Abstract
Sepsis suffers from lack of specific clinical symptoms which contribute to one of the major causes of mortality. In the present study, our aim was to evaluate the role of a recent biomarker Procalcitonin (PCT) in predicting organ dysfunction. 71 patients admitted with sepsis were included in the study. PCT levels were measured at 0, 24, 72 h and 7th day and sequential organ failure assessment score (SOFA) scores were calculated. PCT levels significantly decreased (p < 0.001) in 89.3 % of surviving patients, whereas, in 60 % non surviving patients the PCT level increased significantly (p < 0.001). A significant positive correlation between PCT and SOFA score was observed in survivors at each hour. These observations indicate that PCT concentration is significantly associated with severity of multi organ dysfunction and also helps in determining the prognosis of septic patients.
Keywords: Procalcitonin, Sepsis, Organ dysfunction, Biomarker, Prognosis
Introduction
Sepsis is one of the most common causes of morbidity and mortality in intensive care unit (ICU) patients and one of the major complications of sepsis is multi organ dysfunction. Due to lack of specific symptoms and sensitive laboratory biomarkers, most of the time, appropriate treatment is delayed. Procalcitonin (PCT), a 13 kDa protein has been studied as a useful biomarker of systemic inflammatory response to infection for more than a decade now [1–3]. It is the prohormone of the hormone calcitonin and its production during inflammation is linked to the bacterial endotoxin and to inflammatory cytokines [4]. Till date there is no other biomarker other than PCT which can differentiate between an infectious or non infectious origin of systemic inflammation. Oberhoffer et al. [5] has observed the in vitro expression of mRNA coding PCT in monocytes stimulated by endotoxin or inflammatory cytokines [5]. It has also been observed that the expression of the PCT producing CALC-1 gene increases in multiple extra thyroid tissues in bacterial infection [6]. A recent study [7] has established that PCT guided antibiotic therapy has reduced the antibiotic exposure in patients by 20–70 % without any negative effect. Hence, with the background of usefulness of PCT in sepsis and its close relationship with the severity of the disease [2, 8], our aim was to observe whether a correlation between the level of PCT and the extent of organ dysfunction exist.
Materials and Methods
The study protocol was approved by the institutional ethics committee. A total number of 71 patients admitted with fresh sepsis to the intensive care unit (ICU), New Delhi during July 2010 to July 2011 with clinically suspected bacterial infection and fulfilling at least two criteria of systemic inflammatory response syndrome (SIRS) [9] were included in the study. Informed consent from all patients or their relatives was taken before enrollment in the study. At admission, patients’ demographics, principal diagnosis and all clinical parameters were recorded. APACHE II (Acute Physiology, Age, Chronic Health Evaluation) and SAPS II scores (Simplified Acute Physiology Score) were calculated from the worst values of standard clinical and biological parameters within first 24 h of ICU admission. APACHE II and SAPSII are measures to assess the severity of disease in adult patients admitted to intensive care units; higher score signifies a more severe disease and a higher risk of death. The severity of organ dysfunction was assessed by the sequential organ failure assessment (SOFA) which is based on six different scores, one each for the respiratory, cardiovascular, hepatic, coagulation, renal and neurological systems. SOFA score is well established as an effective index to describe the severity of organ dysfunction in critically ill patients (Table 1) [10]. Blood samples were drawn at 0, 24, 72 h and on day 7 for estimation of PCT. PCT was estimated using BRAHMS PCT sensitive Kryptor assay which is based on time resolved amplified cryptate emission technology. SOFA scores were also recorded simultaneously and were categorized in four increasing order, such as, 1–6, 7–12, 13–18, and 19–24. Patients were followed up for 28 days and were then classified as survivors or non survivors.
Table 1.
The sequential organ failure assessment (SOFA) score
| SOFA score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Respiration (PaO 2/FiO2) | >400 | ≤400 | ≤300 | ≤200 | ≤100 |
| Coagulation (platelets × 103/μL) | >150 | ≤150 | ≤100 | ≤50 | ≤20 |
| Liver (bilirubin mg/dL) | <1.2 | 1.2–1.9 | 2.0–5.9 | 6.0–11.9 | >12.0 |
| Cardiovascular | No hypotension | MAP < 70 mm Hg | Dopa ≤ 5, or dob | Dopa > 5 epi ≤ 0.1, or norepi ≤ 0.1, | Dopa > 15 epi > 0.1, or norepi > 0.1 |
| Central nervous system (Glasgow Coma Scale) | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Renal creatinine mg/dL (urine output mL/day) | <1.2 | 1.2–1.9 | 2.0–3.4 | 3.5–4.9 (or <500 mL/day) | >5 (or <200 mL/day) |
Statistical Analysis
For statistical analysis the statistical package for the social sciences (SPSS, version 17) was used. Normal deviation test was applied to determine the percentage of patients who had either decreased or increased PCT levels over a period of time. Spearman correlation was applied to find out the association between PCT concentrations and corresponding SOFA scores. Student t test and Mann–Whitney test was used for normal and non normal data respectively. Any p value less than 0.05 was considered significant.
