Abstract
Hyperhomocysteinemia has been shown to be an independent risk factor for cardiovascular disease as well as retinal vascular occlusion. Because of the epidemiological, dietary, genetic and environmental diversity among the different countries, each country should establish the reference interval of homocysteine of their own population for recommending appropriate medical decision limits. Hence a total of 1,288 apparently healthy subjects including 636 male and 652 female were enrolled in the present study to determine the reference intervals of homocysteine in an Indian population. Results of the study were presented as mean, standard deviation, median and 2.5th and 97.5th percentile with the 0.90 confidence interval of each percentile values of homocysteine along with decade-wise changes.
Keywords: Homocysteine (Hcys), Hyperhomocysteinemia (HHcys), Reference interval, Confidence interval (CI), Percentile
Introduction
Atherosclerosis induced cardiovascular disease is the leading cause of mortality and morbidity throughout the world [1–6]. The traditional risk factors for atherosclerosis are elevated total cholesterol, LDL-cholesterol [7, 8], triglyceride [9], apolipoprotein B [10], lipoprotein (a) [11], reduced HDL-cholesterol [12] and apolipoprotein A [13]. Since 1969, McCully suggested that moderate levels of hyperhomocysteinemia (HHcys) might be associated with atherosclerosis [14, 15].
All circulating homocysteine (Hcys) is primarily derived from dietary methionine, which acts as a methyl group donor in the form of S-adenosyl methionine. On donating the methyl group it forms S-adenosyl Hcys which is then converted to Hcys. Hcys is a sulfur-containing nonprotein amino acid that is either metabolized to cystathionine by the transsulfuration pathway, requiring vitamin B6, or it is converted back to methionine by transmethylation, requiring vitamin B12 and folate [16].
HHcys is associated with a greater risk of cardiovascular disease [17]. Approximately 10 % of the population’s risk of coronary artery disease is attributable to Hcy [18]. HHcys is also a risk factor for atherosclerosis in the retinal vasculature [19–21].
There are various mechanisms reported regarding endothelial dysfunction by Hcys. These include decreased bioavailability of nitric oxide [22], altered expression of various thrombotic factors, mitogenic effect on arterial smooth muscle cells [23], and expression of acute stressrelated genes [24]. Moreover, the high pKa of the sulfhydryl group (pKa = 10.0) of Hcys is responsible for the formation of stable disulfide bonds with protein cysteine residues and, in the process, alters or impairs the function of many proteins. Albumin, fibronectin, transthyretin, annexin II, and factor V have now been identified as molecular targets for Hcys [25]. Metabolic conversion of Hcys to a chemically reactive metabolite, Hcys-thiolactone is suggested to contribute to Hcys toxicity in humans (Hcys-thiolactone hypothesis) [26] leading to endothelial dysfunction.
According to the centre disease control of prevention, and described in the Laboratory Procedure Manual of Abbott Axsym System [27] the normal concentration of total homocysteine varies between 4.6 and 8.1 μmol/L, for subjects aged under 30 years without regard to gender, however moderate hyperhomocysteinemia is considered for values greater than 16 μmol/L. These values are confirmed in studies reviewed by Kilmer McCully and also in his book The Heart Revolution [14, 15] where hyperhomocysteinemia is classified as: moderate—between 15 and 30 μmol/L; intermediate—between 30 and 100 μmol/L; and severe—over 100 μmol/L [27]. Most laboratories use 15 μmol/L as the cutoff point, between normal and abnormal values, without considering the age of the patient. However, a study conducted from 1991 to 1994 found that the reference range for serum total homocysteine concentration increased with age even among adults [28].
Current reference data on circulating Hcy concentrations are based on studies conducted on foreign population [28–30]. Reference data on Hcy concentrations based on a representative Indian sample are lacking. Therefore the objective of the present study was to quantitate the plasma hcys in healthy Indian male and female in order to establish reference interval.
