Abstract
In the present study, the effect of red (Gracillaria corticata), green (Ulva fasciata) and brown (Sargassum ilicifolium) seaweeds alcoholic extract, against five important human cancer cell lines (MCF-7, MDA-MB-231, HeLa, HepG2, and HT-29) proliferation, apoptosis and cell cycle arrest were evaluated. The reducing activity and total polyphenol content were also investigated. MTT assay was used for cytotoxicity test. Morphological alterations were examined using phase contrast, fluorescent and electron microscopy. All the extracts were antiproliferative against all the cancer cell lines, dose-dependently, with G. corticata methanol extract (GCME) having the greatest inhibition activity against MCF-7 cell line. The percentage of apoptosis increased from 18 to 78 %. The cell cycle analysis also showed that GCME can induce apoptosis which confirm by TEM. Algal extract reducing activities were as follows: G.corticata > S. ilicifolium > U. fasciata. The GCME is a good source of potential complementary and alternative functional food for prevention and treatment of cancer.
Keywords: Antioxidant, Apoptosis, Cytotoxicity, Persian Gulf, Seaweeds
Introduction
Seaweeds are considered very attractive sources for the screening of biologically active compounds, due to their huge biodiversity and safety, as they have long been used in traditional Asian foods and folk medicine. Many kinds of crude or partially purified polysaccharides from various algae were tested for and they showed antitumor activity against experimental tumours [1, 2]. The epidemiological data are supported by animal model studies showing protective effects of dietary algae against skin, intestinal [3–5] and mammary [3, 6] cancer. Seaweeds have been reported to reduce the risk of cancer in animal studies, which may involve effect on cell proliferation and antioxidant activity. The mechanism of anticancer activity through which algae exert their effects is complex because of their remarkable structural diversity, which entails multiple interactions [3, 7].
Various dietary antioxidants have shown remarkable promise as effective agents for chronic disease prevention and treatment by reducing oxidative stress, which has been involved in the development of many chronic diseases including cancer. Therefore modification in dietary habits for reducing the incidence of these diseases, especially by increasing consumption of functional foods rich in antioxidants are increasingly supported.
An algal antioxidant-mediated mechanism [8] enhances the host’s defence by increasing natural killer-cell activity [9], activation of nonspecific immune system [2, 10], inhibition of the cell growth in the G1 phase, inducing terminal differentiation [5, 11, 12], inhibition of the complex process of angiogenesis [13] and induction of apoptosis [14] were hypothesized as a contributing factors in the inhibition of carcinogenesis.
Chemical composition of edible seaweeds varies with species, habitats, maturity and environmental conditions like climate [15]. In recent years, there has been increasing demand for seaweed products, as anticancer drugs. Numerous mechanisms for modulation of carcinogenesis by seaweed have been proposed; however, as yet none have been established, therefore there is a need to investigate the anti-proliferative effect of these. Accordingly the effect of three species namely, Gracillaria corticata (Rhodophyta), Ulva fasciata (Chlorophyta) and Sargassum ilicifolium (Phaeophyta), obtained from Persian Gulf waters (Sistan-o-Balochestan province-Chabahar City), was investigated on five important cancer cell lines MCF-7, (human breast carcinoma cell line, oestrogen positive) MDA-MB-231, (human breast carcinoma cell line, oestrogen negative) HeLa, (human cervical adenocarcinoma cell line) HepG2, (human hepatocellular carcinoma cell line) and HT-29, (human colon carcinoma) to determine in vitro apoptosis and cell cycle effects.
Materials and Methods
Raw Materials
Specimens of the three seaweed species from the coastal areas of Chabahar (Sistan-o-Balochestan province, Iran) were washed and stored at −20 °C. Ground, freeze-dried seaweed samples were methanol-extracted [15], filtered and rotary-evaporated at 40 °C to give a viscous mass (stored at −20 °C).
Chemicals
The 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma–Aldrich Canada (Oakville, ON). RPMI 1640 growth medium, l-glutamine, sodium bicarbonate, non-essential amino acids, sodium pyruvate, foetal bovine serum (FBS) and phosphate buffered saline (PBS) were obtained from Invitrogen Corporation (Burlington, ON), methanol was from Merck (Damstadt, Germany). All reagents were of analytical grade.
