Abstract
The role of exercise in health is well known; here we discuss the specific role of exercise in vaccination responses. Chronic exercise or high levels of physical activity have been shown to be related to improved vaccination responses in older adults, illustrating improved immune function, and conferring potentially significant public health benefit. Acute exercise has recently been examined as a potential adjuvant to vaccination; its promise for clinical use warrants further investigation, given current data.
Keywords: vaccination, exercise, physical activity, immune function
Regular exercise is one of the most well-known behavioral public health targets which is easily explained by the litany of disease conditions which can be ameliorated by exercise. The interaction of inflammation and immune function have been linked to exercise effects on metabolic syndrome, insulin sensitivity, cardiovascular disease, breast and colon cancer, dementia and depression, as well as all-cause mortality.1 The pathway for association with inflammation clearly includes obesity, in particular abdominal adiposity, but it is not surprising that there may also be a direct role for exercise in maintaining immune function. However, the effects of exercise on immune function are far from simple. In addition to the immune consequences of regular exercise, an acute bout of exercise can elicit profound changes in the immune system, including circulating cell numbers, with specific increases in certain subsets, and the release of cytokines by the working myoctes themselves.2,3
Vaccination responses can be understood as a measure of integrated immune function, elicited by antigen exposure and measured by antibody titer and/or cell-mediated response. Research which examines alterations in vaccine responses is simultaneously assessing the integrated immune functionality alongside the public health outcomes of disease prevention. The clinical and public health importance of understanding the factors which elicit changes in vaccination responses is evident when the impact of vaccination programs is considered; for example, it was recently estimated that in the first decade of the 21st century, an extraordinarily large number of deaths of children aged < 5 y — 2.5 million — were prevented by vaccinations.4 This commentary will discuss the role of exercise as a factor in the vaccination response, and consider the future directions for research.
The effects of chronic exercise (amount of physical activity or level of cardiovascular fitness) on vaccination function have been evaluated in both cross-sectional studies and more importantly in randomized controlled trials (RCTs). Observational studies, which classify participants according to physical fitness (e.g., VO2max test) or physical activity (e.g., self report / physical activity questionnaire), have been conducted in both young and older adults. In young adults, Shuler et al.5 examined antibody titers in response to influenza vaccination in college students. Measures of both physical fitness and physical activity were taken, but neither was found to be associated with magnitude of antibody response. In contrast, several cross-sectional studies of older adult populations have all reported greater antibody responses to vaccinations in participants with high levels of physical fitness6 or physical activity.7-9 This contrast of significant effects of chronic exercise on vaccination responses being elicited in older adult populations but not in younger populations is exemplified by Smith et al.8 who compared the immune response to a novel antigen (Keyhole limpet hemocyanin, KLH) in both young and older men. They showed that older active men demonstrated stronger antibody and cell-mediated responses to KLH than sedentary older men, while responses were similar regardless of activity habits in younger men. In RCTs, similar positive effects of exercise can be seen. Woods et al.10 demonstrated that 10 mo of cardiovascular exercise (60–70% maximal oxygen uptake, 45–60 min, 3 × week) in previously sedentary older adults resulted in increased seroprotection maintenance (at 24 weeks post-influenza vaccination) compared with participants who took part in flexibility training over the same period. Responses to novel antigens have also been observed to be increased by chronic exercise. After KLH vaccination IgG1 and IgM concentrations were greater in participants who had completed 10 mo cardiovascular training (VO2max increase 11%) than in control participants (flexibility exercise, 1% VO2max improvement).11 The literature supports the hypothesis that regular exercise improves immune function, which is reflected in greater antibody or cell-mediated responses to vaccination, especially in older adults. Although clinical benefit is only inferred from the quantified antibody or cell-mediated responses, a recent population-based cohort study supports the finding. Siu et al.12 reported that moderately active and highly active individuals as defined by survey responses were less likely to experience an influenza-coded visit to a physician’s office or emergency department than inactive individuals. Younger adults appear to show less effect of chronic exercise on immune function when examining vaccine responses, but it is worth noting that the robust response to most vaccinations in young healthy adults may well mask any more subtle effects of exercise, whereas in older adults with weaker immune function and greater variability, the immune-enhancement effects are notable. Finally, even with low activity levels, fitness in younger adults is likely to still be well above that found in older adults.
Encouraging physical activity on a population wide basis for improved health has been a priority for a number of years. Yet, the continuing decline in the proportion of the population meeting physical activity goals describes the lack of success and is of major public health concern. Thus, although regular exercise is a desirable intervention, for immune function as well as other health benefits, it is one that is hard to implement on a population level.
