Abstract
Japanese encephalitis chimeric virus vaccine (JE-CV) is a licensed vaccine indicated in a single dose administration for primary immunization. This controlled phase III comparative trial enrolled children aged 36–42 mo in the Philippines. 345 children who had received one dose of JE-CV in a study two years earlier, received a JE-CV booster dose. 105 JE-vaccine-naïve children in general good health were randomized to receive JE-CV (JE-vaccine naïve group; 46 children) or varicella vaccine (safety control group; 59 children). JE neutralizing antibody titers were assessed using PRNT50. Immunological memory was observed in children who had received the primary dose of JE-CV before. Seven days after the JE-CV booster dose administration, 96.2% and 66.8% of children were seroprotected and had seroconverted, respectively, and the geometric mean titer (GMT) was 231 1/dil. Twenty-eight days after the JE-CV booster dose seroprotection and seroconversion were achieved in 100% and 95.3% of children, respectively, and the GMT was 2,242 1/dil. In contrast, only 15.4% of JE-CV-vaccine naïve children who had not received any prior JE vaccine were seroprotected seven days after they received JE-CV. One year after receiving the JE-CV booster dose, 99.4% of children remained seroprotected. We conclude that JE-CV is effective and safe, both as a single dose and when administrated as a booster dose. A booster dose increases the peak GMT above the peak level reached after primary immunization and the antibody persistence is maintained at least one year after the JE-CV booster dose administration. Five year follow up is ongoing.
Keywords: Japanese encephalitis vaccines, attenuated vaccines, humoral immune response, active immunization, booster immunization
Introduction
Japanese encephalitis (JE) is a mosquito-borne viral disease that is seasonally endemic in many countries in Southeast Asia, with three billion people living in endemic areas.1,2 Although most infections are sub-clinical, infection with JE virus (JEV) can cause a febrile illness associated with central nervous system inflammation.2 Only 1 in 250 JEV infections is symptomatic in susceptible Asians;1 20–30% of cases are fatal and 30–50% of survivors experience neurological or psychiatric sequelae.3 JE affects mainly children and teenagers, although adult cases are occasionally reported.4,5
JE is a vaccine-preventable disease and several JE vaccines are currently in use.1,6,7 Mouse brain-derived inactivated JE vaccines (MBDVs) to be used with a 2 to 3 dose primary schedule have been the main vaccines to prevent JE for many years and have been used extensively in Asia, despite concerns about reactogenicity.7 New inactivated JE vaccines produced in Vero cells have been licensed or are in development.8,9 A live attenuated virus grown in primary hamster kidney cells, manufactured in China (Chengdu Biological Products Institute, People’s Republic of China), was licensed in 1988 and has only recently been made available in Asian countries outside China; this vaccine is recommended in some countries as a one dose in a primary immunization schedule followed by booster dose administration.
A serological correlate of protection based on neutralizing antibodies is accepted and recommended for evaluation and licensure of JE vaccines; a threshold of 1:10 using a 50% plaque reduction neutralization test (PRNT50) is accepted as evidence of protective immunity by the JE expert community.10,11 The threshold was recently confirmed by passive transfer of human sera in mice that were challenged with wild-type JE virus strains.12 This correlate of protection has been accepted by Health Authorities for the licensure of two new vaccines (IXIARO® from Intercell and JE-CV, IMOJEV®, from Sanofi Pasteur13).
The live, attenuated, JE chimeric virus vaccine (JE-CV) has been developed by replacing the pre-membrane and envelope coding sequences from the yellow fever vaccine virus (strain 17D) genome with the corresponding sequences from the SA14–14–2 JEV strain; JE-CV is grown in Vero cells.14,15 In adults, a single dose of JE-CV has been shown to be safe and elicits a protective immune response in 99% of the recipients one month after the vaccine injection; more than 93% of those vaccinated were seroprotected 15 d after the injection.16 The protective immune response was well maintained over time with high seroconversion rates and the persistence of neutralizing antibodies to the vaccine and wild type JE virus strains up to 60 mo after a single dose administration.17, 18
Successive trials of JE-CV in naïve pediatric populations in Asia have shown that a single dose of JE-CV elicits a safe and protective immune response in more than 95% of the children one month after the vaccination;19 the assessment of the long-term persistence of the antibody response in children immunized with a primary single dose administration is ongoing. In 2010 JE-CV was approved by the Therapeutic Goods Administration (TGA) in Australia and by the Thai Food and Drug Administration for the prophylaxis of JE in individuals aged 12 mo and over. No JE vaccines are currently licensed in the Philippines.
Although a threshold of protection has been defined, vaccinated individuals who seroconvert following primary immunization may remain protected even if their JE neutralizing antibody titer subsequently decreases below the threshold of protection.14,11 A rapid rising immune response at time of re-exposure to JE virus in previously exposed individuals might support the possibility of continued relative protection, if neutralizing antibody titers reach a sufficient level to neutralize the virus before it crosses the blood brain barrier. The demonstration of the maintenance of a memory immune response in vaccinated subjects is therefore of high interest. Immunological memory is best assessed in vaccinees with a neutralizing titer below the level of seroprotection by administering a booster dose of vaccine and comparing the kinetics and magnitude of the response with a primary immune response. This methodology was used to assess immunological memory following vaccination with the SA14–14–2 live-attenuated JE vaccine.20
As part of the clinical development of JE-CV, we previously conducted a study (NCT00735644) to assess the immunogenicity and safety of a single dose of JE-CV among 1,200 children aged 12 to 18 mo.21 Up to 400 children in the Philippines vaccinated with JE-CV in that study were subsequently offered a booster vaccination with JE-CV in this study (Group 1; JE-CV-primed group). The booster dose of JE-CV was given to Group 1 study subjects 2 y after primary immunization. We assessed the persistence of antibody before the booster dose, and immunological memory and the booster effect 7 and 28 d after vaccination, then yearly. In addition, the immune response in a subset of children who seroconverted after primary immunization with JE-CV, and presented at baseline before booster vaccination with neutralizing titer below the protective threshold, was compared descriptively with an immunogenicity control group comprising newly enrolled JE-vaccination-naïve children of the same age to whom we administered a single dose of JE-CV as a primary immunization. This is the primary report up to 1 y post vaccination (for the booster group) and day 28 for the two control groups. The study is ongoing and future reports will provide details of the persistence of the immune response over 5 y in the subjects who received JE-CV as a booster.