Materials and Methods
A total of 1,288 apparently healthy subjects including 636 male and 652 female from West Bengal were enrolled in the study. The age of reference individuals ranged from 20 to 81 years. Reference individuals were selected from those persons who accompanied the patients attending the out patient department of R. G. Kar Medical College and Hospital. The institutional ethics committee approved the study and informed consent was obtained from all the study populations, in accordance with the Declaration of Helsinki. A detailed questionnaire on family history, social status, and dietary habits, including other habits such as smoking, alcohol intake, history of systemic diseases, and drug history was completed by all the study subjects. Hypertension, diabetes mellitus, cardiovascular disease, dyslipidemia, renal disease, liver disease were ruled out in the present study based on the biochemical tests apart from the questionnaire.
Measurement of Plasma Hcys
Venous blood samples were drawn into EDTA-containing tubes after the participants fasted overnight. Plasma was separated immediately from blood cells by centrifugation at 2,000×g for 10 min. Total plasma Hcys was estimated enzymatically with a Reagent kit, supplied by Lilac Clinical chemistry division [31].
Other biochemical tests—fasting plasma glucose, lipid profile (total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol), liver function test (ALT, AST, Total Bilirubin, Direct Bilirubin, Total protein, Albumin), kidney function test (Urea, Creatinine) were performed.
Mean, standard deviation, median and 2.5 and 97.5th percentile with the 0.90 confidence interval of each percentile values of hcys are presented along with decade-wise changes. Statistical analysis was performed using SPSS software.
Results
The mean total plasma Hcys levels for all ages were 11.46 ± 2.56 μmol/L in healthy male and 11.41 ± 2.48 in healthy female. The median values of 11.67 μmol/L in male and 11.4 μmol/L in female were quite similar to mean values in male and female respectively (Table 1).
Table 1.
Total plasma Hcys levels (μmol/L) in reference Indian male and female
| n | Mean | SD | Median | Min–Max | 2.5th percentile | 97.5th percentile | |
|---|---|---|---|---|---|---|---|
| Total plasma Hcys | |||||||
| Male | 638 | 11.46 | 2.56 | 11.67 | 4.1–20.8 | 6.42 | 16.5 |
| Female | 652 | 11.41 | 2.48 | 11.4 | 5.1–22 | 6.55 | 16.27 |
| Total | 1288 | 11.44 | 2.53 | 11.5 | 4.1–22 | 6.5 | 16.38 |
The 2.5th and 97.5th percentile values of total plasma Hcys level in reference Indian population were 6.5 (0.90 CI = 6.3–6.7) and 16.38 (0.90 CI = 16.2–16.6) respectively (Table 2).
Table 2.
Percentile values of total plasma Hcys level (μmol/L) with 0.90 confidence interval in reference Indian male and female
| Percentile | Percentile value | Lower confidence limit | Upper confidence limit | |
|---|---|---|---|---|
| Total plasma Hcys | ||||
| Male | 2.5th | 6.42 | 6.1 | 6.7 |
| 97.5th | 16.5 | 16.2 | 16.8 | |
| Female | 2.5th | 6.55 | 6.3 | 6.8 |
| 97.5th | 16.27 | 16 | 16.5 | |
| Total | 2.5th | 6.5 | 6.3 | 6.7 |
| 97.5th | 16.38 | 16.2 | 16.6 |
Decade-wise analysis of over all total plasma Hcys levels showed steady increase of mean values from 20–29 years to advancing decades until 5th decade in reference population although it was not same for male and female (Table 3).
Table 3.
