Abstract
Breast cancer is one of the most frequent cancer types within women population. Hydroxyurea (HU) is a chemotherapy compound for treatment of patients with cancer diagnosis, including breast cancer associated with several adverse effects. In this study, we applied nanotechnology to decreased drug side effects along with improvement of therapeutic index. Liposomation is widely used in modern pharmacological developments in order to enhance the effects of the drugs. To achieve this, in this study a mixture of phosphatidylcholine and cholesterol was made up and HU was added to the resultant mixture, was then pegylated using Polyethylene Glycol 2000 to increase resistance, applicability and solubility. The mean diameters of nanoliposomal and pegylated nanoliposomal HU were measured by Zeta sizer device and obtained about 402.5 and 338.2 nm. The efficiency of non-pegylated and pegylated liposomal HU was 70.8 and 64.2, respectively. Releasing HU in both formulations was estimated about 25.8 and 21.7 %. Also, this study investigated the cytotoxicity effect of nanoliposomal and pegylated nanoliposomal HU using MTT assay. Results of this investigation showed that the cytotoxic properties of pegylated HU was 3.6 % more than those non-pegylated form, while was 38.93 % more than ordinary from of HU. This study showed that the stability, releasing pattern and cytotoxicity of the pegylated nanoliposomal HU is better than that of nanoliposomal HU.
Keywords: Breast cancer, Hydroxyurea, Liposome, Pegylated liposome
Introduction
Cancer is now one of the most common diseases around the world. Generally, breast cancer is amongst the most frequent types of cancer with about 18 % prevalence in women. In the other hand, it is considered as the main cause of mortality and morbidity in women [1, 2]. Current therapy methods of breast cancer such as surgery, radio therapy and chemotherapy improved survival of women suffering breast cancer [3]. Among chemotherapy agents, Hydroxyurea (HU) is commonly used as an anti-cancer agent [4]. For instance, HU is an effective reagent which was widely applied for treatment of acute angina, breast cancer, several hematological malignancies including chronic myelogenous leukemia (CML), sickle cell anemia, myeloproliferative disorders, and other known cancers previously [5–9]. In addition to its beneficial properties several disadvantages and side effects such as skin disorders were reported to be associated HU in consumers [10, 11].
In this context, some effective methods were developed to either lessen HU side effects or increase its efficiency. The liposomal technology is amongst successful and the newest revolutionized cancer diagnosis and treatment [12].
Presently, various drug carriers are applied in clinical trials and liposome is one of the widely accepted ones. Liposomes are colloidal, vesicular structures composed of one or more lipid bilayers surrounding an equal number of aqueous compartment [13]. Liposomes are able to pass successfully through biological barriers protection of drugs against destructive conditions and compounds, delivering the drug to the target organ and tissue and release a therapeutic load in the optimal dosage range of drug [14].
Therefore, present investigation aimed to provide pegylated and non pegylated liposomal form of HU to improve the drug therapeutic index as well as lessening its adverse effects.
Materials and Methods
Materials
The HU, phosphatidylcholine, cholesterol, polyethylene glycol 2000 (PEG 2000) and MTT (0.5 mg/ml), all were purchased from sigma company (SIGMA, USA). Ethanol and Isopropanol purchased from Merck Company (Merck, Germany). The RPMI-1640 culture medium and breast cancer cell line (MCF-7) were purchased from Invitrogen (Invitrogen, USA) and cell bank of Pasteur institute Iran, respectively.
Preparation of Pegylated and Non-pegylated Liposomal Drug
To produce pegylated liposomal HU, phosphatidylcholine and cholesterol (1:10) were dissolved in 10 ml of ethanol 98 % and the resultant was centrifuged at 300 rpm at room temperature to gain a transparent, yellow suspension. Two different formulations of liposomal drug and pegylated liposomal drug were prepared. To prepare liposomal drug, the upper phase of the resultant solution, using rotary evaporator (Heidoiph, Germany), was discarded, and then normal saline and 8 mg HU was added to the final solution and followed by a second centrifugation at 300 rpm at room temperature. A control solution was also prepared without HU. In addition, two solutions similar to prepared solutions were pegylated using PEG 2000 and were sonicated (Bandelin Sonorex Digitec, 60 HZ) for 5 min to reduce the size of liposomes [15]. The size of particles was also measured by zeta sizer system (Malvern Instruments Ltd.) [16].
Entrapment Efficiency
To determine the amounts of encapsulated HU, 1 ml (contains a 32 mg of HU) of each formulations were centrifuged at 13,000 rpm for 2 h at 4 °C and the optical density of the upper phase of solutions were measured at 214 nm wavelength by Spectrophotometer (UV-160IPC, Shimadzu, Japan). The following formula was used for calculation of the encapsulation [17].