Results
Out of 71 patients included in the study, 56 patients survived and rest 15 patients succumbed to their underlying disease during the observation period of 28 days. The overall mortality rate was 21.12 %. The demographic profile and principal diagnosis of the enrolled patients at the time of admission are given in Tables 2 and 3 respectively. Pneumonia (16 %) was the most common source of sepsis in our study followed by urinary tract infection (11 %).
Table 2.
Clinical characteristics of enrolled patients at the time of admission
| Characteristics | Survivors (n = 56) | Non survivors (n = 15) | p value |
|---|---|---|---|
| Sex (M/F) | 30/26 | 10/5 | 0.366 |
| Age (years) | 54.5 (18–85) | 57 (25–85) | 0.526 |
| APACHE II | 21.5 (9–41) | 29 (3–20) | 0.026 |
| SAPS II | 41 (25–81) | 53 (19–93) | 0.041 |
| SOFA | 8 (1–17) | 10 (3–20) | 0.272 |
| PCT (ng/mL) | 11.56 (0.05–290) | 2.025 (0.45–674) | 0.054 |
All values are given as median. Values in parenthesis represent range. p value < 0.05 is considered significant
Table 3.
Clinical diagnosis of the patients
| Diagnosis | No. of patients (n = 71) |
|---|---|
| Pneumonia | 16 |
| UTI | 11 |
| Respiratory tract infection | 8 |
| Pancreatitis | 6 |
| Encephalopathy | 6 |
| Cellulitis | 4 |
| Meningitis with sepsis | 4 |
| Acute Renal failure/Organ injury | 3 |
| Peritonitis | 1 |
| Pulmonary disease | 2 |
| HELLP syndrome (haemolysis-elevated liver enzymes-low platelets) | 1 |
| Others | 9 |
At the time of admission there was no significant difference in age, sex ratio and SOFA score between survivors and non survivors. However, there was a significant difference in APACHE II and SAPS II (Table 2).
With subsequent observation from 0 h to day 7 we found that 89.3 % of surviving patients had a significant decrease (p < 0.001) in their PCT level as compared to non survivors, whereas, in 60 % non surviving patients the PCT level increased significantly (p < 0.001) as compared to only 10.7 % increase in survivors (Table 4).
Table 4.
Percentage of surviving and non surviving patients with their respective change in PCT levels
| Day 0 to day 7 | Survivors | Non survivors | p |
|---|---|---|---|
| Percentage decrease | 89.3 % (50/56) | 40 % (6/15) | <0.0001 |
| Percentage increase | 10.7 % (6/56) | 60 % (9/15) | <0.0001 |
Figures in parenthesis describe number of survivor or non survivor patients out of respective total number of patients
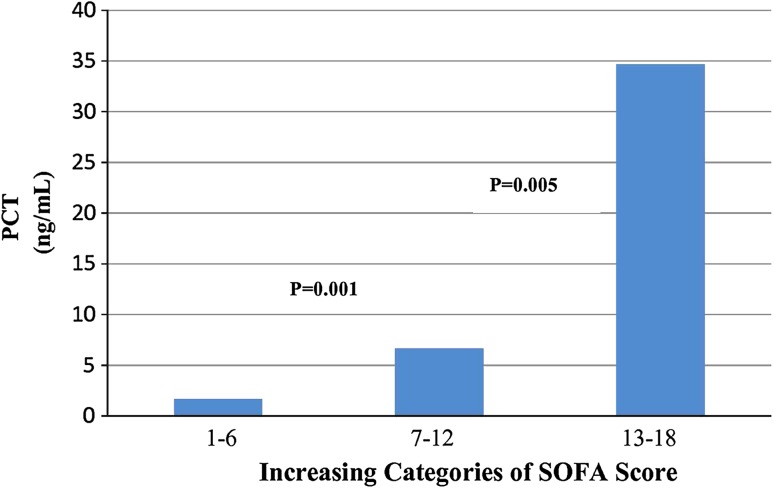
SOFA score was calculated for each patient and the scores were categorized into four groups, such as, 1–6, 7–12, 13–18, and 19–24. To study the correlation between SOFA and PCT, patients were assigned to these above mentioned scores categories. Out of 71 patients none were in the group of 19–24. The PCT levels within the three groups of SOFA score is shown in Fig. 1 which depicts that the median concentrations of PCT increases significantly with increasing SOFA scores. Further when we evaluated the correlation coefficient between PCT and SOFA score in both survivors and non survivors at different time periods the observation was as follows:
Fig. 1.