Decade-wise total plasma Hcys levels (μmol/L) in reference Indian male and female
| Age group in years | 20–29 | 30–39 | 40–49 | 50–59 | ≥60 | Over all |
|---|---|---|---|---|---|---|
| n | ||||||
| Male | 120 | 122 | 126 | 140 | 128 | 636 |
| Female | 122 | 138 | 142 | 126 | 124 | 652 |
| Total | 242 | 260 | 268 | 266 | 252 | 1288 |
| Total plasma Hcys | ||||||
| Male | 10.8 ± 1.9 | 11.2 ± 2.4 | 12 ± 3 | 11.6 ± 2.5 | 11.6 ± 2.6 | 11.46 ± 2.56 |
| Female | 10.9 ± 1.8 | 11.9 ± 1.9 | 11.3 ± 3.2 | 12 ± 2.4 | 11.6 ± 2.5 | 11.41 ± 2.48 |
| Total | 10.8 ± 1.9 | 11.2 ± 2.2 | 11.6 ± 3.2 | 11.8 ± 2.5 | 11.6 ± 2.5 | 11.44 ± 2.53 |
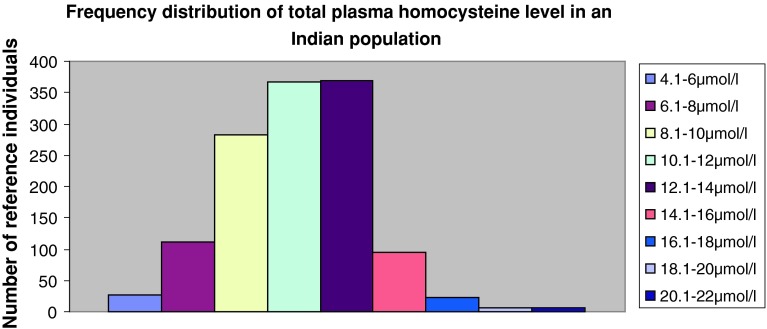
The frequency distribution of total plasma Hcys levels showed normal Gaussian distribution. (Figure 1).
Fig. 1.
Frequency distribution of total plasma homocysteine level in an Indian population
Discussions
Atherosclerosis induced cardiovascular disease is steadily increasing in South East Asian countries and also in India [32]. Retinal vein occlusion due to atherosclerosis has also become the 2nd most common retinal vascular disorder after diabetic retinopathy [33]. Apart from the traditional risk factors, Hcys was also found to be a predisposing factor for atherosclerosis [14, 15]. HHcys is not only associated with a greater risk of cardiovascular disease [17, 18] but also a risk factor for atherosclerosis in the retinal vasculature [19–21]. Folic acid, pyridoxine (vitamin B6), and cobalamin (vitamin B12) reduce homocysteine levels and may help to reverse endothelial injury associated with elevated total homocysteine [34]. So that Hcys level should be monitored routinely in all diagnostic laboratories for both the risk assessment and as follow-up investigations subsequent to the vitamin supplementations in atherosclerosis induced disease. The concentrations of Hcys is very much dependent upon ethnicity, specific dietary habits, and genetic make up, advancing age, gender, life style, and environmental factors. Current reference data on circulating Hcy concentrations are based on studies conducted on foreign population [28–30]. Reference data on Hcy concentrations based on a representative Indian sample are lacking. Therefore the study aimed to determine reference interval for total plasma homocysteine level in an Indian population.
Selhub j et al. [28] observed that reference ranges for serum total homocysteine concentration increased with age; these ranges were 4.3–9.9 μmol/L for male participants and 3.3–7.2 μmol/L for female participants of 12–19 years of age and from 5.9 to 15.3 μmol/L for men and 4.9 to 11.6 μmol/L for women of 60 years of age or older. A high homocysteine concentration was defined as at least 11.4 μmol/L for male participants and at least 10.4 μmol/L for female participants in their study.
Reference intervals were also showed to be similar for both genders in a Portuguese study. The reference range for homocysteine in young Portuguese adults was 6.2–11.6 μmol/L, regardless of gender [29].
Another study based on Greek children of 6–15 years of age showed no statistically significant difference in total Hcy level in between gender [30].
Similar results were also found in our study (Table 1). Total plasma Hcys levels showed steady increase of mean values from 20 to 29 years to advancing decades until 5th decade (Table 3) with the over all mean value in male, female, and total population were 11.46 ± 2.56, 11.41 ± 2.48 and 11.44 ± 2.53 μmol/L respectively. Although the decade-wise changes in total plasma Hcys levels did not show the steady increase in females. Differences in the body size and estrogen status may contribute to the differences in plasma total Hcy between male and female [35, 36] in our study.
