Abstract
Routine laboratory investigations play an important role in estimating the risk of mortality in intensive care unit (ICU) patients. The significance of urea:albumin ratio (UAR) in predicting the stay and mortality of ICU patients is not known. It is a retrospective study of patients admitted to ICU (n = 412) with non-chronic kidney disease (non-CKD). Receiver-operating characteristics (ROC) analysis for predicting mortality was carried out to find area under curve (AUC) and threshold levels. Analysis of survival probability was carried out by Kaplan–Meier method and Log-rank test. The AUC to predict mortality were 0.695, 0.767 and 0.791 for serum albumin, urea and UAR, respectively. The threshold levels for albumin, urea and UAR were 2.8 g/dL, 53 mg/dL, and 23.44 mg/g, respectively. The highest odds ratio (OR) of 9.75 to predict mortality at threshold level was observed for UAR, while OR were 7.0 and 3.62 for serum urea and albumin, respectively. The serum urea above and albumin below threshold level were associated with increase in ICU stay of >3 days but the highest OR of 4.73 to predict stay of >3 days was observed for UAR. Kaplan–Meier survival analysis shows significant (p < 0.001) difference at the threshold value of UAR. Serum urea and albumin are found to be an independent predictor for the mortality and stay; however an increased UAR value is the best parameter in predicting mortality and stay in ICU patients with non-CKD illness.
Keywords: Urea:albumin ratio (UAR), Critical patients, Non-CKD, Stay, Mortality, Threshold level
Introduction
The intensive care unit (ICU) is the most active division in a hospital. Increased mortality is associated with the severity of the patient’s illness and underlying conditions. Since patients in the ICU are critical, it becomes increasingly important to identify the patients who need extended stay or are at high risk of mortality, so that the treatment provided to these patients may be critically monitored to have favourable prognosis. There are various methods to measure high risk in terms of outcome, length of stay, ventilator-free days at hospital and by using APACHE II scores [1] at the time of admission. Routine laboratory investigations play an important role in estimating the risk of mortality in ICU patients. Earlier studies have shown that estimating blood urea at the time of admission can be used for mortality risk assessment in patients with acute coronary syndromes along with other cardiac markers [2, 3] and also in the early assessment of acute pancreatitis [4].
The purpose of this study is to determine the prognostic significance of serum urea, serum albumin levels and urea:albumin ratio (UAR) in estimating the ICU stay and mortality of patients admitted in ICU with varied disorders excluding the patients of chronic kidney disease (CKD).
Materials and Methods
It is a retrospective study of patients admitted from 01 Mar 2011 to 31 Dec 2011 in the ICU of a tertiary care hospital. The study included all patients who were admitted to medical and surgical ICU excluding CKD patients diagnosed on the basis of history, renal symptoms and an abnormal renal biochemical profile for more than 3 months [5].
The study group comprised of 412 patients admitted with a diagnosis other than CKD. The clinical diagnosis of patients in surgical and medical ICU is shown in Table 1. Patients had median age of 55 years, with male comprising 65 % of total study population. There was no inclusion or exclusion criteria based on age, disease severity, time of admission or duration of hospitalization. The institutional appointed ethical committee approved the research protocol. Data of biochemical investigations including serum urea, albumin on the first day of admission were retrieved from hospital records, for each patient along with their length of ICU stay in terms of days and mortality or survival.
Table 1.
Diagnosis-related groups of patients admitted in ICU
| Disease (n = 412) | Cases (%) |
|---|---|
| Surgical ICU: (203) | ~50 |
| Carcinoma (60) | 15 |
| Head injury (40) | 10 |
| Intestinal obstruction (15) | 4 |
| Obstructive jaundice (12) | 3 |
| Meningioma (10) | 2 |
| Orthopaedic surgery (28) | 7 |
| Other (38) | 9 |
| Medical ICU: (209) | ~50 |
| Endocrine—metabolic (46) | 11 |
| Gastrointestinal disorders (34) | 8 |
| Sepsis (30) | 7 |
| Central nervous disorders (30) | 7 |
| Cardiovascular disorders (28) | 7 |
| Respiratory disorders (22) | 5 |
| Other (19) | 5 |
The statistical analysis was performed with Epi Info and MedCalc 32 bit demo version software. The results were considered as statistically significant at p < 0.05. Receiver-operating characteristics (ROC) analysis was used to assess the ability of various levels and calculate threshold level (that maximized the combined sensitivity and specificity) of continuous variables i.e. serum urea, serum albumin and UAR to predict ICU mortality. Survival curves were created using the Kaplan–Meier method and the Log-rank test was used for comparing the curves. The impact of the independent variable in predicting mortality and ICU stay in days was determined by the odds ratio (OR) and the 95 % CI, which were calculated using the binary logistical regression in univariate analysis using Epi Info.