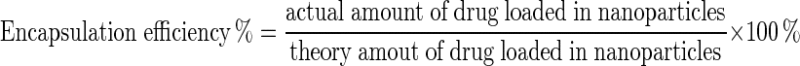 |
To obtain respected standard curve, different concentrations of HU were prepared and their optical density were measured at 214 nm wavelength using Spectrophotometric method.
In Vitro Release Study
For the assessment of the HU release pattern from liposomes, 1 ml (contains a 32 mg of HU) from each formulation (liposomal drug and pegylated liposomal drug) and 10 ml PBS (pH 7.4) were added to the dialysis bags and bags were left on a shaker for 24 h at 37 °C, finally the amounts of released HU in PBS were measured using Spectrophotometric method and regarding the rate of HU releasing was obtained by standard curve [18].
Cytotoxicity Assay
100 μl of MCF-7 cells suspension contained 10,000 cells was added to the well of 96-well plates and incubated at 37 °C, 5 % CO2. Following 24 h, the culture media was removed and substituted various concentrations of different drug formulations (liposomal HU and its control, pegylated liposomal HU and its control) and then incubated for a further 24 h. Subsequent to 24 h again culture supernatants were removed and MTT solution was added and after 3 h of incubation, the amethyst crystal (formazan) was dissolved in 100 μl Isopropanol. The optical absorption was measured at 570 nm by a spectrophotometer and IC50 was calculated using Pharm software [19].
Statistical Analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data were statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and significant difference was set at p < 0.05.
Results
Determination of Particle Sizes
The mean diameter of liposomes for liposomal HU was 402.5 nm and for pegylated liposomal HU was 338.2 nm.
Entrapment Efficiency
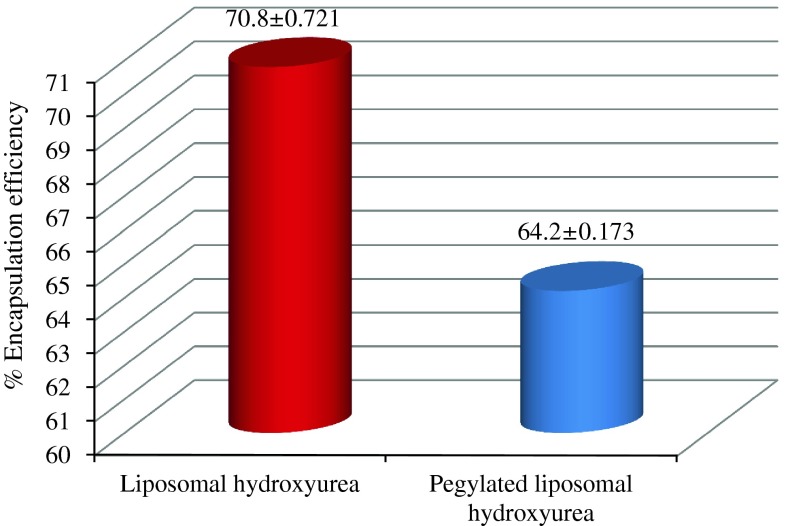
The encapsulation percentage was calculated according to the standard curve for each drug formulation. Using the encapsulation formula (Formula 1), the amount of non-encapsulated was subtracted from the initial amount, divided to the total drug amount and multiplied to 100. Our results demonstrated that the percent of encapsulation was 64.2 and 70.8 % for pegylated and non-pegylated liposomal drugs, respectively (Fig. 1).
Fig. 1.
