Abstract
Dietary and lifestyle factors have been shown to have a profound effect on paraoxonase-1 (PON1) activity. Cigarette smoke has been shown to inhibit its mass and activity where as resveratrol has been shown to enhance it. We exposed hepatoma derived cell line (HepG2) to resveratrol and nicotine in varying doses and measured PON1 enzymatic activity and PON1 gene expression. In addition, total protein content of HepG2 cells was also measured. Resveratrol in a dose of 15 μmol/l or above significantly increased the PON1 enzyme activity (p > 0.001) where as nicotine in a dose of 1 μmol/l or higher significantly reduced it (p < 0.05). The resveratrol in this dose also enhanced the PON1 gene expression whereas nicotine decreased it as compared to controls. However, the protein conent of cells was not changed suggesting that they were not cytotoxic in the doses used. Till date the antioxidant vitamins have shown disappointing results against LDL oxidation and cardiovascular protection. However, the effect of resveratrol on PON1 gene expression and activity was significant, suggesting increase in PON1 activity and enhanced gene expression may be its alternative mechanism for offering protection against cardiovascular disease and may be an potential pharmacological agent which can be used for this.
Keywords: Paraoxonase1, PON1 gene expression, HepG2 cell line, Resveratrol, Nicotine
Introduction
The oxidation of low density lipoprotein cholesterol (LDL-C) is central to the current theories on initiation and progression of atherosclerosis [1]. High density lipoprotein cholesterol (HDL-C) has been shown to inhibit LDL-C oxidation in vitro. PON1 is a HDL associated enzyme secreted by liver and plays an important role in preventing LDL from peroxidation. In addition, it also leads to hydrolysis of its oxidized form, thereby playing a role in prevention of atherosclerosis [2].
In experimental studies cigarette smoke has been shown to decrease serum PON1 level and activity suggesting it to be detrimental to it [3–5]. James et al. [4] showed that PON1 serum concentration and activity was reduced in smokers as compared to non-smokers. Ex-smokers had activity and concentration comparable to those in non-smokers, suggesting a reversible effect of smoking stoppage.. Polyphenolic antioxidants such as resveratrol, are present in relatively high concentration in grapes and red wine [6, 7]. It has been shown to inhibit lipid peroxidation in vivo [8]. Other biological properties of it include inhibition of platelet aggregation, cellular proliferation, and migration in the vascular wall [9] and induction of PON1 gene. It also inhibits the inflammatory response by suppressing prostaglandin biosynthesis [10].
We investigated whether the PON1activity in HepG2 cell line was affected significantly or not by presence of resveratrol and nicotine. In addition, we also observed their effect on PON1 gene expression.
Materials and Methods
Cell Culture
Human liver hepatoma derived cell line (HepG2) was used in the study. The cells were maintained in minimum essential medium (MEM) containing 2 mM l-glutamine, Earle’s balanced salt solution (Hyclone), 1.5 g/l sodium bicarbonate, 50U/ml penicillin, 50 μg/ml streptomycin and 10 % heat inactivated fetal calf serum (FBS) (PAA, USA) at 37 °C in a humidified atmosphere of 5 % CO2.
PON1 Enzymatic Activity
HepG2 (5 × 105 cells per 6-well dish) were treated with varying concentrations of resveratrol and nicotine for 48 h in usual culture medium. After this period, the medium was withdrawn and cells were washed with phosphate buffered saline (PBS). New medium containing heated fetal calf serum (90 min at 56 °C) which resulted in the loss of serum-associated PON1 activity) was then added. After 24-hour incubation, PON1 secreted enzymatic activity was measured by method as previously described [11].
Preparation of Cell Lysate
Cells were harvested by scrapping and were washed twice with (PBS) without Ca+ or Mg+ ions. After pelleting, the cells were suspended in 300 ml of lysis buffer. After 5 min. of incubation on ice, the lysed cells were centrifuged for 5 min at 10,000 rpm (Eppendorf, USA; mini spin). The supernatant was collected and stored at −20 °C. Protein concentration was then measured by BCA assay kit (Bio-Rad).