Results
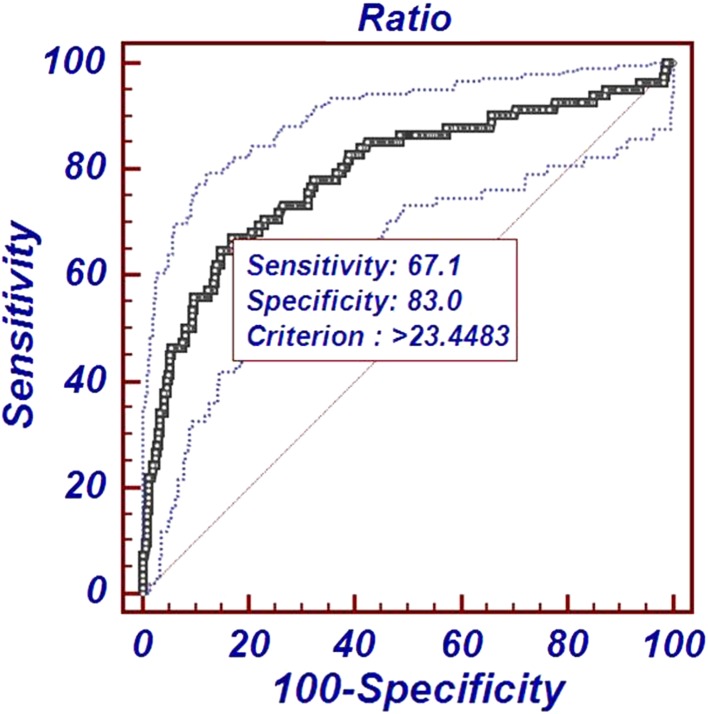
The ROC curve analysis established that the area under the curve for UAR was 0.791 (95 % CI 0.784–0.829) which is higher than that of serum urea 0.767 (95 % CI 0.723–0.803) and serum albumin 0.695 (95 % CI 0.648–0.739), respectively as depicted in Figs. 1 and 2. The threshold level (that maximized the combined sensitivity and specificity) for serum albumin, serum urea and UAR was 2.8 g/dL, 53 mg/dL, and 23.44 mg/g, respectively. At the threshold, sensitivity (true positive cases) for predicting mortality was 71 and 67 % for serum urea and UAR, respectively; whereas specificity (true negative cases) was 74 and 83 % for serum urea and UAR, respectively.
Fig. 1.
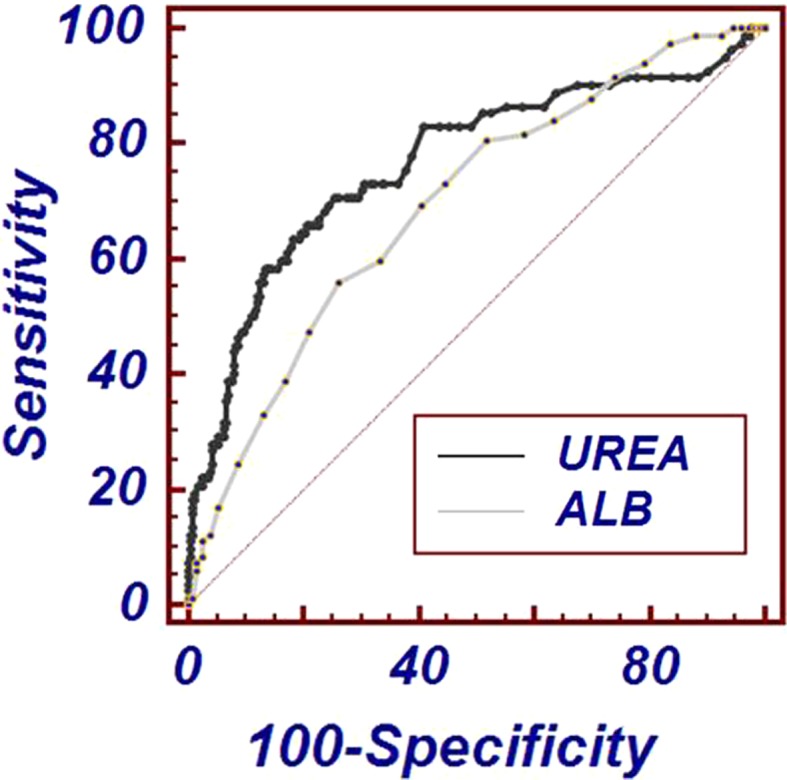
Receiver-operating characteristic curves of serum urea and albumin for predicting ICU mortality
Fig. 2.