This study was registered, ClinicalTrials.gov: NCT01190228.
Results
Study population
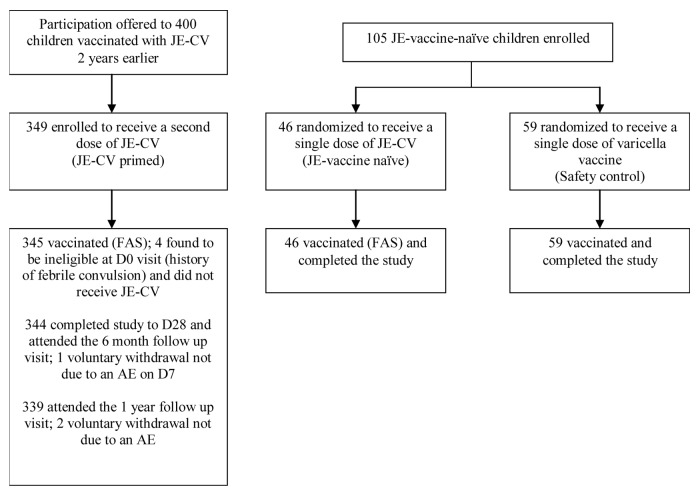
Between August and October 2010, 345 children who had been previously vaccinated with a single dose of JE-CV and were eligible for inclusion were enrolled (Group 1; JE-CV primed group; Figure 1); these constituted the full analysis set (FAS) for the JE-vaccine exposed children. A further 105 JE-vaccine-naïve children in general good health were enrolled and randomized to receive either JE-CV (Group 2, JE-vaccine naïve group; 46 children constituting the FAS set of JE-vaccine naïve children) or varicella vaccine (Group 3; Safety Control group; 59 children). Demographic characteristics at baseline were similar across vaccine groups (Table 1). All children were Asian and their mean age was approximately 39 mo.
Figure 1. Disposition of children. JE-CV, Japanese encephalitis chimeric virus vaccine; FAS, full analysis set; D, day; AE, adverse event
Table 1. Demographic characteristics at baseline.
| JE-CV Booster Dose (JE-CV previously vaccinated) | JE-CV First Dose (JE-vaccine naïve) | Varicella Vaccine (Safety control) | |
|---|---|---|---|
| Number | 3451 | 461 | 592 |
| Gender, n (%) | |||
| Male | 173 (50.1) | 21 (45.7) | 30 (50.8) |
| Female | 172 (49.9) | 25 (54.3) | 29 (49.2) |
| Age, mean (SD) months | 39.6 (1.71) | 39.3 (1.99) | 39.3 (2.09) |
| Body mass index, mean (SD) kg/m2 | 15.3 (1.75) | 14.7 (1.38) | 14.8 (1.49) |
Analysis populations
The per protocol analysis set (PP) for the JE-CV primed group included 340 children; the most common reasons for exclusion were inclusion/exclusion criteria deviations, blood samples for serology not taken, and vaccination in this study not done.
In the JE-vaccine naïve group, 14/46 (30.4%) children were flavivirus seropositive (presence of anti-dengue or protective level of anti-JE antibodies) before vaccination; 11/46 (23.9%) children were seropositive for any dengue serotype and 7/46 (15.2%) were seropositive for JE. The PP for the JE-vaccine naïve group excluded children who had pre-existing JE neutralizing antibodies and therefore comprised 39 children. Children in the safety control with varicella arm were not tested for presence of pre-existing flavivirus antibodies.
For the analysis of booster response the PP population was used, while for antibody persistence this was primarily FAS. For safety analysis the FAS and both control groups were primarily considered.
Antibody persistence
On D0, two years after their JE-CV primary vaccination, 80.3% of Group 1 (277/345 FAS; 273/340PP) had protective levels of JE neutralizing antibodies (≥ 10 1/dil) and geometric mean titer (GMT) was 39.4 1/dil (95% confidence interval [CI]: 33.7; 46.0).
Immune response to a booster dose
Seven days after booster vaccination, 96.2% (95% CI: 93.6; 97.9) of children in Group 1 were seroprotected (Fig. 2). The GMT had increased by 5.87-fold between D0 and D7 and was 231 1/dil (95% CI: 191; 279); seroconversion was achieved by 66.8% (95% CI: 61.5; 71.8) of children on D7.