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The sodium dodecyl sulphate polyacrylamide gel for electrophoresis was prepared as shown below. Protein profile of cell lysate was analyzed on SDS-PAGE, according to the method described by Laemmli [12]. Molecular weight was determined by the relative migration of standard proteins on SDS-PAGE.
Preparation of sodium dodecyl sulphate polyacrylamide gel (10 %)
| Acrylamide stock solution | (30 %) |
| Stacking gel buffer | 1.0 M Tris–HCl (pH 6.8) |
| Separating gel buffer | 1.5 M Tris–HCl (pH 8.8) |
| APS | 10 % in distilled water |
| Sodium dodecyl sulphate | 10 % in distilled water |
| Running buffer | |
| Sample buffer | (5×) |
Procedure
The glass plates, spacers and comb were thoroughly washed with detergent solution and dried. The repellant was applied on both glass plates. 0.75 mm thick spacers were adjusted and plates were clamped together and separating gel solution was poured between the glass plates. The gel was allowed to polymerize for 20 min at room temperature. Then 4 % stacking gel was poured and wells were made using 0.75 mm thick comb. After polymerization, samples (treated with 5× Laemmli buffer and boiled for 7 min in a water bath) were loaded in the wells. Samples were resolved at 10–15 mA in stacking gel and 18–21 mA in separating gel. The gel was stained for 30 min with staining solution followed by destaining for 1–2 h.
Separating gel (10 ml)
| Components | Volume |
|---|---|
| DDW | 3.3 ml |
| 30 % Acrylamide | 4 ml |
| 1.5 M Tris–HCl (pH 8.8) | 2.5 ml |
| 10 % SDS | 100 μl |
| 10 % APS | 100 μl |
| TEMED | 10 μl |
Stacking gel (3 ml)
| Components | Volume |
|---|---|
| DDW | 2.1 ml |
| 30 % Acrylamide | 0.5 ml |
| 1.5 M Tris–HCl (pH 6.8) | 0.38 ml |
| 10 % SDS | 30 μl |
| 10 % APS | 30 μl |
| TEMED | 3 μl |
Immunological Detection of Paraoxonase (PON1)
The cell lysate was assessed for PON1. The samples containing 50 μg protein were boiled in Laemmli buffer for 5 min and were subjected to electrophoresis (10 % SDS-PAGE) followed by transfer to a PVDF (polyvinylidene fluoride) membrane. The blots were further blocked with 5 % Bovine Serum Albumin (Sigma Chemicals, USA) overnight at 4 °C. It was then washed twice, for 10 min each, with PBS and one washing with PBS plus 2 % Tween-20. The membrane was further incubated with primary PON1 antibody (1:1000 Abnova, USA) at 37 °C for 3 h. After incubation the PVDF membrane was washed twice with PBS plus 2 % Tween-20, followed by incubation for 2 h at 37 °C with horse-radish peroxidase (HRP) conjugated mouse antigoat antibody (1:5000 Santa Cruz Biotechnology, C.A, USA). After 1 h of incubation, the blot was again washed thrice with PBS plus 2 % Tween-20 at 10 min interval time. Afterwards the blot was incubated with the mixture of ECL plus detection reagents A and B (Biological Industries) in a ratio of 1:1 for 1 min. Finally, the X-ray film was exposed to PVDF membrane and developed. The change in band intensity in case of each sample was quantified by scanning densitometry using Scion image 4.3 software.
The HepG2 cell line was procured from National Center for Cell Sciences (Pune), India. The study was reviewed by institutional review board and was approved. The statistical analysis was carried out by Student 2-tailed t tests using the SPSS (Version 16.0) software.