Reference intervals for total plasma homocysteine in Indian population may benefit in future in formulating medical decision limits and the guidelines in predicting future risk for Coronary heart disease and retinal vascular disease and also open up the scope for further research on effect of varying physiological states (body size, estrogen concentration etc.) on Hcys.
Contributor Information
Kapil D. Lahiri, Phone: +919038405409, Email: kapildeb.lahiri@gmail.com
Himadri Datta, Email: himadri.datta@gmail.com.
Harendra N. Das, Email: haren_doc@yahoo.co.in
References
- 1.Tunstall-Pedoe H, Kuulsmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infraction and coronary deaths in the World Health Organization MONICA Project: registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.CIR.90.1.583. [DOI] [PubMed] [Google Scholar]
- 2.O’Flaherty FordE, Allender S, Scarborough P, Capewell S. Coronary heart disease trends in England and Wales from, 1984 to 2004: concealed leveling of mortality rates among young adults. Heart. 2008;94:178–181. doi: 10.1136/hrt.2007.118323. [DOI] [PubMed] [Google Scholar]
- 3.Marmot MG, Syme SL, Kagar A, Kato H, Cohen JB, Belsky J. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: prevalence of coronary and hypertensive heart disease and associated risk factors. Am J Epidemiol. 1975;102:514–525. doi: 10.1093/oxfordjournals.aje.a112189. [DOI] [PubMed] [Google Scholar]
- 4.Howard BV, Lee ET, Cowan LD, Fabsitz RR, Howard WJ, Oopik AJ, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians: the strong heart study. Am J Cardiol. 1996;78:1400–1405. doi: 10.1016/S0002-9149(96)00642-X. [DOI] [PubMed] [Google Scholar]
- 5.Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KGMM, et al. Heterogeneity of coronary heart disease risk factors in Indian Pakistani, Bangladeshi and European origin populations: cross sectional study. Br Med J. 1999;319:215–220. doi: 10.1136/bmj.319.7204.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronary heart disease statistics. London: British Heart Foundation; 2007. [Google Scholar]
- 7.Pekkanen J, Lenn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, et al. Ten year mortality from cardiovascular in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 8.Saddlemire AE, Denny CH, Greenlund KJ, Coolidge JN, Fan AZ, Croft JB. Trends in cholesterol screening and awareness of high blood cholesterol—United States, 1991–2003. CDC MMWR Morb Motal Wkly Rep. 2005;54:865–870. [PubMed] [Google Scholar]
- 9.Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol. 2001;153:490–499. doi: 10.1093/aje/153.5.490. [DOI] [PubMed] [Google Scholar]
- 10.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High Apolipoprotein B, low Apolipoprotein A–I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 11.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke a meta-analysis of observational studies. Stroke. 2007;38:1959–1966. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 12.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 13.Rahmani M, Raiszadeh F, Allahverdian S, Kiaii S, Navab M, Azizi F. Coronary artery disease is associated with the ratio of apolipoprotein A-I/B and serum concentration of apolipoprotein B, but not with paraoxonase enzyme activity in Iranian subjects. Atherosclerosis. 2002;162:381–389. doi: 10.1016/S0021-9150(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 14.McCully KS. Homocysteine, vitamins, and prevention of vascular disease. Mil Med. 2004;169(4):325–329. doi: 10.7205/milmed.169.4.325. [DOI] [PubMed] [Google Scholar]
- 15.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein JD. Inborn errors of sulfur-containing amino acid metabolism. J Nutr. 2006;136(suppl 6):1750S–1754S. doi: 10.1093/jn/136.6.1750S. [DOI] [PubMed] [Google Scholar]