Shows the entrapment efficiency of pegylated and non pegylated liposomal drug formulations
In Vitro Release Study
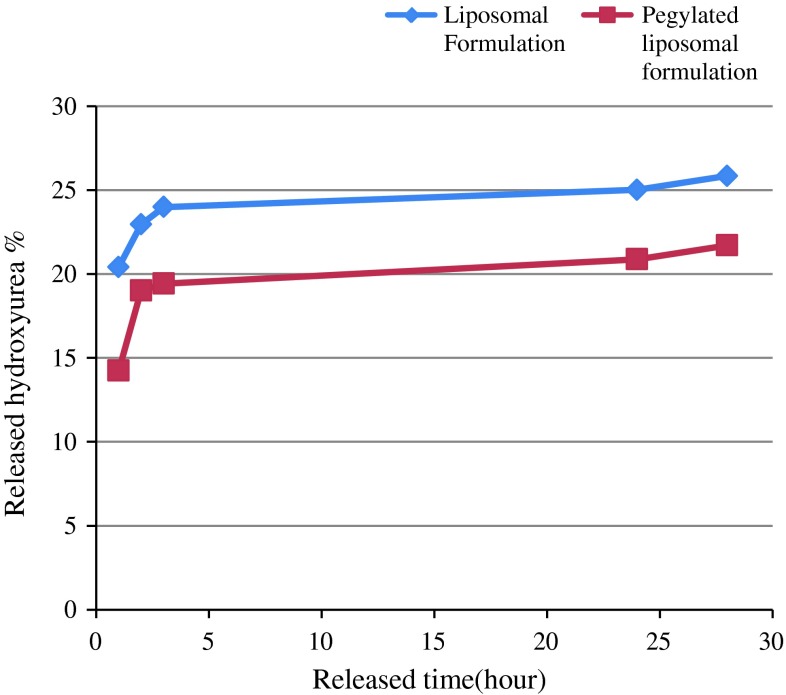
The releasing of HU from the two different formulations (liposomal HU and pegylated liposomal HU) in PBS buffer was determined after 1, 2, 3, 24 and 28 h (Fig. 2). Our findings indicated that 25.85 and 21.71 % of the drug were released into the PBS in liposomal formulation and pegylated liposomal formulation, respectively.
Fig. 2.
Indicated the rate of drug release in different formulations (liposomal and pegylated liposomal) at various time points
Cytotoxicity Assay
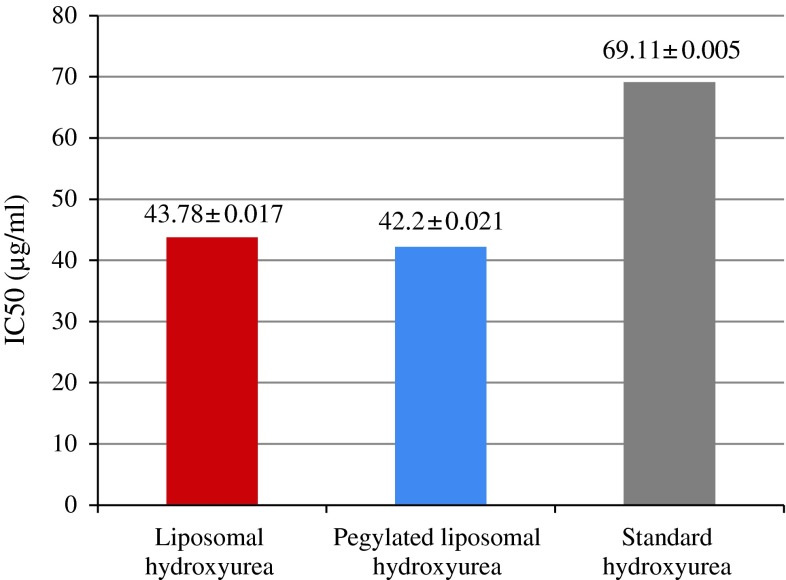
Figure 3 indicates the respected IC50 for different forms of HU including standard drug, liposomal drug and pegylated liposomal form.
Fig. 3.
Indicates the respected IC50 for different forms of HU including standard HU, liposomal HU and pegylated liposomal HU
Discussion
It is now well evidenced that carriers have a potential capability to deliver therapeutic reagents to the cancer tissues or organs [20]. The targeted drug delivery is considered as a promising method for treatment of several disease states. The availability of lipidic carriers especially liposome for many pharmacological therapeutic compounds makes them promising targets for therapeutic reasons. Due to the fact that it is easier to target a cancerous tissue and thus decreases the toxicity of drugs in neighboring healthy tissues in comparison with other carriers, liposomal carriers were used [21].
Promising results of liposomal based carriers in therapy or prevention of a wide spectrum of diseases in laboratory animals and human disorders are indicative for prominent role will play by these compounds in near future [22]. Liposomes are differentiated from the other types of carriers by following properties: (a) they can easily pass through several biological barriers and also have the ability to protect drugs against destructive reagents and conditions (b) liposome carriers have the ability of targeted delivery [14].
Rom and colleagues demonstrated the increasing therapeutic efficiency of Doxorubicin in liposomal in comparison with standard form on melanoma cell lines [23].
In addition a similar study by Chen et al. [24] indicated that Adriamycin in liposomal form targeted diffuse breast cancer cells, however, not only massive amounts of Liposomes were absorbed by tumor cells, but also the rate of cellular toxicity was significantly increased. Interestingly, any type of allergic reaction or toxicity was not observed by the investigators in patients. In another study undertaken by Giota and colleagues, it is demonstrated that the therapeutic effects of liposomal Doxorubicin were more than the free form of the drug.