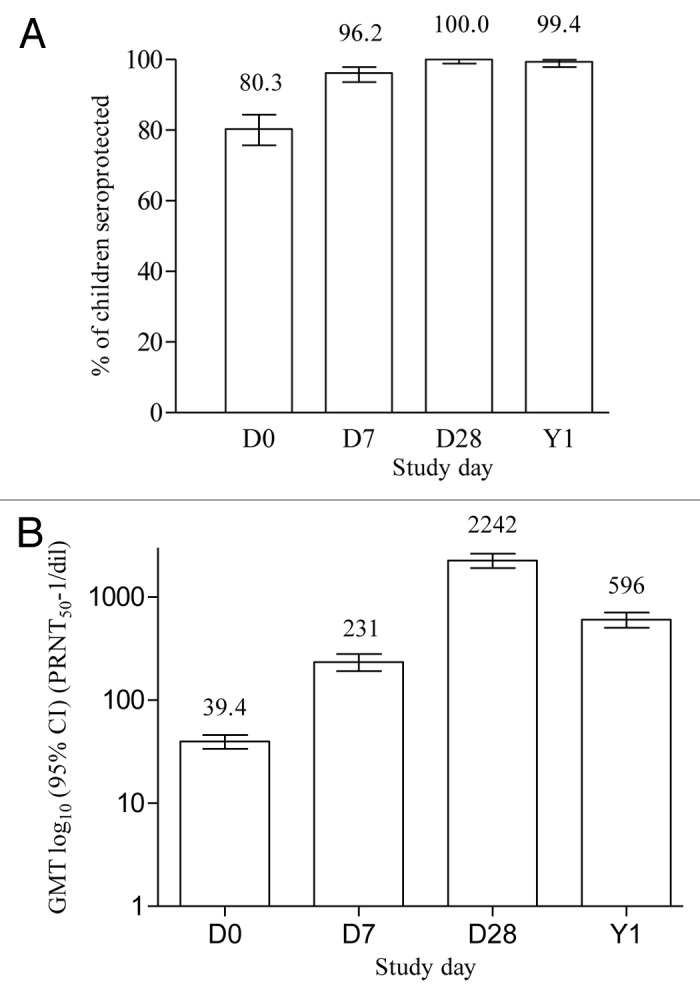
Figure 2. Seroprotection and GMT levels after a booster dose of JE-CV. D, day; Y, year; GMT, geometric mean titer; PRNT50, plaque reduction neutralization test; error bars, 95% confidence intervals. One dose of JE-CV was given to eligible children who had previously received a single dose of JE-CV two years earlier. JE neutralizing antibody titers were assessed using a PRNT50. A neutralizing antibody titer of ≥ 10 1/dil is accepted as evidence of protection.11 Results are for the PP (n = 340) for D0, D7, and D28 and for the FAS (n = 339) for Y1.
Titers continued to increase in the JE-CV primed group, and three weeks later on D28 100% (95% CI: 98.9; 100) of children were seroprotected (Fig. 2). The GMT had increased by 57.0-fold between D0 and D28 and was 2,242 1/dil (95% CI: 1,913; 2,628); the seroconversion rate was 95.3% (95% CI: 92.5; 97.3) on D28.
For children with antibody titers ≥ 10 on D0 (n = 277) before the administration of the JE-CV booster dose, the GMT on D28 was 2,999 (95% CI: 2557; 3517) 1/dil and for children with antibody titers < 10 1/dil at study onset (n = 68) this was 716 (95% CI: 500; 1025) 1/dil.
One year after the JE-CV booster vaccination, almost all the children (99.4% [95% CI: 97.9; 99.99]) in the FAS remained seroprotected (Fig. 2). The GMT decreased approximately 4-fold from D28 to Y1 to 596 1/dil (95% CI: 502; 708).
In the earlier study, 14 children in the JE-CV vaccinated group did not seroconvert within 28 d of the first JE-CV vaccination. Five out of these 14 seroconverted between then and enrollment in the present study, two years after primary immunization with JE-CV. The other 9 children exhibited a rapid protective immune response within seven days after the administration of a booster dose of JE-CV with antibodies above the level required for seroprotection. All 14 were seroprotected by D28 after the second vaccination, with a mean GMT of 290 1/dil (95% CI: 118; 713).
Immune response to a booster dose in JE-CV primed children who were seronegative at baseline
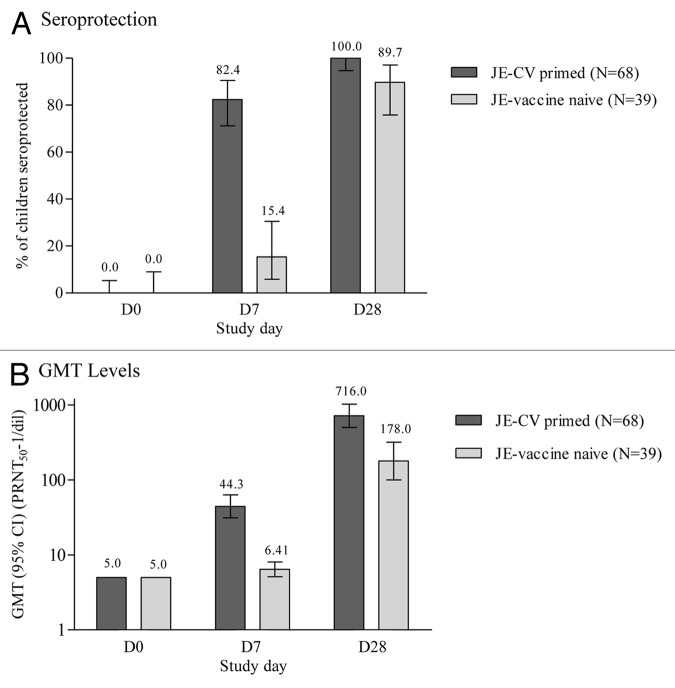
To better distinguish an anamnestic or memory response from a primary response, neutralizing titers were assessed on D7, an early timepoint at which a primary response is still low while a secondary response is already significant, in the subgroup of children who presented with antibody titers below the threshold for protection (< 10 1/dil) at baseline. Their antibody response following booster vaccination was descriptively compared with the primary immune response of children who had not previously received a JE vaccine (JE-vaccine naïve). Among the 68 children in the subgroup of the JE-CV primed group (FAS) who presented with JE neutralizing antibody titers below the protective level at baseline (Fig. 3), 82.4% (95% CI: 71.2; 90.5) were seroprotected on D7 compared with 15.4% (95% CI: 5.9; 30.5) in the JE-vaccine naïve group (Group 2) who received JE-CV for the first time. The seroprotection rate on D28 in this subgroup of Group 1 was 100% (95% CI: 94.7; 100.0); the GMT had increased 8.8-fold and 143-fold on D7 and D28, compared with D0, respectively. In comparison in Group 2, the seroprotection rate was 89.7% on D28 (95% CI: 75.8; 97.1), and GMT had increased less than 2-fold and 35.6-fold on D7 and D28 compared with D0, to values of 6.41 (95% CI: 5.11; 8.05) and 178 (95% CI: 99.7; 318), respectively (Fig. 3).