Results
Effect of the Resveratrol and Nicotine on PON1-Enzymatic Activity
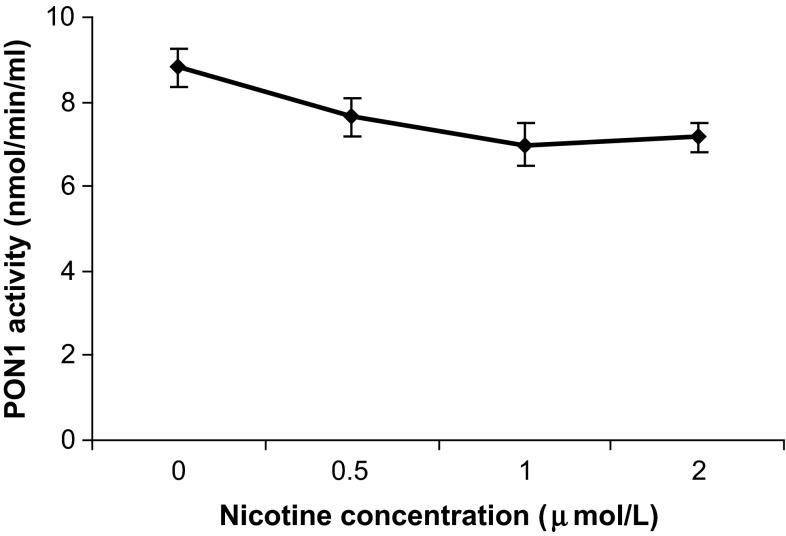
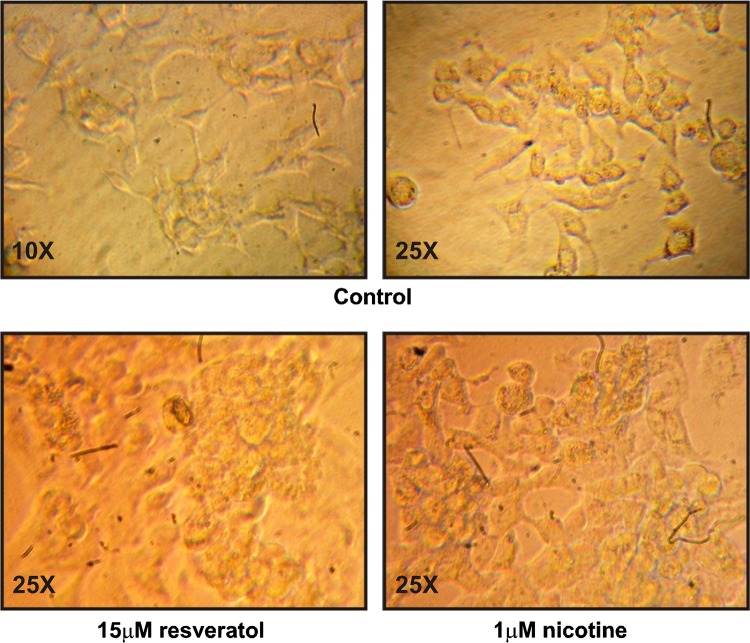
In the initial set of experiments, we carried out dose response to determine the effect of different doses of resveratrol i.e. 0, 1, 5, 10, 15 and 20 μmol/l and nicotine i.e. 0, 0.5, 1 and 2 μmol/l on PON1-enzymatic activity after 48 h of incubation. The results of the experiments are shown in Figs. 1 and 2 respectively. Resveratrol in a dose of 15 μmol/l or above significantly increase PON1 enzymatic activity (>2 times) (p < 0.0001) whereas nicotine in a dose of 1 μmol/l or above significantly decreased it (p < 0.05. However no morphological changes were observed in HepG2 cells (Fig. 3).
Fig. 1.
The effect of resveratrol on PON1 activity in HepG2 cells
Fig. 2.
The effect of nicotine on PON1 activity in HepG2 cells
Fig. 3.