In more recent investigations, Chi-Chang Chang and his research team reported that liposomal curcuminoids were effective on several breast cancer cell lines, including MCF-7, MDA-MB-4355, MDA-MB-231 and also liposomal form of drug decreased the rate of cell survival [21].
In the present study we examined the effects of liposomal HU and pegylated liposomal HU on the MCF-7 breast cancer cell line. The technology of liposome pegylation is a new method for improvement of pharmacokinetics of therapeutic proteins [25]. Pegylated liposomes covalently bind to proteins as the carrier and several lines of evidence demonstrated that pegylated therapeutic compound are more stable in circulation and are not easily removed by reticuloendothelial system (RES) and this formulation increases the adherence of drug to the cancer cell. In the other words it will result in enhancement of cellular drug absorption [9, 26–29].
Irma and colleagues showed that the efficiency and pegylated liposome at high-doses Ciprofloxacin is well tolerated by rat [30]. In more recent studies Yatuv Rate and colleagues demonstrated that pegylation Liposomes of both forms increased pharmacokinetics of these formulations [25].
Conversely, enhanced efficiency of standard form of Oxaliplatin compared to its pegylated form has been reported by Yang et al. [31].
In the present research we assessed the toxicity of HU in liposomal formulation and pegylated liposomal formulation by MTT analysis.
Determination of the diameter size by zeta sizer (DLS) demonstrated that the size of particles in liposome samples compared to its pegylated form is 64.3 nm smaller. It appears that PEG 2000 causes a more regular structure of liposome as well as due to variation in liposome load and PEG 2000, the pegylated form is smaller.
Our results with regard to encapsulation indicated that the size was decreased approximately 6.5 % in encapsulated form. This reduction is as a result of preventative effects of PEG 2000 on drug penetration. It is also likely that polyethylene residues penetrate into the liposome and decrease free spaces of liposomal structure and in turn lower amounts of HU liposomated later.
To determine the release rate of HU at different times we applied dialysis and showed that release process includes two various phases, quick and slow diffusion. We showed that the maximum release occurred during the first two hours. Initial release of liposomal drug was faster than pegylated liposomal drug. Consistent with our finding Mu and Feng [32] indicated that the initial release rate decreases with enhanced leading rate.
The cytotoxic effects of both formulations (liposome and pegylated liposome) were determined by MTT on MCF-7 cells. Our findings showed that liposome and pegylated form, without drug, had no cytotoxic effects on MCF-7 cells and IC50 of HU liposomal form was more than its pegylated form and both of them had increasing in IC50 compare to standard HU. Obviously, pegylation decreased IC50 and this can come from inhibitory effects of PEG, Additionally, PEG demonstrated the increasing solubility of drug and consequently better contact with the target cells.
Conclusion
Nanoliposomal and pegylated nanoliposomal formulation of paclitaxel were successfully prepared by reverse phase evaporation method. The results showed that the appropriate amount of drugs was encapsulated and cytotoxicity of these two formulations (liposomal and pegylated liposomal) was better than free drug. Furthermore, the in vitro release study indicated that new formulation of paclitaxel have a slower releasing pattern.
Contributor Information
Soheil Ghassemi, Email: ghassemi_so@yahoo.com.
Azim Akbarzadeh, Phone: +00982166465406, FAX: +00982166465132, Email: azimakbarzadeh1326@gmail.com.