Figure 3. Immune response after vaccination with JE-CV in children who were seronegative at baseline. GMT, geometric mean titer and 95% CI; PRNT50, plaque reduction neutralization test; FAS for JE-CV primed children and PP for JE vaccine naïve children; error bars, 95% confidence intervals. One dose of JE-CV was given to children who previously received a single dose of JE-CV two years earlier, and to JE-vaccine naïve children. JE neutralizing antibody titers were assessed using a PRNT50. This figure shows the immune response in children who were seronegative at the start of the study.
Safety and reactogenicity
The safety analysis was descriptive. Slightly more solicited injection site reactions and slightly fewer solicited systemic reactions were reported in the JE-vaccine naïve group (Group 2) than in the two other groups, however the sample size of this group was small. The most frequently reported unsolicited adverse event (AE) was upper respiratory tract infection.
There were no deaths and no vaccine-related serious adverse events (SAEs) reported within 6 mo (JE-vaccine naïve and safety control groups) or 1 y (JE-CV primed group) after vaccination. Two SAEs were reported in the JE-CV primed group within 28 d after booster vaccination, one case of clinically suspected dengue fever (fever, signs of bleeding with epistaxis, and clear thrombocytopenia) and one case of generalized febrile convulsion. The febrile convulsion occurred two days after vaccination in a 3-y-old child and was associated with an upper respiratory tract infection; the child recovered from the febrile convulsion on the same day, without hospitalization. The child with suspected dengue fever presented with grade 3 fever six days after vaccination, followed by an episode of epistaxis, petechial rash, thrombocytopenia, without neurological signs. The child was hospitalized and recovered within 11 d. A third SAE was reported in the JE-CV primed group 45 d after booster vaccination; a child was hospitalized with clinically suspected dengue fever and recovered within 7 d. None of the SAEs was considered related to the vaccination by the investigator. There were no immediate AEs and no child had an unsolicited adverse reaction (AR). No other AEs of special interest (AESIs), other than febrile convulsion, were observed during the study. AESI as defined for the JE-CV clinical development includes hypersensitivity and allergic reactions, neurological events (including febrile convulsions), and vaccine failure.22
Solicited injection site reactions (Table 2) were reported for similar percentages of children in the JE-CV-primed and safety control groups (23.5% and 23.7%, respectively), and appeared slightly higher in the JE-vaccine naïve group (30.4%), although confidence intervals of all three point estimates overlapped. Injection site pain was the most frequent solicited injection site reaction (20.3–28.3% of children). Injection site erythema was reported in all groups, while injection site swelling was only reported by children in the JE-CV-primed (5.5%) and safety control (1.7%) groups. Most injection site reactions were mild in intensity (easily tolerated, with both swelling and erythema < 2.5 cm in diameter), occurred within three days of vaccination, and most lasted between one and three days.
Table 2. Solicited injection site and systemic reactions after vaccination: Safety analysis set.
| JE-CV – Booster dose (JE-CV previously vaccinated) n = 345 | JE-CV – First dose (JE-vaccine naïve) n = 46 | Varicella vaccine (Safety control) n = 59 | ||||
|---|---|---|---|---|---|---|
| Children experiencing at least one: | % | (95%CI) | % | (95%CI) | % | (95%CI) |
| Solicited reaction | 37.2 | (32.1, 42.6) | 39.1 | (25.1, 54.6) | 40.7 | (28.1, 54.3) |
| Injection site reaction | 23.5 | (19.2, 28.4) | 30.4 | (17.7, 45.8) | 23.7 | (13.6, 36.6) |
| Injection site pain | 21.2 | (17.0, 25.9) | 28.3 | (16.0, 43.5) | 20.3 | (11.0, 32.8) |
| Injection site erythema | 8.4 | (5.7, 11.9) | 2.2 | (0.1, 11.5) | 6.8 | (1.9, 16.5) |
| Injection site swelling | 5.5 | (3.4, 8.5) | 0 | (0.0, 7.7) | 1.7 | (0.0, 9.1) |
| Systemic reaction | 24.7 | (20.2, 29.6) | 15.2 | (6.3, 28.9) | 28.8 | (17.8, 42.1) |
| Fever | 14.2 | (10.7, 18.4) | 2.2 | (0.1, 11.5) | 15.3 | (7.2, 27.0) |
| Headache | 11.9 | (8.7, 15.8) | 10.9 | (3.6, 23.6) | 13.6 | (6.0, 25.0) |
| Malaise | 14.2 | (10.7, 18.4) | 13.0 | (4.9, 26.3) | 10.2 | (3.8, 20.8) |
| Myalgia | 5.5 | (3.4, 8.5) | 4.3 | (0.5, 14.8) | 3.4 | (0.4, 11.7) |
CI, confidence interval. Solicited injection site reactions were collected daily up to D7; solicited systemic reactions were collected up to D28
Overall, solicited systemic reactions (Table 2) were reported by similar percentages of JE-CV primed children in Group 1 and safety control groups (24.7% and 28.8%, respectively) and by a slightly lower percentage in the JE-vaccine naïve children in Group 2 (15.2%). Fever, malaise, and headache were the most frequent solicited systemic reactions after JE-CV vaccination in Group 1 and in the safety control group. After JE-CV vaccination in Group 2, malaise and headache were also the most frequent solicited systemic reactions; fever, however, was reported at a lower rate, although the sample size of this group was small and formal statistical testing was not performed. Most reactions were grade 1 or 2 in intensity (mild or moderate), appeared within three days of vaccination (fever in the JE-CV primed group occurred between eight and 14 d after vaccination), and lasted between one and three days.