Receiver-operating characteristic curve of UAR with 95 % CI for predicting ICU mortality
Serum albumin and urea as continuous variables in the regression model showed that both were associated with the increased risk of mortality: At threshold level the OR for serum albumin and urea was 3.62 (95 % CI 2.19–5.98) and 7.00 (95 % CI 5.11–11.94), respectively. Highest OR of 9.75 (95 % CI 5.67–16.77) was observed for UAR at threshold level of 23.44 mg/g.
OR for ICU stay of more than 3 days was 2.06 (95 % CI 1.34–3.17) and 3.42 (95 % CI 2.20–5.34) for serum albumin and urea, respectively, while the highest OR of 4.73 (95 % CI 2.85–7.84) for UAR at a cutoff for stay of more than 3 days was observed.
Survival curves were created using the Kaplan–Meier method. Comparison in percentage probability of survival in two groups, one with UAR less than 23.44 mg/g and another with UAR greater than 23.44 mg/g is shown in Fig. 3. Log-rank test shows a significant difference between the survival curves with p < 0.001.
Fig. 3.
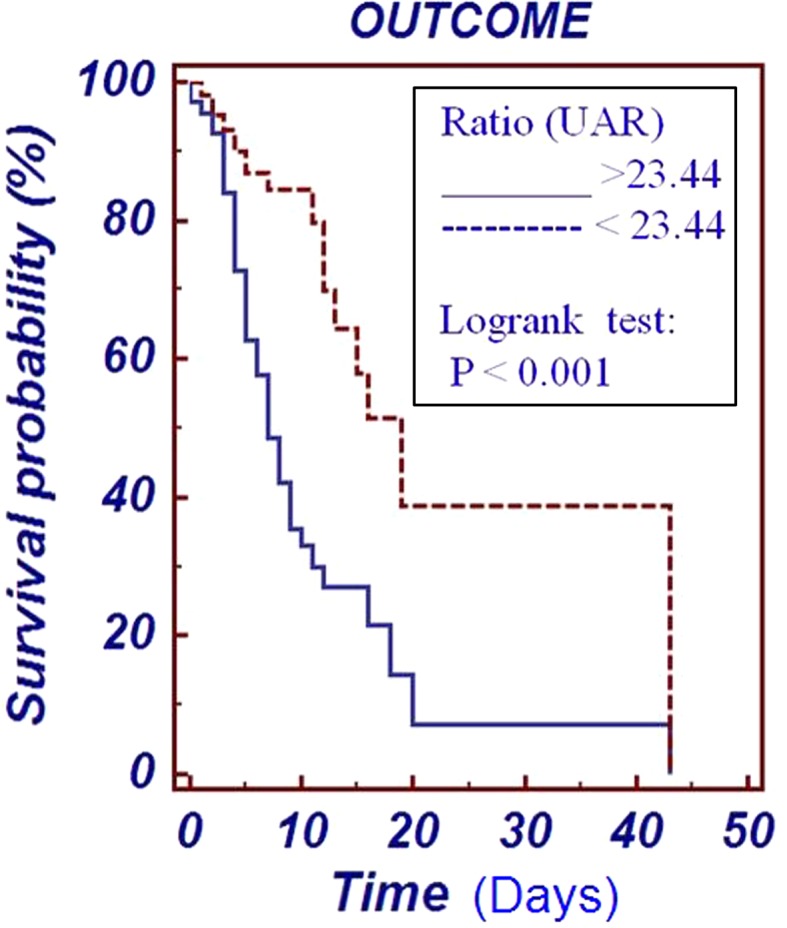
Kaplan–Meier curve for survival probability in ICU patients with UAR at cut-off value of 23.44 mg/g
Discussion
Intensive care is characterized by increased sophistication, resulting in spiraling costs. Auditing treatment in the ICU is thus an integral component in health care planning and management. There is a need to accurately predict prognosis, so that the physician can be guided in clinical decision-making, including the appropriateness of therapy [6].
This study shows that, an increase in serum urea and decrease in serum albumin levels are associated with a high risk of ICU mortality. However, it is observed that an increase in UAR is a better predictor of mortality in ICU patients with non-CKD illness.