PCT concentrations of patients within three categories of the sequential organ failure assessment (SOFA) score. Indicated are median obtained from 71 patients from day 1 to day 7. p values indicated are comparison with the subsequent category
There was a positive correlation at all time periods between the PCT level and SOFA score; however the correlation was significant only in the survivor group (Table 5).
Table 5.
Correlation between PCT and SOFA score in survivor and non survivors
| 0 h | 24 h | 72 h | Day 7 | |
|---|---|---|---|---|
| Survivors | r = 0.38 | r = 0.37 | r = 0.401 | r = 0.42 |
| p = 0.004 | p = 0.005 | p = 0.002 | p = 0.002 | |
| n = 56 | n = 56 | n = 56 | n = 54 | |
| Non survivors | r = 0.135 | r = 0.477 | r = 0.592 | r = 0.543 |
| p = 0.404 | p = 0.072 | p = 0.055 | p = 0.266 | |
| n = 15 | n = 15 | n = 11 | n = 6 |
p < 0.05 is considered significant
r Correlation coefficient, n number of patients
Discussion
In this study, we investigated whether the change in the level of PCT over a period of time has any outcome on the prognosis and whether a correlation between the level of PCT and the extent of organ dysfunction exist in patients during systemic inflammation. The study population was not large and the mortality rate (21.12 %) was low, which is attributable to better sepsis management and use of sepsis bundles in our ICU [11]. Two major studies which were undertaken in various centers, reported a mortality rate in patients with sepsis between 16.8 and 34 % [12, 13]. In this study, we have observed that, in a significant number of patients (89.3 %) the PCT level decreased with time and eventually survived the 28 day observation period, whereas the outcome of increase of PCT in 60 % patients was lethal. Thus it can be said that if there is no significant decrease or if there is an increase in the PCT level in patients under treatment, an alert should be raised on reassessment of the therapy [14]. In 2006, Jensen et al. [15] studied PCT measurements in critical care patients and found that an increase in PCT value following the first observation was an independent predictor of mortality. There are some other studies which have associated high PCT values with poor prognosis [16, 17].
The other objective of our study was to explore if there is any correlation between PCT and SOFA scores in sepsis patients. Our results indicate that, PCT levels are directly associated with organ dysfunction in sepsis as assessed by SOFA score. The significant positive correlation was observed only in the survivor group. The non significant positive correlation in the non surviving patients may be because of the small group size. These observations elucidates that PCT represents a beneficial and predictive indicator of sepsis complications such as multi organ dysfunction. Routine analysis of PCT levels seems to aid early recognition of these sepsis complications.
Conclusion
Our study has certain important observations which might guide the clinicians in making early decisions since early diagnosis of sepsis and its associated complications is of utmost importance for greater clinical success. The observations definitely indicate that serum PCT measurements should be used as a part of the comprehensive clinical assessment, which would improve the management and consequently the survival of patients with sepsis.
Acknowledgments
We are grateful to Indian Council of Medical Research (ICMR), New Delhi for the sanction of a research project and financial support.
References
- 1.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26(Suppl 2):S148–S152. doi: 10.1007/BF02900728. [DOI] [PubMed] [Google Scholar]
- 3.Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection and sepsis: clinical utility and limitations. Crit Care Med. 2008;36(3):941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 5.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis related cytokines in vitro. J Lab Clin Med. 1999;134:49–55. doi: 10.1016/S0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 6.Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-I gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin. 2011;27:253–263. doi: 10.1016/j.ccc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C reactive protein as markers of sepsis. Crit Care Med. 2003;1:1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74.
- 10.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter prospective study. Working group on “sepsis related problems” of the European society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Khilnani GC, Hadda V. Management of severe sepsis: role of “bundles”. Indian J Chest Dis Allied Sci. 2009;51:27–36. [PubMed] [Google Scholar]
- 12.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 13.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. doi: 10.1001/jama.1997.03550030074038. [DOI] [PubMed] [Google Scholar]
- 14.Anand D, Das S, Srivastava LM. Procalcitonin—a novel sepsis biomarker. Asian J Med Res. 2012;1:6–8. [Google Scholar]
- 15.Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596–2602. doi: 10.1097/01.CCM.0000239116.01855.61. [DOI] [PubMed] [Google Scholar]
- 16.Rammaert B, Verdier N, Cavestri B, Nseir S. Procalcitonin as a prognostic factor in seven acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2009;14:969–974. doi: 10.1111/j.1440-1843.2009.01597.x. [DOI] [PubMed] [Google Scholar]
- 17.Hillas G, Vassilakopoulos T, Plantza P, Rasidakis A, Bakakos P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator associated pneumonia. Eur Respir J. 2010;4:805–811. doi: 10.1183/09031936.00051309. [DOI] [PubMed] [Google Scholar]