The percentage of children with unsolicited AEs was comparable in the three vaccine groups (19.6–24.9%). The most frequent AE was upper respiratory tract infection (10% of all JE-CV vaccinations), in line with expected observations in this age range. The most frequently reported unsolicited AEs in each of the vaccine groups were in the categories “Infections and infestations” (18.3%, 15.2%, and 18.6% of children in the JE-CV-primed, JE-vaccine naïve, and safety control groups, respectively) and “General disorders and administration site conditions” (4.6%, 4.3%, and 1.7% in the JE-CV primed, JE-vaccine naïve, and safety control groups, respectively).
Grade 3 (i.e., ‘severe’) solicited systemic reactions were reported for seven children in the JE-CV primed group (2%) and one child in the JE-vaccine naïve group (2.2%); 7/8 reactions were fever (one headache), and all resolved quickly with medication. Where tested there was no confirmation of any cases with an associated JE-vaccine viremia. There were no severe solicited systemic reactions reported for children in the safety control group.
Discussion
Long-term follow up of the seroprotective immune responses conferred by vaccination against JE is important for people who live in endemic areas given the ongoing exposure and risk for severe clinical outcome. Clinical studies to date have shown that, based on a serological correlate of protection accepted by the WHO,11 JE-CV elicits a protective immune response one month after a single dose for primary immunization in 99% of adults and 95% of children.16,17,19 The immune response conferred by this single dose administration of JE-CV is well maintained over time in adults,17 and no booster is needed before at least 5 y. In children, the peak GMT is lower than in adults and the seroprotective level is not maintained as well.19 Therefore the need for a booster vaccination in children to maximize protection needs to be considered. In this study we sought to evaluate the persistence of immunological memory 2 y after primary immunization and to assess the booster response in children previously vaccinated with JE-CV.
Almost all (95.9%) of the 345 eligible children enrolled to receive a booster dose of JE-CV had had protective JE neutralizing antibody titers one month after the first vaccination, and most (80.3%) were still seroprotected 2 y later at the time just before the booster vaccination. Similar results were observed in a Phase II study in Thailand19 where more than 80% of children aged 12–24 mo were still seroprotected two years after a single JE-CV dose as primary immunization (unpublished data).
Children previously vaccinated with a single dose of JE-CV exhibited a relatively rapid increase in titer to a level far above that reached after primary immunization (GMTs of 231 1/dil on D7, and 2,242 1/dil on D28, vs. 168 1/dil on D28 post-primary immunization). This is typical of an anamnestic response. Based on the GMT reached post-booster, there is no indication that the neutralizing antibody titers at the time of booster (GMT of 40 1/dil) dampened the anamnestic response. The Day 28 GMTs were lower in subjects with titers below 10 1/dil before the booster vaccination than in those with titers of 10 1/dil or more. In another study done in yellow fever 17D vaccinees, a booster response showed a plateau, resulting in a reduced post-to-pre ratio of neutralizing titers for subjects with higher titers at the time of booster. However, the long-term response was better in subjects with high titers at the time of booster compared with subjects with lower titers.23 There is therefore no indication that a booster given in the presence of higher neutralizing antibody titers would be negatively impacted.
In this study, 82.4% of the children with a titer below the seroprotective threshold at baseline were capable of mounting a protective response within 7 d of receiving the JE-CV booster dose, indicating the persistence of immunological memory 2 y after primary immunization despite the absence of measureable neutralizing antibodies. By comparison, 15.4% of the subjects from the control group negative at baseline were seroprotected at the same timepoint, a level higher than observed in a study of the live attenuated SA14–14–2 JE vaccine in which no subjects in the naïve control group had detectable antibody 7 d after vaccination.20 This rapid onset of seroprotection following primary immunization of children with JE-CV can be compared with the 93.6% seroprotection level reached in adults 15 d after primary immunization.16 The persistence of immunological memory after JE-CV immunization is comparable with the response observed in children who were given a booster dose of the live attenuated SA14–14–2 JE vaccine 6 y after receiving a single dose of the same vaccine;20 seven days after the booster, 76.5% (13/17) of children who were seronegative for JE neutralizing antibody before the SA14–14–2 booster dose were seroprotected.
The anamnestic response following natural infection might be quick enough to confer protection from disease.11 Such a protection by the memory response has been demonstrated for hepatitis B.24 It is explained by an incubation time of the disease far longer than the time needed to recall a protective memory response by the incoming antigen.25 Incubation is far shorter for JE than for hepatitis B, but protection or at least attenuation of the disease is possible if the passage of the virus through the blood brain barrier can be prevented by a quick recall of immunological memory by the infecting JE virus. Such a protection by the memory response has been shown in a preclinical model of mice vaccinated with a DNA vaccine against JE.26 However, a definitive answer will only be provided by epidemiology studies.