A majority of studies have shown that serum urea has a role in predicting mortality in some specific diseases such as acute coronary syndrome [2, 3] and acute pancreatitis [4]. Recently a study has established serum albumin as a prognostic indicator in heart failure patients [7]. However, there are no studies on an optimal approach regarding the use of routine laboratory tests like serum urea and albumin in predicting the mortality in ICU patients who have a wide spectrum of diseases. We restricted our study to non-CKD illness as serum urea is a known marker for CKD and its levels would be raised.
This study evaluated serum albumin, urea, and UAR as prognostic markers in ICU patients for several reasons. Firstly, as routine laboratory tests, these measurements are widely carried out with rapid assay method and therefore without added cost. Secondly, both serum urea and albumin are markers that can reflect changes in intravascular volume status. Thirdly, serum urea and albumin are incorporated in several clinical scoring systems for predicting mortality [8]. Therefore, the current study represents an opportunity to systematically compare the prognostic accuracy of serum albumin, urea and UAR measurements in the early assessment of non-CKD ICU patients.
There are several mechanisms by which changes in serum urea may be related to mortality in ICU. Serum urea may reflect the underlying physiologic state of the patient including intravascular volume depletion and pre-renal azotemia, subclinical renal dysfunction or a state of ongoing negative nitrogen balance related to increased protein catabolism induced in critically ill patients [9]. Most ICU patients are hyper catabolic, with high urea generation rates.
The decrease in serum albumin level may occur due to decrease in the rate of albumin synthesis, which is affected by both nutrition [10] and inflammation. Given that albumin is a negative acute phase protein and most ICU patients have ongoing inflammatory processes, levels of albumin as acute phase marker will decrease. We have taken the serum albumin levels on the day of admission so that no artifactual reduction occurs due to intravenous fluid treatment or total parental nutrition in subsequent days of ICU stay. Irrespective of the specific mechanism, this study interprets that patients with high serum urea and/or low albumin levels with increased UAR at the time of admission to ICU are at increased risk of mortality. In addition the patients who survive, require longer time of intensive management of ICU. It has been observed that OR for ICU stay of more than 3 days is 4.7 for UAR value higher than threshold level, determined to predict mortality.
Conclusion
An increased level of UAR is a significant risk factor for assessing the stay and outcome in patients admitted to ICU with non-CKD illness. Therefore, UAR can be an important guide in attending such patients with critical evaluation and extensive treatment for an improved outcome.
Acknowledgment
The study did not receive any specific grant from any funding agency in public, commercial or not-for-profit sector.
Conflict of interest
The authors have no conflict of interest.
References
- 1.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med J. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ruslan TS, Marya GG, Svetlana VS. Blood urea nitrogen and creatinine levels at admission for mortality risk assessment in patients with acute coronary syndromes. Emerg Med J. 2010;27:105–109. doi: 10.1136/emj.2008.068155. [DOI] [PubMed] [Google Scholar]
- 3.Kirtane AJ, Leder DM, Waikar SS, Chertow GM, Ray KK, Gibson M, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol. 2005;45:1781–1786. doi: 10.1016/j.jacc.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 4.Bechien UW, Richard SJ, Xiaowu S, Darwin LC, Peter AB. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. J Gastroentrol. 2009;137:129–135. doi: 10.1053/j.gastro.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Perrone RD, et al. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. J Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Knaus WA, Wagner DP, Lynn J. Short term mortality predictions for critically ill hospitalized adults: science and ethics. Science. 1991;254:389–394. doi: 10.1126/science.1925596. [DOI] [PubMed] [Google Scholar]
- 7.Su W, An T, Zhou Q, Huang Y, Zhang J, Zhang Y, et al. Serum albumin is a useful prognostic indicator and adds important information to NT-proBNP in a Chinese cohort of heart failure. Clin Biochem. 2012;45:561–565. doi: 10.1016/j.clinbiochem.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DA, D’Amico G, Singer M. The pancreatitis outcome prediction (POP) score: a new prognostic index for patients with severe acute pancreatitis. Crit Care Med. 2007;35:1703–1708. doi: 10.1097/01.CCM.0000269031.13283.C8. [DOI] [PubMed] [Google Scholar]
- 9.Shaw JH, Wolfe RR. Glucose, fatty acid, and urea kinetics in patients with severe pancreatitis: the response to substrate infusion and total parenteral nutrition. Ann Surg. 1986;204:665–672. doi: 10.1097/00000658-198612000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsch R, Frith L, Black E, Hoffenberg R. Regulation of albumin synthesis and catabolism by alteration of dietary protein. Nature. 1968;217:578–579. doi: 10.1038/217578a0. [DOI] [PubMed] [Google Scholar]