- 17.Nygard O, Vollset SE, Refsum HM. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1536–1543. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 18.Boushey CJ, Beresford SAA, Wilson PW, Rush D, Rosenberg IH. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 19.Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Loewenstein A. Hyperhomocysteinemia in patients with nonarteric anterior ischemic optic neuropathy: central retinal artery occlusion and central retinal vein occlusion. Ophthalmology. 2000;107:1588–1592. doi: 10.1016/S0161-6420(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 20.Wenzler EM, Rademakers AJ, Boers GH, Cruysberg JR, Webers CA, Deutman AF. Hyperhomocysteine in retinal artery and retinal vein occlusion. Am J Ophthalmol. 1993;115:162–167. doi: 10.1016/s0002-9394(14)73919-4. [DOI] [PubMed] [Google Scholar]
- 21.Brown BA, Marx JL, Ward TP, Hollifield RD, Dick JS, Brozetti JJ, et al. Homocysteine: a risk factor for retinal venous occlusive disease. Ophthalmology. 2002;109(2):287–290. doi: 10.1016/S0161-6420(01)00923-X. [DOI] [PubMed] [Google Scholar]
- 22.Weiss N. Mechanisms of increased vascular oxidant stress in hyperhomocysteinemia and its impact on endothelial function. Curr Drug Metab. 2005;6(1):27–54. doi: 10.2174/1389200052997357. [DOI] [PubMed] [Google Scholar]
- 23.Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol. 2006;26(3):508–513. doi: 10.1161/01.ATV.0000201039.21705.dc. [DOI] [PubMed] [Google Scholar]
- 24.Jakubowski H. Pathophysiological consequences of homocysteine excess. J Nutr. 2006;136(Suppl 6):1741S–1749S. doi: 10.1093/jn/136.6.1741S. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen DW, Catanescu O, Barbato JC. Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clin Chem Lab Med. 2005;43(10):1076–1083. doi: 10.1515/CCLM.2005.188. [DOI] [PubMed] [Google Scholar]
- 26.Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr. 2006;136:1726S–1730S. doi: 10.1093/jn/136.6.1726S. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention: Laboratory Procedure Manual. Total Homocysteine (tHcy). Total homocysteine in plasma NHANES 1999–2000. Available from http://www.cdc.gov/nchs/data/nhanes/frequency/lab06_met_homocysteine.pdf [Updated 2008 July 7; cited 2011 Dec 9].
- 28.Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, et al. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131(5):331–339. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 29.Brandao MP, Pimentel FL, Cardoso MF. Serum homocysteine concentrations in Portuguese young adults reference interval. Acta Med Port. 2011;24:271–278. [PubMed] [Google Scholar]
- 30.Papandreou D, Mavromichalis I, Makedou A, Rousso I, Arvanitidou M. Reference range of total serum homocysteine level and dietary indexes in healthy Greek schoolchildren aged 6–15 years. Br J Nutr. 2006;96(4):719–724. [PubMed] [Google Scholar]
- 31.Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH, et al. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39:1764–1779. [PubMed] [Google Scholar]
- 32.Patel K, Bhopal R. The epidemic of coronary heart disease in South Asian populations: causes and consequences. Birmingham: South Asian Health Foundation; 2004. p. 164. [Google Scholar]
- 33.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Study. Trans Am Ophthalmol Soc. 2000;98:133–141. [PMC free article] [PubMed] [Google Scholar]
- 34.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–246. doi: 10.1016/0002-9343(94)90149-X. [DOI] [PubMed] [Google Scholar]
- 35.Brattstrom L, Lindgren A, Israelsson B, Anderson B, Anderson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Intern Med. 1994;236:633–641. doi: 10.1111/j.1365-2796.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 36.Wouters MG, Moorrees MT, van der Mooren MJ, Blom HJ, Boers GH, Schellekens LA, et al. Plasma homocysteine and menopausal status. Eur J Clin Invest. 1995;25:801–805. doi: 10.1111/j.1365-2362.1995.tb01687.x. [DOI] [PubMed] [Google Scholar]