Evaluation of Antioxidant Activity Using TPTZ
The FRAP (Ferric reducing antioxidant power assay) procedure which described by Benzie and Strain was followed [16].
Measurement of Total Phenolic Contents
Total phenolic were determined calorimetrically using Folin–Ciocalteu reagent as described by Velioglu et al. [17] with slight modifications.
Cell Lines and Cell Culture
A normal cell line Vero cell (ATCC CCl-81, African green monkey kidney cell line) was used to test the cytotoxic effect of crude extracts. Vero cell was cultured in the growth medium (GM) that contained the Eagle’s Minimum Essential Medium (MEM, GIBCO, USA), supplemented with 10 % FBS and 0.1 % gentamicin, 20 mM HEPES and 2 mM of glutamine and harvested and plated in flat bottom 96-well plates at the density of 1.5 × 103 cell/ml in GM and incubated at 37 °C in a humidified incubator containing 5 % CO2 for 24 h. After removing old medium, the cells were treated with varying concentrations (0, 300 μg/ml) of test samples solution in MEM containing 1 % FBS (MM) and incubated at 37 °C for 3 days. Cells overlaid with only MM were used as control. The morphological alterations of the cells in their shape, level of adhesion were inspected daily under inverted microscope (Nikon CMM 214, Japan). The maximal non-toxic concentration (MNCC), defined as the maximal concentration of the extract that did not exert toxic effect detected by microscopic monitoring, was recorded at the end of treatment.
Human cancer cell lines, purchased from the American Type Culture Collection (ATCC, Rockville, MD), were cultured in RPMI 1640 medium (GIBCO, USA), and supplemented with FBS and gentamicin as above. The anti proliferative activities were determined by the MTT assay. In brief, cells were plated in 96-well plates (1 × 10³ cells per well), incubated for 24 h at 37 °C, were treated with various concentrations of seaweed extract and incubated for 24, 48, and 72 h. MTT solution was added and the optical density was read with an ELISA reader.
Morphological Assessment of Apoptosis
In this experiment, morphological alterations induced by extracts were examined using phase contrast, fluorescent and electron microscopy.
Scanning Electron Microscopy (SEM)
In each T-plate, treated and untreated cells were washed with PBS, fixed and dehydrated, then mounted onto stubs using standard procedure before coating with gold in a sputter coater for 1.5 min and viewed immediately using SEM or stored in a silica gel desiccators.
Transmission Electron Microscopy (TEM)
The adhered cells were detached, solidified, fixed, and dehydrated, then infiltrated with acetone: resin mixture, using standard procedure and were embedded for polymerization. Thick sectioning (1 μm) were performed using glass knife on the ultra microtome. The sections were stained and examined under light microscope for ultrathin sectioning, and specific areas were selected, picked up, stained, washed, and finally were stained with lead acetate and washed with double distilled water before viewing under the transmission electron microscope.
Acridine Orange (AO) and Propidium Iodide (PI) Staining
MCF-7 cells (plated at a density of 5 × 105 cells in a 35 mm dish) were exposed to GCME, then harvested with the PI DNA stain according to the kit protocol, or stain with AO and PI for observation of chromatin condensation The percentage of viable, necrotic and apoptotic population for each time point were determined from more than 500 cells. The population of each cell cycle phase were counted by flow cytometry (FACScan flow cytometer with Cell Quest software).
Hoechst 33342 Staining
Treated and untreated cells were fixed, washed, and incubated for 15 min at 37 °C with Hoechst 33342 dye (5 μg/ml in PBS). Cells are visualized using an Olympus BHZ, RFCA microscope (Japan) equipped with fluorescent light source with an excitation wavelength of 330 nm and a barrier filter of 420 nm. A minimum of 200 cells are counted at the same preparation but five different areas. Experiments are repeated at least three different times and classified in the following criteria: Live cells: normal nuclei, blue/green pale chromatin with organized structure, apoptotic cells:(early apoptotic cells) can be identified by the presence of chromatin condensation within the nucleus and intact nuclear boundaries, bright blue chromatin that is highly condensed, marginated; (late apoptotic cells) exhibit nuclear fragmentation into smaller nuclear bodies within an intact cytoplasmic membrane.