In the JE-CV vaccinated group, the GMT reached 2,242 1/dil on day 28 post booster with 100% seroprotection and was still at 596 1/dil with 99.4% seroprotection 1 y after administration of the JE-CV booster. A very similar antibody kinetic was observed after the administration of JE-CV as a booster vaccination in children aged 2–5 y in Thailand previously primed by an inactivated JE vaccine given in a 2-dose immunization schedule, according to the National Schedule of Immunization.19 In this trial, the GMT reached 2,707 1/dil on day 28 post-vaccination and was 454 1/dil one year later;19 it then remained stable and was 461 1/dil corresponding to 100% seroprotection three years later (FAS) (unpublished data), suggesting a plateau of the antibody titers at a level far above the seroprotection threshold from 1 y post booster administration. These results indicate that the JE-CV booster is able to recall memory cells induced by priming with a killed or a live-attenuated JE vaccine and for an interval of 12 or 24 mo between primary immunization and booster. It suggests that the administration of a JE-CV booster to children will achieve a long-lasting protection similar to the one observed in adults after a single dose17,18 or after a single dose plus booster 6 mo later.17
We observed that 30.4% of the children in the JE-vaccine naïve group had pre-existing neutralizing antibodies against JE or dengue viruses at baseline; among those, 15.2% (7/46) had pre-existing JE neutralizing antibodies before their first JE-CV vaccination. No JE vaccine is licensed or marketed in the Philippines and therefore none of these subjects would have received a JE vaccine. As the pre-existing JE antibody titers were not induced by a JE vaccination, the antibodies are possibly due to natural exposure to wild-type JE viruses, although they could also be due to cross-reactivity with some other flaviviruses. Some antibodies directed against epitopes present within the envelope protein have been shown to cross-neutralize a range of flaviruses.28 This possible explanation due to cross reactivity could be is supported by the low to moderate level of antibodies in some of these children and the measure of antibody titers against dengue virus in 23.9% of subjects, indicating these children have been exposed to other non-JEV flaviviruses.
The safety profile of JE-CV given as a booster dose is good and similar to that of JE-CV given as a single-dose administration for primary immunization. Furthermore, the safety profile of JE-CV given as a first or booster dose to children 36 to 42 mo of age is similar to that of a licensed varicella vaccine and for children 2 to 5 y of age similar to a licensed hepatitis A vaccine.19 There were no related SAEs, no immediate AEs, and no withdrawals for AEs in any group in either study. The majority of solicited systemic and injection site reactions were reported as mild or moderate; the most frequently reported severe AE was fever in both studies.
There was one case of febrile convulsion. While febrile convulsions have been reported as unrelated SAEs in some of the previous studies evaluating JE-CV in pediatric populations, it should be remembered that febrile convulsions in general are the most common seizure disorders in children and the finding of a single case fits into expected observations.22 During the clinical development of this vaccine, no identified safety risk has been observed in more than 4,400 subjects receiving JE-CV, including approximately 2,500 adults and 1,900 children.
Conclusion
An immunological memory and a robust anamnestic response were observed following the administration of a booster dose of JE-CV given to children two years after having received at 12 to 18 mo of age a single dose of JE-CV for primary immunization. The kinetic of the antibody response was similar to the one observed when JE-CV was given as a booster after a primary immunization with an inactivated JE vaccine. The three-year follow-up data of this latter study and the results obtained with JE-CV in adults suggest that a JE-CV booster will provide long-lasting protection to children. The JE-CV booster was shown to be well tolerated, with an overall safety profile similar to that of licensed varicella and hepatitis A vaccines.
Patients and methods
Study design and population
The study was performed in accordance with the Declaration of Helsinki (version in force at the time of the study) and Good Clinical Practice as defined by the International Conference on Harmonization. The Institutional and Ethical Review Board of the Research Institute for Tropical Medicine (RITM), Manila, the Philippines, approved the protocol. The child’s parent or guardian provided signed informed consent to the study before exposure to any study procedures.
This was an open, controlled, multicenter, phase III trial involving children aged 36–42 mo on the day of inclusion. A previous study (NCT00735644) assessed the safety and immunogenicity of a single dose of JE-CV in 1,200 children aged 12 to 18 mo; up to 400 children vaccinated with JE-CV in three satellite sites of the RITM in the earlier study were recruited into this study reported here and vaccinated with a booster dose of JE-CV, two years after the first single dose vaccination (Group 1; JE-CV primed group). JE-vaccine-naïve children of the same age range were enrolled and randomized to receive either a single dose of JE-CV (Group 2; JE-vaccine naïve group; immunogenicity control) or a varicella vaccine (Group 3, safety control group).
Children were in good general health and were excluded if they fulfilled any of the following criteria: active, planned, or recent activity in any vaccine study; receipt of antiviral medication in the preceding two months; receipt of any blood product in the preceding three months; immunodeficiency from any cause; history of central nervous system disorder or disease, including seizures or febrile seizures; known hypersensitivity to any vaccine components; for Group 1, receipt of any other JE vaccine since the completion of the previous study; for the Groups 2 and 3, varicella or flavivirus infection or vaccinations.
Procedures
At the first visit (D0) eligibility was confirmed and blood was taken to evaluate the baseline immune status before vaccination (Groups 1 and 2 only). There was no randomization for Group 1 as it comprised children who had been enrolled at these sites when participating in an earlier study and were offered a JE-CV booster dose in this study. JE-vaccine-naïve children were randomized to either an immunogenicity control group (Group 2) and administered JE-CV as primary vaccination, or a safety control group (Group 3) and administered a varicella vaccine, using the permuted block method with stratification on study center; group allocation was determined by an independent voice response system (IVRS) that provided information on the control vaccine to be administered. The appropriate study vaccines were reconstituted and injected subcutaneously by qualified personnel into the child’s deltoid region.