Even a single acute bout of exercise is well known to elicit significant changes in the immune system.3 Also referred to as a stress response, the increases in epinephrine, cortisol, heart rate and blood pressure are all part of our response to exercise. Related to these changes are the well-known leukocytosis response, and a transient increase in cytokines. In addition, exercise that causes muscle damage results in increase in ‘danger signals’ such as heat-shock proteins, and induces trafficking of leukocytes to the tissue. Finally, muscle contractions are also known to increase flow in lymphatic vessels to secondary lymphoid organs. All these changes evoked by acute stress / exercise have been hypothesized to be part of an activation of immune surveillance in anticipation of antigen entry.13,14 Recent animal data supports this hypothesis, with acute stressors being shown to enhance cellular and humoral immune responses to antigen exposure.15,16 The development of the acute stress-induced immunoenhancement hypothesis has now been extended to human studies, with particular reference to vaccination.17
A series of studies have been published examining the effect of a bout of exercise prior to vaccination on immune response in young healthy adults. The initial study compared an exercise task (cycling) and a psychological stress task prior to influenza and meningococcal vaccination. Antibody responses were not uniformly enhanced, with women showing increased responses in both the exercise and stress groups to influenza vaccine compared with the control group,18 contrasting with an increased response to meningococcal vaccine in men in exercise and stress groups.19 Similar non-uniform responses in subsequent studies led to the suggestion that exercise acted to enhance responses only when the control response was relatively poor. Subsequent studies investigated effects of a muscle damaging exercise task on influenza vaccination responses. Interestingly, responses to a full dose vaccine in young healthy adults did not show any significant differences,20 however, when a reduced dose of vaccine was used enhancements were found to 2 of 3 influenza strains (but not to the most immunogenic strain).21 These findings supported the hypothesis that sub-optimal responses (represented by responses to a reduced dose of vaccine), are susceptible to enhancement by acute exercise.
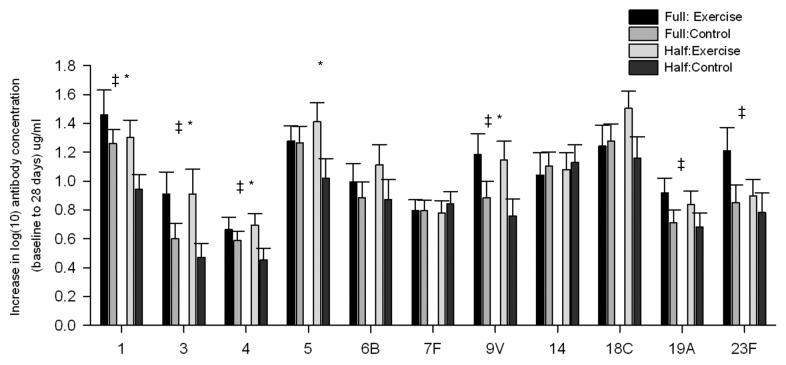
Most recently, this hypothesis was directly tested using a study design comparing exercise vs. rest prior to receipt of a full or half dose pneumococcal vaccine.22 Young healthy adults either rested or completed an upper-body resistance-band based task (15 min). Half of each group received a full dose vaccine expected to elicit a strong response in this cohort, the other half received 50% of the full dose, expected to produce a weaker response. When collapsed across doses, exercise groups were found to show stronger responses than control groups. However, when examining only the groups receiving the full dose, both control and exercise groups showed similar antibody responses. While in the groups that received the half dose of vaccine, 5 of 11 strains measured showed greater responses in the exercise compared with control groups. Interestingly, when examining the strains that revealed differences, it was the strains with larges difference in full vs. half dose control responses that were enhanced by exercise (see Fig. 1).
Figure 1. Change scores for pneumococcal strains from baseline to 28 d for all groups. ‡, significant effect of exercise over both full and half dose; *significant effect of exercise within half dose groups.
The data for acute exercise as an adjuvant to vaccination is encouraging, and deserves further attention for development. Of primary interest are two questions: (1) is exercise an appropriate and effective adjuvant in at-risk populations; and (2) what is the most effective task in terms of type, intensity, duration and timing. Several populations are of immediate interest for potential use of exercise as a behavioral adjuvant. A predictable accompaniment of aging is the decline in immune function known as immunosenescence. This results not only in higher rates of inflammation related disease but also the severity and frequency of infectious disease, which can be inferred from the well known reduced responses to vaccination in older adults. Many developed nations include routine vaccination against influenza and pneumococcus for adults aged over 65 y, reflecting the fact that deaths related to pneumonia and influenza are the 6th leading cause of death in older adults.23 Because of the reduced immune responses in the elderly, interventions to improve their vaccine responses warrant investigation. Similarly, benefit may accrue for other populations with known reduced immune function such as HIV+ patients, or with increased risk of infection such as pregnant women, using a simple, cheap and safe intervention.
The question of the best task for such an intervention has received little attention. One study reported no difference in magnitude of enhancement when muscle-damaging exercise of different intensities was used,21 however, other studies have cited insufficient exercise intensity as an explanation for the lack of effect when a walking protocol was used.24 Currently no study has compared different forms of exercise (such as aerobic vs. muscle damaging weights exercise), or duration of exercise. Measurements of aspects of the response to exercise such as blood flow and inflammatory markers have been included to a limited extent with associations with immune response reported.18,25 However, it holds that for clinical reliability, significant further investigation of mechanisms to inform the exercise task design is required. If a simple and appropriate exercise task could be effectively combined with vaccine administration to enhance responses in at risk populations, the public health implications would support implementation.