Statistics Analysis
All data were expressed as mean ± SEM. One-way analysis of variance (ANOVA; SPSS 16.0 for Windows; SPSS Inc., Chicago, IL) was used to test for differences between different treatment concentrations as well as between seaweed species. Where differences did exist, the source of the differences at a p < 0.05 significance level was identified by the Student–Newman–Keuls multiple range test.
Results
Antioxidant Activity and Total Phenolic Contents
The results of antioxidant activity of seaweeds extract by using FRAP (ferric reducing antioxidant power) assay expressed as FRAP value. These values represented mmol. FeII per 100 g dried plant (Table 1). Table 1 also shows total phenolic contents of three species of seaweeds using Folin–Ciocalteu method. Total phenolic contents are expressed as mg gallic acid equivalents per 100 g dried plant.
Table 1.
Antioxidant activity (mmol FeII/100 g dried plant), total phenolic contents (mg gallic acid/100 g dried plant), IC50 values (μg/ml), time course study of MCF-7 cell population incubation with 0.1 % DMSO (control group), or 30 μg/ml GCME determined from AO/PI staining, and percentage of each phase of cell cycle in 0 μg/ml (control), 30 μg/ml, 60 μg/ml, and 120 μg/ml GCME treatment group for 48 h
| Algae | Gracillaria corticata | Sargassum ilicifolium | Ulva fasicata | |
|---|---|---|---|---|
| Total phenolic contents | 13.00 ± 0.25 | 55.95 ± 4.33 | 7.30 ± 0.27 | |
| Antioxidant activity | 77.32 ± 11.36 | 11.08 ± 1.03 | 0.96 ± 0.54 | |
| IC50 (μg/ml) | 24 | 30 ± 0.2 | 37 ± 0.5 | 50 ± 0.2 |
| Time (h) | 48 | 27 ± 0.4 | 37 ± 0.1 | 43 ± 0.3 |
| MCF-7 | 72 | 25 ± 0.3 | 37 ± 0.2 | 33 ± 0.4 |
| HeLa | 24 | 37 ± 0.2 | 60 ± 0.2 | 77 ± 0.3 |
| 48 | 32 ± 0.4 | 53 ± 0.1 | 67 ± 0.7 | |
| 72 | 27 ± 0.3 | 45 ± 0.9 | 54 ± 0.2 | |
| MDA-MB-231 | 24 | 53 ± 0.5 | 62 ± 0.2 | 104 ± 0.5 |
| 48 | 53 ± 0.4 | 52 ± 0.4 | 94 ± 0.8 | |
| 72 | 45 ± 0.2 | 42 ± 0.8 | 84 ± 0.6 | |
| HepG2 | 24 | 102 ± 0.2 | 120 ± 0.2 | 90 ± 0.9 |
| 48 | 97 ± 0.4 | 120 ± 0.8 | 100 ± 0.7 | |
| 72 | 92 ± 0.1 | 110 ± 0.5 | 100 ± 0.1 | |
| HT-29 | 24 | 250 ± 0.0 | 300 ± 0.7 | >400 |