References
- 1.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 2.Vo AT, Millis RM. Epigenetics and breast cancers. Obstet Gynecol Int. 2012;2012:602–620. doi: 10.1155/2012/602720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–417. doi: 10.1016/j.biotechadv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Friedrisch JR, Prá D, Maluf SW, Bittar CM, Mergener M, Pollo T, et al. DNA damage in blood leukocytes of individuals with sickle cell disease treated with hydroxyurea. Mutat Res. 2008;649:213–220. doi: 10.1016/j.mrgentox.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, et al. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;17(148):939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meo A, Cassinerio E, Castelli R, Bignamini D, Perego L, Cappellini MD. Effect of hydroxyurea on extramedullary haematopoiesis in thalassaemia intermedia: case reports and literature review. Int J Lab Hematol. 2008;30:425–431. doi: 10.1111/j.1751-553X.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 8.Rice L, Baker KR. Current management of the myeloproliferative disorders: a case-based review. Arch Pathol Lab Med. 2006;130:1151–1156. doi: 10.5858/2006-130-1151-CMOTMD. [DOI] [PubMed] [Google Scholar]
- 9.Cervantes F, Mesa R, Barosi G. New and old treatment modalities in primary myelofibrosis. Cancer J. 2007;13:377–383. doi: 10.1097/PPO.0b013e31815a7c0a. [DOI] [PubMed] [Google Scholar]
- 10.Zaccaria E, Cozzani E, Parodi A. Secondary cutaneous effects of hydroxyurea: possible pathogenetic mechanisms. J Dermatolog Treat. 2006;17:176–178. doi: 10.1080/09546630600780494. [DOI] [PubMed] [Google Scholar]
- 11.Sirieix ME, Debure C, Baudot N, Dubertret L, Roux ME, Morel P, et al. Leg ulcers and hydroxyurea: forty-one cases. Arch Dermatol. 1999;135:818–820. doi: 10.1001/archderm.135.7.818. [DOI] [PubMed] [Google Scholar]
- 12.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 13.Maurer N, Fenske DB, Cullis PR. Developments in liposomal drug delivery systems. Expert Opin Biol Ther. 2001;1:923–947. doi: 10.1517/14712598.1.6.923. [DOI] [PubMed] [Google Scholar]
- 14.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17:367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Yang T, Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, et al. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J Control Release. 2007;120:169–177. doi: 10.1016/j.jconrel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Patel R, Singh SK, Singh S, Sheth NR, Gendle R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J Pharm Sci Res. 2009;1:71–80. [Google Scholar]
- 17.Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–4033. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Jawahar N, Venkatesh DN, Sureshkumar R, Senthil V, Ganesh GNK, Vinoth P, et al. Development and characterization of PLGA-nanoparticles containing carvedilol. J Pharm Sci Res. 2009;1:123–128. [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Mohanty C, Das M, Kanwar JR, Sahoo SK. Receptor mediated tumor targeting: an emerging approach for cancer therapy. Curr Drug Deliv. 2011;8:45–58. doi: 10.2174/156720111793663606. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Yang WT, Ko SY, Hsu YC. Liposomal curcuminoids for transdermal delivery: iontophoresis potential for breast cancer chemotherapeutics. Dig J Nanomater Bios. 2012;7:59–71. [Google Scholar]
- 22.Goyal P, Goyal K, Vijaya Kumar SG, Singh A, Katare OP, Mishra DN. Liposomal drug delivery systems–clinical applications. Acta Pharm. 2005;55:1–25. [PubMed] [Google Scholar]
- 23.Eliaz RE, Nir S, Marty C, Szoka FC., Jr Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res. 2004;64:711–718. doi: 10.1158/0008-5472.CAN-03-0654. [DOI] [PubMed] [Google Scholar]
- 24.Chen JH, Ling R, Yao Q, Li Y, Chen T, Wang Z, et al. Effect of small-sized liposomal Adriamycin administered by various routes on a metastatic breast cancer model. Endocr Relat Cancer. 2005;12:93–100. doi: 10.1677/erc.1.00871. [DOI] [PubMed] [Google Scholar]
- 25.Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia. Int J Nanomedicine. 2010;5:581–591. doi: 10.2147/ijn.s8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA. 1988;85:6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–225. [PubMed] [Google Scholar]
- 28.Zalipsky S, Brandeis E, Newman MS, Woodle MC. Long circulating, cationic liposomes containing amino-PEG-phosphatidylethanolamine. FEBS Lett. 1994;353:71–74. doi: 10.1016/0014-5793(94)01013-7. [DOI] [PubMed] [Google Scholar]
- 29.Cheong I, Zhou S. Tumor-specific liposomal drug release mediated by liposomase. Methods Enzymol. 2009;465:251–265. doi: 10.1016/S0076-6879(09)65013-8. [DOI] [PubMed] [Google Scholar]
- 30.Bakker-Woudenberg IA, ten Kate MT, Guo L, Working P, Mouton JW. Improved efficacy of ciprofloxacin administered in polyethylene glycol-coated liposomes for treatment of Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 2001;45:1487–1492. doi: 10.1128/AAC.45.5.1487-1492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C, Liu HZ, Fu ZX, Lu WD. Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol. 2011;11:21. doi: 10.1186/1472-6750-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu L, Feng SS. PLGA/TPGS nanoparticles for controlled release of paclitaxel: effects of the emulsifier and drug loading ratio. Pharm Res. 2003;20:1864–1872. doi: 10.1023/B:PHAM.0000003387.15428.42. [DOI] [PubMed] [Google Scholar]