Children were observed for 30 min after vaccination to monitor any immediate AEs. Parents/guardians were given a digital thermometer for axillary temperature measurement and a ruler for measuring injection site reactions. They were provided with a diary card to record information about solicited injection site and systemic reactions up to seven and 14 d, respectively, after vaccination, and unsolicited AEs up to 28 d after vaccination. Blood samples for immunogenicity assessment were taken at visits seven and 28 d after vaccination (Groups 1 and 2), and then annually from the day of vaccination (Group 1 only). Safety data were collected by telephone three and 14 d after vaccination, and reviewed at the D7 and D28 visits. There was an additional phone call/home visit 6 mo after vaccination. All SAEs were recorded up to 6 mo after vaccination and related SAEs and deaths from 6 mo to 5 y were recorded for children in the Group 1.
Vaccines
JE-CV was manufactured by Sanofi Pasteur at Government Pharmaceutical Organization – Mérieux Biological Products (GPO-MBP), Thailand, and reconstituted using 0.4% sodium chloride diluent for injection; each 0.5mL dose administered to children in the Groups 1 and 2 contained 4.79 log10 plaque forming units of virus. The control vaccine to be administered to Group 3 was a licensed, live, attenuated varicella vaccine (OKAVAX® Biken, Research Foundation for Microbial Diseases of Osaka University), and was administered according to standard practice and the manufacturer’s recommendations. Varicella vaccination is not included in the Expanded Program on Immunization (EPI) in the Philippines, although it is both licensed and recommended.
Serology
JE neutralizing antibody levels were assessed by a PRNT50 assay using JE-CV as the challenge virus (Focus Diagnostics Inc.). The final endpoint neutralization titer is the inverse of the highest serial dilution of serum that can neutralize ≥ 50% of JE challenge virus.19
Antibody levels to dengue serotypes 1–4 were measured in the children enrolled for receiving JE-CV for the first time. Anti-dengue IgG and IgM antibody responses were assayed by the Centre for Vaccine Development (CVD), Mahidol University, Thailand using dengue IgM and IgG enzyme linked immunosorbent assays (ELISA) (Focus Diagnostics), and dengue neutralizing antibody responses were assayed using a PRNT50 with a challenge of each dengue serotype 1–4.
Outcome measures
A serological correlate of protection based on neutralizing antibodies against JE is accepted and recommended by the World Health Organization (WHO);11 a threshold of 10 1/dil is considered as the level of protection. JE-neutralizing antibody responses were expressed in terms of the seroprotection rate and GMT at each timepoint. The seroconversion rate and the geometric mean titer ratio (GMTR) between pre- and post-vaccination timepoints (D28/D0 and D7/D0) were also calculated. Seroprotection is defined as a neutralizing antibody titer ≥ 10 1/dil11 and seroconversion as achievement of this antibody level of 10 or more 1/dil in children who were seronegative at baseline (titer < 10 1/dil). In children who presented with pre-existing antibody titers at baseline (i.e., considered as positive), seroconversion is defined as ≥ 4-fold rise in antibody titers.
Safety evaluations were descriptive and included time to onset and intensity of solicited (events or reactions prelisted in the diary card and the case report form [CRF]) and unsolicited AEs. All solicited reactions and all injection site AEs (whether or not pre-listed in the CRF) were considered to be related to vaccination. ARs were unsolicited AEs considered by the investigator to be related to vaccination. The following were denoted as AESIs (Adverse Events of Specific Interest): hypersensitivity/allergic reactions, neurological events including febrile convulsions, encephalopathy, encephalitis, acute disseminated encephalomyelitis, myelitis, GBS, peripheral neuropathy, facial (Bell’s) palsy, and vaccine failure. AEs were coded using the Medical Dictionary for Regulatory activities (MedDRA version 12.0) preferred term.
Statistical methods
No hypothesis was defined in this trial and the results are descriptive. The sample size was arbitrarily defined. All children who participated in the earlier study at the three study sites in the Philippines and who received a single dose administration of JE-CV were invited to participate and therefore could be enrolled in the JE-CV primed group. For safety, it provided a 95% probability of observing events that had an occurrence of 0.75%. The sample size for the Groups 2 and 3 was arbitrarily defined; a total of 45 and 60 children were to be included in these two groups, respectively, providing a 95% probability of observing events that had an occurrence of 4.9%.
The PP set included all children who received a dose of JE-CV and had no protocol deviations and was used for analysis of the immunogenicity at D7 and D28. Children in the JE-vaccine naïve group were excluded from the PP population if they were seropositive for JE at baseline. The FAS included all children who received a dose of JE-CV and was used for all immunogenicity analyses and subgroup analysis. The safety analysis set (SAS) included children who received the JE-CV or the varicella vaccine.
It was assumed that the log10 transformation of the titers/ratios followed a normal distribution. The mean and 95% interval were calculated on log10 (titers/ratios) using the usual calculation for normal distribution and then, antilog transformations were applied to the results to provide GMTs/GMTRs and their 95% CIs. 95% CI of percentages were computed using the exact binomial distribution.
Acknowledgments
The authors are grateful to the children and their parents, and to the following for their contributions to this work: Dr. Edison Alberto, Dr. Nelia Malubay, Dr. Eisel Palestroque and study teams at the trial centers; D.r Sutee Yoksan, at the Center for Vaccine Development at Mahidol University, for dengue antibody titrations and flavivirus viremia assessments; T. Laot, F. Sillan, I. Bruyere, S. Personnic, H. Aurell, C. Monfredo, M. Bayet, C. Guillaume, H. DyTioco, M.A. Verdan, S. Orlando, A. Padieu-Sequeira, at Sanofi Pasteur; and B. Thawornrungroaj at GPO-MBP. This manuscript was prepared with the assistance of professional medical writers, Melanie Lee and Sam Hampson, and with funding from Sanofi Pasteur.