| 48 | 220 ± 0.1 | 350 ± 0.8 | >400 | |
| 72 | 130 ± 0.2 | 280 ± 0.3 | >400 | |
| Time (h) | %Viable | %Apoptotic | %Necrotic | |
| Control MCF-7 + 0.1 % DMSO | 0 | 98 + 0.3 | 0.6 + 0.2 | 0.3 + 0.3 |
| 24 | 92.6 ± 0.1 | 5.0 ± 1.7 | 2.1 ± 0.6 | |
| 48 | 91.0 ± 1.2 | 7.0 ± 1.2 | 2.0 ± 0.5 | |
| 72 | 85.3 ± 1.4 | 9.9 ± 2.2 | 5.1 ± 1.3 | |
| Treatment MCF-7 + 30 μg/ml GCME | 0 | 98.8 + 0.3 | 0.5 + 0.3 | 0.6 + 0.4 |
| 24 | 48.8 ± 1 | 51.87 ± 6.6* | 1.1 ± 0.0 | |
| 48 | 25.8 ± 1.6 | 67.5 ± 3.3* | 6.7 ± 1.8 | |
| 72 | 12.4 ± 4.7 | 78.7 ± 1.7* | 8.3 ± 2.5 | |
| % of each phase | ||||
| Group | Sub G1 | G1 | S | G2/M |
| Control | 5.25 ± 1 | 60.54 ± 2.1 | 28.70 ± 4.7 | 9.20 ± 1.2 |
| 30 μg/ml GCME | 9.50 ± 1.1* | 53.60 ± 4.1 | 28.2 ± 5.9 | 9.99 ± 1 |
| 60 μg/ml GCME | 14.2 ± 1.8* | 49.1 ± 3.6 | 29.2 ± 4.3 | 8.18 ± 1.3 |
| 120 μg/ml GCME | 25.1 ± 2* | 47.20 ± 8.1 | 29.1 ± 5.1 | 7.60 ± 2.1 |
Values are expressed as mean ± standard deviation, n = 3
*Significantly different at p < 0.05
Cytotoxicity on Normal and Cancer Cell
The results of the cytotoxicity tested by observing cellular morphological change showed that metanolic extract of all these three seaweed species tested had no toxicity to normal cell Vero cell line, but dose- and time-dependently inhibited the proliferation of the five cancer cell lines. (Table 1). The IC50 values calculated for (GCME) on MCF-7, HeLa, MDA-MB-231, HepG2, and HT-29 cells was 30 ± 0.2, 37 ± 0.2, 53 ± 0.5, 102 ± 0.2, and 250 ± 0.0 μg/ml after treatment for 24 h, respectively.
Microscopic Results
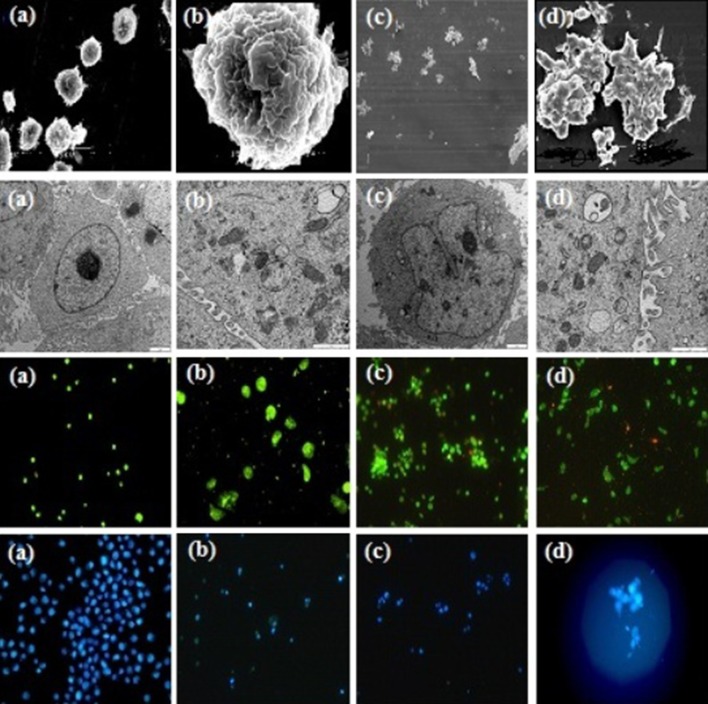
The series of photographs in Fig. 1 illustrates some of the possible morphologies seen with SEM, TEM, AO/PI staining, and Hoechst 33342 nuclear staining and fluorescence microscopy. In observation under SEM, typical apoptotic characteristics were found, including cell membrane blebing, microvillus disappearance or reduction, and separated apoptotic bodies. In addition, treated MCF-7 cells were observed under TEM, and shrinkage of cells, and condensation of chromosomes was found. Figs. 2, 3.
Fig. 1.