Photographs (×25) of HepG2 control and treated cells with resveratrol and nicotine
Effect of the Resveratrol and Nicotine on PON1-Gene Expression
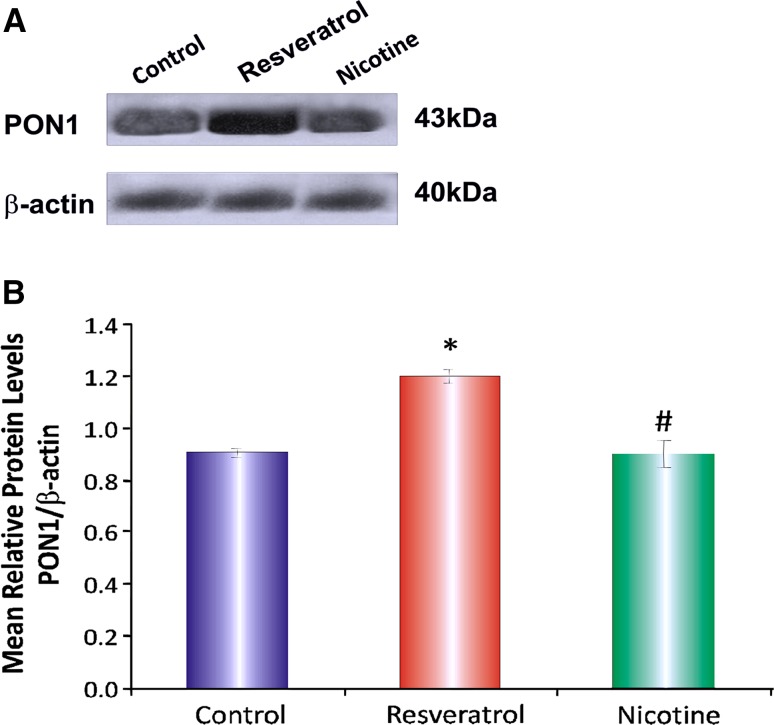
The effect of resveratrol and nicotine on PON1 gene expression in HepG2 cells was investigated by western-blot analysis. Resveratrol increased PON1 gene expression in HepG2 cells as compared to control cells whereas, nicotine decreased PON1 gene expression (Fig. 4) as compared to resveratrol treated cells. The total protein content of HepG2 cells was not affected by both, suggesting resveratrol and nicotine are not cytotoxic at the doses used in the study.
Fig. 4.
Mean protein levels (PON1/Actin) in HepG2 cells
Discussion
Paraoxonase-1 (PON1) is a high density lipoprotein (HDL) associated enzyme, secreted by the liver and its status has been related to individual’s susceptibility to cardiovascular disease (CVD) [13]. Moderate wine consumption appears to have potential beneficial effects in preventing CVD [14]. Several in vivo and in vitro studies have proposed certain molecular mechanisms which could explain the potential beneficial effects of red wine in CAD and these mechanisms are mostly related to the antioxidant properties of some of its polyphenolic compounds such as resveratrol [6, 7]. However, it is still not clear what plasma concentration of it shall be achieved after wine consumption which will enhance the PON1 enzyme activity and gene expression and what concentration is sufficient [15, 16].
Resveratrol has been shown to inhibit lipid peroxidation in vivo [8] which in part could be due to its direct chemical antioxidant activity as it has been shown to inhibit LDL oxidation in vitro [14]. However, this antioxidant effect may not be the sole explanation as antioxidant vitamins have not been shown to be protective for CVD in clinical trials [17.]. The induction of PON1 gene by resveratrol and enhancement in its activity could be new mechanism for its potential anti-atherogenic effect.
Other biological properties of resveratrol are inhibition of platelet aggregation, cellular proliferation and migration in vascular wall [8]. Resveratrol inhibits the inflammatory response by suppressing the prostaglandin biosynthesis [10] and by down regulating the gene expression of intracellular adhesion molecule (ICAM-1) and NF-k-B signaling pathway (an indirect marker of oxidative stress) [18]. The NF-k-B signaling pathway can be evoked by oxidative stress which in turn can be inhibited by resveratrol [9]. TNF-α induced activation of NF-k-B is also attenuated by resveratrol and this is probably mediated by an antioxidant effect [19] which in turn reduces the platelet activation [20] and the sequence can explain the resveratrol induced inhibition of oxidative stress in platelet induced by TNF-α [21]. Resveratrol also inhibits angiotensin II (A-II) induced cardiomyocyte hypertrophy due to its antioxidant effects because as it inhibits A-II induced production of ROS (reactive oxygen species).