Glossary
Abbreviations:
- JE
Japanese encephalitis
- JE-CV
JE chimeric virus vaccine
- PRNT50
50% plaque reduction neutralization test
- FAS
full analysis set
- PP
per protocol analysis set
- SAS
safety analysis set
- GMT(R)
geometric mean titer (ratio)
- CI
confidence interval
- (S)AE
(serious) adverse events
- AR
adverse reaction
- AESI
AE of special interest
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial disclosure
The study sponsor and manufacturer of the investigational vaccine, Sanofi Pasteur, was involved in the trial design, the management and analysis of data, and, in the decision to publish. This manuscript was prepared in close collaboration with all the authors and with the assistance of medical writers, funded by Sanofi Pasteur.
Disclosure of Potential Conflicts of Interest
Emmanuel Feroldi, Mark Boaz, Sophia Gailhardou, Claude Meric and Alain Bouckenooghe are employees of Sanofi Pasteur.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23087
References
- 1.Halstead SB, Jacobson J. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines, 5th ed. Philadelphia (PA): Saunders Elsevier, 2008:311–52. [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Fischer M, Lindsey N, Staples JE, Hills S, Centers for Disease Control and Prevention (CDC) Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-1):1–27. [PubMed] [Google Scholar]
- 4.Olsen SJ, Supawat K, Campbell AP, Anantapreecha S, Liamsuwan S, Tunlayadechanont S, et al. Japanese encephalitis virus remains an important cause of encephalitis in Thailand. Int J Infect Dis. 2010;14:e888–92. doi: 10.1016/j.ijid.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Borah J, Dutta P, Khan SA, Mahanta J. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with Acute Encephalitis Syndrome. J Clin Virol. 2011;52:45–9. doi: 10.1016/j.jcv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther. 2008;8:95–106. doi: 10.1517/14712598.8.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–64. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 8.Kaltenböck A, Dubischar-Kastner K, Schuller E, Datla M, Klade CS, Kishore TSA. Immunogenicity and safety of IXIARO (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine. 2010;28:834–9. doi: 10.1016/j.vaccine.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Toriniwa H, Komiya T. Long-term stability of Vero cell-derived inactivated Japanese encephalitis vaccine prepared using serum-free medium. Vaccine. 2008;26:3680–9. doi: 10.1016/j.vaccine.2008.04.076. [DOI] [PubMed] [Google Scholar]
- 10.Oya A. Japanese encephalitis vaccine. Acta Paediatr Jpn. 1988;30:175–84. doi: 10.1111/j.1442-200X.1988.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 11.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine. 2005;23:5205–11. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, et al. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO(®)) induced neutralizing antibody titers. Vaccine. 2011;29:5925–31. doi: 10.1016/j.vaccine.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 13.Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–64. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 14.Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis. 2003;188:1213–30. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 15.Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–18. doi: 10.1016/S0264-410X(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 16.Torresi J, McCarthy K, Feroldi E, Méric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: Randomised controlled phase 3 trials. Vaccine. 2010;28:7993–8000. doi: 10.1016/j.vaccine.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Nasveld PE, Ebringer A, Elmes N, Bennett S, Yoksan S, Aaskov J, et al. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine: randomized, double-blind, 5-year phase II study in healthy adults. Hum Vaccin. 2010;6:1038–46. doi: 10.4161/hv.6.12.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai K, Coudeville L, Bailleux F. Modelling the long-term persistence of neutralizing antibody in adults after one dose of live attenuated Japanese encephalitis chimeric virus vaccine. Vaccine. 2012;30:2510–5. doi: 10.1016/j.vaccine.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, et al. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29:1111–7. doi: 10.1097/INF.0b013e3181f68e9c. [DOI] [PubMed] [Google Scholar]
- 20.Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: immunogenicity and anamnestic responses. Vaccine. 2008;26:1638–43. doi: 10.1016/j.vaccine.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, et al. Single-dose, live-attenuated Japanese encephalitis vaccine in JE-naïve infants aged 12–18 months; a randomized, controlled Phase 3 trial of immunogenicity and safety. Hum Vaccin Immunother. 2012;8:929–37. doi: 10.4161/hv.20071. [DOI] [PubMed] [Google Scholar]
- 22.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 23.Hepburn MJ, Kortepeter MG, Pittman PR, Boudreau EF, Mangiafico JA, Buck PA, et al. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24:2843–9. doi: 10.1016/j.vaccine.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 24.European Consensus Group on Hepatitis B Immunity Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561–5. doi: 10.1016/S0140-6736(99)07239-6. [DOI] [PubMed] [Google Scholar]
- 25.Banatvala JE, Van Damme P. Hepatitis B vaccine -- do we need boosters? J Viral Hepat. 2003;10:1–6. doi: 10.1046/j.1365-2893.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 26.Konishi E, Yamaoka M, Khin-Sane-Win, Kurane I, Mason PW. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J Virol. 1998;72:4925–30. doi: 10.1128/jvi.72.6.4925-4930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura-Kuroda J, Yasui K. Antigenic comparison of envelope protein E between Japanese encephalitis virus and some other flaviviruses using monoclonal antibodies. J Gen Virol. 1986;67:2663–72. doi: 10.1099/0022-1317-67-12-2663. [DOI] [PubMed] [Google Scholar]