Morphological changes of MCF-7 cells under SEM, TEM, and Fluorescence microscope. a, b MCF-7 cells control group. c, d MCF-7 cells treated with GCME
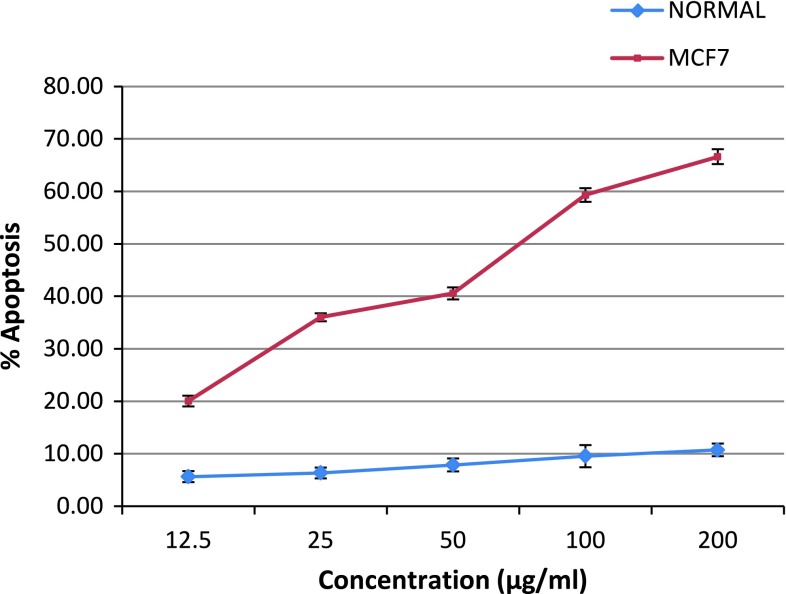
Fig. 2.
Dose course study of MCF-7 cell population incubation with 0.1 % DMSO (normal group) and human breast cancer (MCF-7) cells treated with GCME (0–300 μg/ml). Apoptosis was evaluated using fluorescence microscopy by calculating the percentage of cells showing nuclear morphology of apoptosis after staining with Hoechst 33342. Results were obtained from three independent experiments and expressed as mean ± SD
Fig. 3.
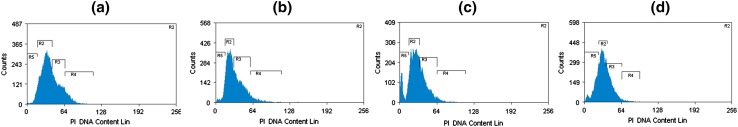
Cell cycle analysis of MCF-7 cells treated with GCME by flow cytometry. MCF-7 cells were incubated with various concentration of GCME a 0 μg/ml (control), b 30 μg/ml, c 60 μg/ml, d 120 μg/ml for 48 h. The cells were then stained with PI and analysed by flow cytometry
Intact membranes exclude PI but allow the uptake of AO, which bind to double stranded DNA and fluoresces green under 488 nm excitation. Untreated cells show a diffuse green fluorescence, while in apoptotic cells; condensed chromatin material resulted in clumps of intense green fluorescent spots within the cell. The characteristic condensation patterns observed were the crescent shape at the nuclear periphery and the more numerous round clumps. Purely necrotic cells stain red with PI with no green fluorescence evident. At specific time intervals (24, 48, and 72 h), the proportion of normal, apoptotic and necrotic cells were scored as a percentage of the total cell population counted and showed in Table 1. In MCF-7 cells treated with GCME, apoptotic cells increased from 0.6 % before treatment to 51 % after 24 h, 67 % after 48 h, and 78 % after 72 h. Percentages of apoptotic cells increased from 18 to 78 % by increasing concentration of seaweeds extract from 15 to 300 μg/ml. This increase did not exist in control cells.
Cell Cycle Analysis
In cells treated with apoptosis inducing agents, a sub population of cells appears before the G1 peak and is referred to as the sub-G1 peak, which is believed to the result of endonuclease activation and subsequent leakage of DNA from the cells. Necrotic cells do not show the immediate reduction in DNA content so a distinction can be made. As shown in Table 1, the sub-G1 population, which indicated apoptotic cells, increased in dose dependent manner from 5.25 % at 0 μg/ml (control) to 9.53 % at 30 μg/ml, 14.2 % at 60 μg/ml, and 25.1 % at 120 μg/ml, after exposure to GCME for 48 h. Although the G1 population decreased along with an increase of sub-G1, the other portion of non-apoptotic cells did not show a significant change.
Discussion
Recently, much attention has been paid to marine organisms for the screening of biologically active compounds. Among these marine organisms, seaweeds are considered to be very attractive sources, due to their huge biodiversity, safety and as they have long been used in traditional Asian foods. Although several reports have suggested that crude seaweed extracts have antiproliferative activity in cancer cell lines, most studies focused on their antioxidant activity. Water-soluble polysaccharides, such as laminarans and fucoidans, are representative anticancer substances extracted from seaweeds [4].