Exercise elicits many responses, including cardiovascular, nervous, immune, endocrine and psychological; these do not operate in isolation but interact, their proper balance intrinsic to health. We argue that the changes induced by acute exercise prepare us for challenge, including antigen challenge such as vaccination, producing a state of improved readiness. A holistic view of homeostasis involves a consideration of complexity. In order to comprehend the potential for benefit under various conditions, it is important that we explore the complete picture while indeed considering the minutiae.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23365
References
- 1.Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587:5559–68. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh NP, Gleeson M, Pyne DB, Nieman DC, Dhabhar FS, Shephard RJ, et al. Position statement. Part two: Maintaining immune health. Exerc Immunol Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- 3.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Ten great public health achievements--worldwide, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60:814–8. [PubMed] [Google Scholar]
- 5.Schuler PB, Lloyd LK, Leblanc PA, Clapp TA, Abadie BR, Collins RK. The effect of physical activity and fitness on specific antibody production in college students. J Sports Med Phys Fitness. 1999;39:233–9. [PubMed] [Google Scholar]
- 6.Keylock KT, Lowder T, Leifheit KA, Cook M, Mariani RA, Ross K, et al. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol. 2007;102:1090–8. doi: 10.1152/japplphysiol.00790.2006. [DOI] [PubMed] [Google Scholar]
- 7.Schuler PB, Leblanc PA, Marzilli TS. Effect of physical activity on the production of specific antibody in response to the 1998-99 influenza virus vaccine in older adults. J Sports Med Phys Fitness. 2003;43:404. [PubMed] [Google Scholar]
- 8.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97:491–8. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57:M557–62. doi: 10.1093/gerona/57.9.M557. [DOI] [PubMed] [Google Scholar]
- 10.Woods JA, Keylock KT, Lowder T, Vieira VJ, Zelkovich W, Dumich S, et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J Am Geriatr Soc. 2009;57:2183–91. doi: 10.1111/j.1532-5415.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- 11.Grant RW, Mariani RA, Vieira VJ, Fleshner M, Smith TP, Keylock KT, et al. Cardiovascular exercise intervention improves the primary antibody response to keyhole limpet hemocyanin (KLH) in previously sedentary older adults. Brain Behav Immun. 2008;22:923–32. doi: 10.1016/j.bbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siu E, Campitelli MA, Kwong JC. Physical activity and influenza-coded outpatient visits, a population-based cohort study. PLoS One. 2012;7:e39518. doi: 10.1371/journal.pone.0039518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhabhar FS, McEwen BS. Bi-directional Effects of Stress on Immune Function: Possible Explainations for Salubrious as well as Harmful Effects. In: Ader R, Felten DL, Cohen N, eds. Psychoneuroimmunology. New York: Academic Press, 2001. [Google Scholar]
- 14.Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–69. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- 15.Dhabhar FS, Viswanathan K. Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am J Physiol Regul Integr Comp Physiol. 2005;289:R738–44. doi: 10.1152/ajpregu.00145.2005. [DOI] [PubMed] [Google Scholar]
- 16.Silberman DM, Wald MR, Genaro AM. Acute and chronic stress exert opposing effects on antibody responses associated with changes in stress hormone regulation of T-lymphocyte reactivity. J Neuroimmunol. 2003;144:53–60. doi: 10.1016/j.jneuroim.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Edwards KM, Burns VE, Carroll D, Drayson M, Ring C. The acute stress-induced immunoenhancement hypothesis. Exerc Sport Sci Rev. 2007;35:150–5. doi: 10.1097/JES.0b013e3180a031bd. [DOI] [PubMed] [Google Scholar]
- 18.Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006;20:159–68. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Edwards KM, Burns VE, Adkins AE, Carroll D, Drayson M, Ring C. Meningococcal A vaccination response is enhanced by acute stress in men. Psychosom Med. 2008;70:147–51. doi: 10.1097/PSY.0b013e318164232e. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JP, Edwards KM, Ring C, Drayson MT, Bosch JA, Inskip A, et al. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav Immun. 2010;24:236–42. doi: 10.1016/j.bbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Edwards KM, Campbell JP, Ring C, Drayson MT, Bosch JA, Downes C, et al. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain Behav Immun. 2010;24:623–30. doi: 10.1016/j.bbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Edwards KM, Pung MA, Tomfohr LM, Ziegler MG, Campbell JP, Drayson MT, et al. Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine. 2012;30:6389–95. doi: 10.1016/j.vaccine.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heron M. Deaths: Leading Causes for 2009. National Vital Statistics Report. Hyattsville, MD.: National Center for Health Statistics, 2012. [Google Scholar]
- 24.Long JE, Ring C, Drayson M, Bosch J, Campbell JP, Bhabra J, et al. Vaccination response following aerobic exercise: can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations? Brain Behav Immun. 2012;26:680–7. doi: 10.1016/j.bbi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007;21:209–17. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]