, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010;51:1007–16. doi: 10.1086/656587. [DOI] [PubMed] [Google Scholar]
- 16.Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004;23:363–8. doi: 10.1183/09031936.04.00063504. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist SA, Nanni A, Levine O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakers. Am J Public Health. 2012;102:596–605. doi: 10.2105/AJPH.2011.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viasus D, Paño-Pardo JR, Pachón J, Riera M, López-Medrano F, Payeras A, et al. Novel Influenza A(H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI). Pneumonia complicating pandemic (H1N1) 2009: risk factors, clinical features, and outcomes. Medicine (Baltimore) 2011;90:328–36. doi: 10.1097/MD.0b013e31822e67a7. [DOI] [PubMed] [Google Scholar]
- 19.Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol. 2010;177:166–75. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koon K, Sanders CM, Green J, Malone L, White H, Zayas D, et al. Co-detection of pandemic (H1N1) 2009 virus and other respiratory pathogens. Emerg Infect Dis. 2010;16:1976–8. doi: 10.3201/eid1612.091697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palacios G, Hornig M, Cisterna D, Savji N, Bussetti AV, Kapoor V, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riquelme R, Riquelme M, Rioseco ML, Inzunza C, Gomez Y, Contreras C, et al. Characteristics of hospitalised patients with 2009 H1N1 influenza in Chile. Eur Respir J. 2010;36:864–9. doi: 10.1183/09031936.00180409. [DOI] [PubMed] [Google Scholar]
- 23.De Serres G, Lampron N, La Forge J, Rouleau I, Bourbeau J, Weiss K, et al. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46:129–33. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med. 1999;159:2437–42. doi: 10.1001/archinte.159.20.2437. [DOI] [PubMed] [Google Scholar]
- 25.Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55:255–8. doi: 10.1093/cid/cis354. [DOI] [PubMed] [Google Scholar]
- 26.Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55:265–7. doi: 10.1093/cid/cis364. [DOI] [PubMed] [Google Scholar]
- 27.Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–67. doi: 10.1586/erv.11.99. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sukhni W, Avarino P, McArthur MA, McGeer A. Impact of public vaccination programs on adult vaccination rates: two examples from Ontario, Canada. Vaccine. 2008;26:1432–7. doi: 10.1016/j.vaccine.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Andrews RM. Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia. Vaccine. 2005;23:2756–61. doi: 10.1016/j.vaccine.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Begun F, Pebody R. Annual pneumococcal polysaccharide vaccine uptake in 65 years old and over for England. Influenza immunization uptake monitoring programme. London: Department of Health and Health Protection Agency, 2008. [Google Scholar]
- 31.Jackson LA, Baxter R, Naleway AL, Belongia EA, Baggs J. Patterns of pneumococcal vaccination and revaccination in elderly and non-elderly adults: a Vaccine Safety Datalink study. BMC Infect Dis. 2009;9:37. doi: 10.1186/1471-2334-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santibáñez TA, Zimmerman RK, Nowalk MP, Jewell IK, Bardella IJ. Physician attitudes and beliefs associated with patient pneumococcal polysaccharide vaccination status. Ann Fam Med. 2004;2:41–8. doi: 10.1370/afm.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121(Suppl 2):S28–35. doi: 10.1016/j.amjmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Nichol KL. Pneumococcal vaccination and revaccination in the elderly population. J Infect Dis. 2010;201:659–61. doi: 10.1086/651376. [DOI] [PubMed] [Google Scholar]
- 35.Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–31. doi: 10.1001/jama.1993.03510150060030. [DOI] [PubMed] [Google Scholar]
- 36.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–24. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 37.Domínguez A, Alonso J, Astray J, Baricot M, Cantón R, Castilla J, et al. Grupo de Trabajo del Proyecto CIBERESP de Casos y Controles sobre la Gripe Pandémica. Rev Esp Salud Publica. 2011;85:3–15. doi: 10.1590/S1135-57272011000100002. [Risk factors of influenza (H1N1) 2009 hospitalization and effectiveness of pharmaceutical and nonpharmaceutical interventions in its prevention: a case-control study] [DOI] [PubMed] [Google Scholar]
- 38.European Commission. Commission decision of 30 April 2009 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Available at: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:110:0058:0059:EN:PDF Accessed on September 15, 2012.
- 39.World Health Organization. WHO(2009b) CDC Protocol of realtime RTPCR for influenza A (H1N1). October 2009. Available at: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf Accessed on September 16, 2012.
- 40.Puig-Barberà J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Pérez-Vilar S, Silvestre-Silvestre E, et al. Surveillance and Vaccine Evaluation Group during the autumn 2009 H1N1 pandemic wave in Castellón, Spain. Effectiveness of seasonal 2008-2009, 2009-2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellón, Spain. A test-negative, hospital-based, case-control study. Vaccine. 2010;28:7460–7. doi: 10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Song JY, Cheong HJ, Heo JY, Noh JY, Choi WS, Park DW, et al. Effectiveness of the pandemic influenza A/H1N1 2009 monovalent vaccine in Korea. Vaccine. 2011;29:1395–8. doi: 10.1016/j.vaccine.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza - primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Assess. 2010;14:313–46. doi: 10.3310/hta14340-05. [DOI] [PubMed] [Google Scholar]
- 43.Katz MH. Interpreting the results. In: Katz MH. Multivariable analysis. A practical guide for clinicians. Third edition. Cambridge: Cambridge University Press, 2011:140-161. [Google Scholar]
- 44.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