Phenolic compounds mostly found in plants, are reported to have several biological effects including antioxidant, antiapoptosis, anti-aging and anti carcinogenic properties and are therefore considered for their important dietary roles as antioxidants and chemoprotective agents.
The present study is the first to report the anticancer activities of extracts from the Persian Gulf seaweed species, red (G. corticata), green (U. fasciata) and brown (S. ilicifolium), using five cancer cell lines model. Our results of cytotoxicity studies showed that the seaweed extracts had no toxic effect on the Vero cells. On the other hand, they significantly affect all five human cancer cell lines. MCF-7 cell line was the most sensitive and HT-29 was the most resistant. These results confirmed that the extracts of seaweeds selectively inhibited the growth of particular cell types or tumour types. In the report of Harada et al. [18], 47 species of alga exhibited strong cytotoxic activity against L1210 cells. They also showed similar cytotoxicity with low cytotoxicity to normal cells. Such selective activity was also conspicuous in other seaweeds reported in their experiment. All these results together with this study suggested that the active substances interact with special cancer-associated receptors or cancer cell special molecule, thus triggering some mechanisms that cause cancer cell death [18].
Previous studies reported that hot-water extracts of several brown algae were effective against some mouse cancer cells and the active principle was found to be polysaccharide fraction. Among 306 species of seaweeds involved in the screening experiment of Harada et al. [18], a remarkably selective cytotoxic activity was found in MeOH extract from a red alga, Amphiroa zonata. In this study it is reported that the chemical properties of the active substance present in the extract was heat stable and soluble in relatively higher polar organic solvents and water. The molecular weight was less than 3000, which shows it is different from the substances such as polysaccharides reported from other studies [18, 19].
Induction of apoptosis is a useful approach in cancer therapies. In apoptotic cells, several cellular and molecular biological features, such as cell shrinkage, DNA fragmentations, and activation of the caspase cascade, are exhibited [20].
In our study typical apoptotic characteristics were found when treated cells were observed under SEM and TEM, including cell membrane blebing, microvillus disappearance or reduction, shrinkage of cells, condensation of chromosomes and apoptotic bodies with complete membrane.
Cytotoxicity of marine algal extracts was evaluated by growth inhibition. When the growth inhibited cells were stained with AO/PI and Hoechst 33342 apoptotic cell death was observed in time and dose depended manner in all cultures. These results suggested that GCME caused irreversible cell damages in cultured cells.
Regulation of the cancer cell cycle is one strategy in the development of anticancer drugs. The result of cell cycle analysis determined by flow cytometry analysis also showed that GCME can induce apoptosis in MCF-7 cells without cell cycle arrest. Kwon et al. [14] have also reported that the ethanolic extracts of Corallina pilulifera showed cytotoxic activity against human cervical adenocarcinoma cell line, HeLa. Their results also showed that the ethanolic extracts of C. pilulifera can induce apoptosis in HeLa cells without cell cycle arrest [14].
Parker et al. [23] have also reported that methanolic extracts of various kelps exhibited dose-dependent inhibition of the growth of human gastric (AGS) and HT-29 colon cancer cells. An in vivo study by Funahashi et al. [27] reported that a cold water extract of L. japonica, administered in place of drinking water to DMBA-treated rats, reduced the incidence of mammary tumours. Moreover, it is also reported that the L. japonica water extract induced apoptosis in several human breast cancer cell lines, potentially related to inhibition of SOD activity by iodine, or effects of other compounds such as polyphenols [6]. A role for algal polyphenols as anticarcinogens and antiproliferative agents is further supported by antitumour promotion activity against ornithine decarboxylase induction by tumour promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) in BALB/c 3T3 fibroblasts with 75–87 % inhibition by Laminariales sp. and 92 % inhibition by P. tenera methanol extracts [21].
A remarkable selective cytotoxic activity was found in methanolic extract from a red alga, G. corticata compared to U. fasciata and S. ilicifolium in this study. Matanjun et al. [15] showed Eucheuma cottonii (red alga) had the highest ash content. These seaweeds contained high amounts of macrominerals and trace elements [15]. The Na/K ratios were very low for E. cottonii and it had the highest iodine content followed by S. polycystum. In comparison, the iodine content of terrestrial vegetables and fruits are approximately 0.02–0.88 μg/g [22].