Resveratrol has been reported to modulate gene expression by binding to {ER (estrogen receptors) [22, 23]}. Estrogen response element-2 (2-ERE)-like sequences are present in PON1 promoter sequence. In our experiment, we observed that resveratrol increased the PON1 gene expression and activity and this may be because of these receptors, which leads to increased expression as well as PON1 activity in HepG2 cells. However, this may not be true and may be through aryl-hydro carbon (AhR) receptor [24]. Our results favour the concept that PON1 gene induction could be one of the reason behind beneficial properties of resveratrol as it nearly increased its activity by more than twofold. It could be possibly be led by direct AhR activation in different promoter contexts with subsequent specific induction of “protective” target genes such as PON1. We suggest further reassessment of the AhR as a pharmacological target in atherosclerosis.
In the same experiment, we observed that nicotine decreased the PON1 activity but expression was not changed. Compounds suggested to be responsible for inhibition of PON1 activity in cigarette smoke may be various reactive aldehydes (acetaldehyde, formaldehyde and a,b-unsaturated aldehydes, such as acrolein and crotonaldehyde), as well as aromatic hydrocarbons [5]. In in-vitro experiments, it has been shown that inhibition of PON1 activity by cigarette-smoke extract can be reversed by reduced glutathione (GSH), N-acetylcysteine, and 2-mercaptoethanol, suggesting that free thiols are possibly central to this inhibitory effect [5].
In conclusion the resveratrol enhances the PON1 gene expression and also enhances its activity by more than twofold in HepG2 cells in vitro. It is worthwhile to evaluate its potential clinically in prevention of atherosclerosis.
References
- 1.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198901053200122. [DOI] [PubMed] [Google Scholar]
- 2.Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–253. doi: 10.1016/0021-9150(94)05524-M. [DOI] [PubMed] [Google Scholar]
- 3.Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, et al. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1 (192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20:2441–2447. doi: 10.1161/01.ATV.20.11.2441. [DOI] [PubMed] [Google Scholar]
- 4.James RW, Leviev I, Righetti A. Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation. 2000;101:2252–2257. doi: 10.1161/01.CIR.101.19.2252. [DOI] [PubMed] [Google Scholar]
- 5.Nishio E, Watanabe Y. Cigarette smoke extract inhibits plasma paraoxonase activity by modification of the enzyme’s free thiols. Biochem Biophys Res Commun. 1997;236:289–293. doi: 10.1006/bbrc.1997.6961. [DOI] [PubMed] [Google Scholar]
- 6.Aviram M, Fuhrman B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann N Y Acad Sci. 2002;957:146–161. doi: 10.1111/j.1749-6632.2002.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 7.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:197501985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 8.Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, aphytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/S0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 9.Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, et al. Resveratrol scavenges reactive oxygen species and effects radical induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 10.Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865–870. doi: 10.1016/S0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD. Paraoxonase 1 (PON1) polymorphisms haplotypes and activity in predicting CAD risk in North-West Indian Punjabis. PLoS ONE. 2011;6:e17805. doi: 10.1371/journal.pone.0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, et al. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 14.Frankel EM, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/S0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 16.Bertelli A, Bertelli AA, Gozzini A, Giovannini L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp Clin Res. 1998;24:133–138. [PubMed] [Google Scholar]
- 17.Durrington PN, Mackness B, Mackness MI. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler Thromb VascBiol. 2002;22:1248–1250. doi: 10.1161/01.ATV.0000027414.34728.1F. [DOI] [PubMed] [Google Scholar]
- 18.Leiro J, Arranz JA, Fraiz N, Sanmartin ML, Quezada E, Orallo F. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol. 2005;5:393–406. doi: 10.1016/j.intimp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF alpha induced activation of coronary arterial endothelial cells: role of NF-kappa B inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zbikowska HM, Olas B. Antioxidants with carcinostatic activity (resveratrol, vitamin E and selenium) in modulation of blood platelet adhesion. J Physiol Pharmacol. 2000;51:513–520. [PubMed] [Google Scholar]
- 21.De Biase L, Pignatelli P, Lenti L, Tocci G, Piccioni F, Riondino S, et al. Enhanced TNF alpha and oxidative stress in patients with heart failure: effect of TNF alpha on platelet O2-production. Thromb Haemost. 2003;90:317–325. doi: 10.1160/TH03-02-0105. [DOI] [PubMed] [Google Scholar]
- 22.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of reveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 23.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 24.Gouedard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase1 gene expressionby an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]