In the developed world, Japan has one of the lowest age adjusted breast cancer mortality rates [23]. Evidence for a dietary link is further supported by the rise in breast cancer incidence seen in Japanese immigrants to the US, and their successive generations, whose rates gradually increase to that of white women in the US [24]. High iodine status may be a key protective factor against the development of breast cancer in Japanese women. One correlation study in Spain found a significant positive association between regions where iodine intake was low and breast cancer mortality rates [25]. Traditional eastern Asian medicine has long used iodine-rich seaweeds as a cancer treatment to ‘‘soften’’ tumors and ‘‘reduce’’ nodulation [5, 26]. An anticarcinogenic role for iodine in experimental animals was suggested by the work of Funahashi and co-workers, who found that administration of Lugol’s iodine or iodine rich Wakame seaweed to rats treated with the carcinogen dimethyl benzanthracene suppressed the development of mammary tumours [27]. The same group demonstrated that seaweed induced apoptosis in human breast cancer cells with greater potency than that of fluorouracil, a chemotherapeutic agent used to treat breast cancer. This finding led the authors to speculate that ‘seaweed may be applicable for prevention of breast cancer’ [6] .
One of the few things on which nutritionists agree is that the incidence of cardiovascular disease, cancer and probably of the neurodegenerative diseases can be diminished by diets rich in fruits, grains and vegetables. It is widely believed that a significant contributor to the age-related development of cancer is the relentless attack of reactive oxygen species upon DNA. Most of this damage is repaired, but a low steady-state level of oxidatively-modified bases remains in DNA. These steady-state levels of DNA base oxidation products are, in theory, sufficient to exert a significant mutagenic. Cancer increased the tissue oxidative stress and E. cottonii extract administration to rat significantly improved the oxidative status [3]. This improved oxidative status contributed to the in vivo tumour suppression response. The cell alleviated oxidative stress either by repairing the damaged nucleotides and lipid peroxidation by-products or by directly reducing the pro-oxidative state via enzymatic and non-enzymatic antioxidants. Seaweed consumption has been shown to increase the endogenous antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and sometimes catalase activities in vivo [3, 15].
Conclusions
This study showed that GCME could inhibit the growth of cancer cells and could induce apoptosis in human breast cancer in time and dose depended manner. Thus, a marine red alga, G. corticata might be abundant source of potential complementary and alternative functional food for prevention and treatment of cancer and it is the most suitable for further research to deal with selective antitumor active substances to human cancer especially breast cancer.
Acknowledgments
This study was funded by the Mashhad Branch, Islamic Azad University of Iran.
Disclosure
No competing financial interests exist.
References
- 1.Mohamed S, Hashim SN, Rahman HA. Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol. 2012;23(2):83–96. doi: 10.1016/j.tifs.2011.09.001. [DOI] [Google Scholar]
- 2.Schumacher M, Kelkel M, Dicato M, Diederich M. Gold from the sea: marine compounds as inhibitors of the hallmarks of cancer. Biotechnol Adv. 2011;29(5):531–547. doi: 10.1016/j.biotechadv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Namvar F, Mohamed S, Fard S. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012;130(2):376–382. doi: 10.1016/j.foodchem.2011.07.054. [DOI] [Google Scholar]
- 4.Noda H, Amano H, Arashima K, Nisizawa K. Antitumor activity of marine algae. Hydrobiologia. 1990;204–205(1):577–584. doi: 10.1007/BF00040290. [DOI] [Google Scholar]
- 5.Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2012;142(3):591–619. doi: 10.1016/j.jep.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Funahashi H, Imai T, Mase T, Sekiya M, Yokoi K, Hayashi H, et al. Seaweed prevents breast cancer? Jpn J Cancer Res. 2011;92(5):483–487. doi: 10.1111/j.1349-7006.2001.tb01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deslandes E, Pondaven P, Auperin T, Roussakis CC, Gu J, Stiger V. Preliminary study of the in vitro antiproliferative effect of a hydroethanolic extract from the subtropical seaweed Turbinaria ornata (Turner J. Agardh) on a human non-small-cell bronchopulmonary carcinoma line (NSCLC-N6). J Appl Phycol. 2000;12(3–5):257–62.
- 8.Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxic. 2006;44(7):1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Fernández LE, Valiente OG, Mainardi V, Bello JL, Vélez H, Rosado A. Isolation and characterization of an antitumor active agar-type polysaccharide of Gracilaria dominguensis. Carbohydr Res. 1989;190:77–83. doi: 10.1016/0008-6215(89)84148-5. [DOI] [PubMed] [Google Scholar]
- 10.Mayer AMS, Gustafson KR. Marine pharmacology in 2003–2004: anti-tumour and cytotoxic compounds. Eur J Cancer. 1990;42(14):2241–2270. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Mabeau S, Fleurence J. Seaweed in food products : biochemical and nutritional aspects. Trends Food Sci Technol. 1993;4(April):927–929. [Google Scholar]
- 12.Gupta S, Abu-Ghannam N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov Food Sci Emerg Technol. 2011;12(4):600–609. doi: 10.1016/j.ifset.2011.07.004. [DOI] [Google Scholar]
- 13.Souza LARD, Dore CMPG, Castro AJG, Azevedo TCGD, Oliveira MTBD, Moura MDFV, et al. Galactans from the red seaweed Amansia multifida and their effects on inflammation, angiogenesis, coagulation and cell viability. Biomed Prev Nutr. 2012;2(3):154–162. doi: 10.1016/j.bionut.2012.03.007. [DOI] [Google Scholar]
- 14.Kwon H-J, Bae S-Y, Kim K-H, Han C-H, Cho S-H, Nam S-W, et al. Induction of apoptosis in HeLa cells by ethanolic extract of Corallina pilulifera. Food Chem. 2007;104(1):196–201. doi: 10.1016/j.foodchem.2006.11.031. [DOI] [Google Scholar]
- 15.Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol. 2008;20(4):367–373. doi: 10.1007/s10811-007-9264-6. [DOI] [Google Scholar]
- 16.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 17.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 18.Harada H, Noro T, Kamei Y. Selective antitumor activity in vitro from marine algae from Japan coasts. Biol Pharm Bull. 1997;20:541–546. doi: 10.1248/bpb.20.541. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-H, Tu C-J, Wu H-T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol Pharm Bull. 2004;27(2):180–184. doi: 10.1248/bpb.27.180. [DOI] [PubMed] [Google Scholar]
- 20.Germain M, Affar EB, Amours DD, Dixit VM, Salvesen GS, Poirier GG. Cleavage of automodified poly (ADP-ribose) polymerase during apoptosis. J Biol Chem. 1999;274(40):28379–28384. doi: 10.1074/jbc.274.40.28379. [DOI] [PubMed] [Google Scholar]
- 21.Okai Y, Higashi-Okai K, Nakamura S, Yano Y, Otani S. Suppressive effects of the extracts of Japanese edible seaweeds on mutagen-induced umu C gene expression in Salmonella typhimurium (TA 1535/pSK 1002) and tumor promotor-dependent ornithine decarboxylase induction in BALB/c 3T3 fibroblast cells. Cancer Lett. 1994;25(87(1)):25–32. doi: 10.1016/0304-3835(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 22.Mahesh DL, Deosthale YG, Narasinga Rao BS. A sensitive kinetic assay for the determination of iodine in foodstuffs. Food Chem. 1992;43(1):51–6.
- 23.Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW. Cancer statistics by race and ethnicity. CA Cancer J Clin. 1998;48(1):31–48. doi: 10.3322/canjclin.48.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian–American women. J Natl Cancer Inst. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 25.Cann SA, van Netten JP, van Netten C. Hypothesis: iodine, selenium and the development of breast cancer. Cancer Causes Control. 2000;11(2):121–127. doi: 10.1023/A:1008925301459. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Liu J, Zhang Y. Developments in cancer prevention and treatment using traditional Chinese medicine. Front med. 2011;5(2):127–133. doi: 10.1007/s11684-011-0137-7. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi H, Imai T, Tanaka Y, Tsukamura K, Hayakawa Y, Kikumori T, et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Jpn J Cancer Res. 1999;90(9):922–927. doi: 10.1111/j.1349-7006.1999.tb00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]