Abstract
Background
Prevention strategies are critical to reduce infection rates in total joint arthroplasty (TJA), but evidence-based consensus guidelines on prevention of surgical site infection (SSI) remain heterogeneous and do not necessarily represent this particular patient population.
Questions/Purposes
What infection prevention measures are recommended by consensus evidence-based guidelines for prevention of periprosthetic joint infection? How do these recommendations compare to expert consensus on infection prevention strategies from orthopedic surgeons from the largest international tertiary referral centers for TJA?
Patients and Methods
A review of consensus guidelines was undertaken as described by Merollini et al. Four clinical guidelines met inclusion criteria: Centers for Disease Control and Prevention's, British Orthopedic Association, National Institute of Clinical Excellence's, and National Health and Medical Research Council's (NHMRC). Twenty-eight recommendations from these guidelines were used to create an evidence-based survey of infection prevention strategies that was administered to 28 orthopedic surgeons from members of the International Society of Orthopedic Centers. The results between existing consensus guidelines and expert opinion were then compared.
Results
Recommended strategies in the guidelines such as prophylactic antibiotics, preoperative skin preparation of patients and staff, and sterile surgical attire were considered critically or significantly important by the surveyed surgeons. Additional strategies such as ultraclean air/laminar flow, antibiotic cement, wound irrigation, and preoperative blood glucose control were also considered highly important by surveyed surgeons, but were not recommended or not uniformly addressed in existing guidelines on SSI prevention.
Conclusion
Current evidence-based guidelines are incomplete and evidence should be updated specifically to address patient needs undergoing TJA.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-013-9369-1) contains supplementary material, which is available to authorized users.
Keywords: prosthetic joint infection, international survey, infection prevention strategies
Introduction
Surgical site infection (SSI) remains one of the most devastating complications in total joint arthroplasty (TJA). It leads to significant patient morbidity and creates a cost burden to society [22, 35]. In the Nordic countries such as Finland, Norway, Denmark, and Sweden, an increase in revisions due to infection has been observed during the last decade [8–10, 18, 23, 34]. Data from the Swedish Knee Arthroplasty Register show that the risk of revision for infection has slightly increased from 2006–2010 as compared to 2001–2005 [34]. In the USA, infection remains a significant cause for revision arthroplasty, and this burden is projected to rise over time as our population ages and arthroplasty procedures become more numerous [2, 3, 21]. Estimated annual cost to hospitals in the USA increased from $320 million in 2001 to $566 million in 2009 [22]. This cost burden was projected at $1.62 billion by 2020 [22].
Prevention strategies are critical to reduce infection rates in TJA, but evidence-based consensus guidelines on prevention of SSI remain heterogeneous and are not specific to TJA populations. The International Society of Orthopedic Centers (ISOC) represents an international group of 18 leading orthopedic tertiary referral centers performing over 50,000 total joint replacements collectively per year. Using this cohort of international experts, we compared clinician opinions on the prevention of SSI in TJA with existing evidence based guidelines on SSI. The purpose of this study is to (1) identify the most important infection prevention strategies in TJA from existing evidence-based guidelines and (2) to compare these recommendations with expert consensus opinion from 28 orthopedic surgeons from the largest international tertiary referral centers for TJA. We hypothesized that clinicians' views on SSI prevention would diverge from existing guidelines, and some concerns of clinicians have not been adequately addressed.
Materials and Methods
Clinician Cohort
Twenty-eight orthopedic surgeons from members of the ISOC were surveyed at the fifth annual meeting in Hamburg, Germany in April 2013. Further information about each institution can be found at www.isocweb.org. Membership criteria for application to ISOC include (1) >5,000 orthopedic procedures per year, (2) >20 orthopedic surgeons on staff who publish collectively >5 peer-reviewed publications per year, (3) commitment to both basic and clinical research, and (4) academic medical center with resident and fellow education. Members of ISOC that were represented included the following institutions: Hospital for Special Surgery (New York, NY, USA); University Hospitals (Leuven, Belgium); Sunnybrook Health Sciences Centre (Toronto, Canada); Clinica Alemana (Santiago, Chile); Helios Endo-Klinik (Hamburg, Germany); Schulthess Klinik (Zurich, Switzerland); Royal National Orthopaedic Hospital (Stanmore, UK); Ganga Hospital (Coimbatore, India); Istituto Ortopedico Rizzoli (Bologna, Italy); I.R.C.C.S. Istituto Ortopedico Galeazzi (Milan, Italy); Instituto Nacional De Rehabilitacion (Mexico City, Mexico); Sint Maartenskliniek (Nijmegen, The Netherlands); University of Cape Town, Division of Orthopaedic Surgery (Cape Town, South Africa); Skåne University Hospital (Lund, Sweden); Australian Mater Hospital (Sydney, Australia); and Australia University of New South Wales (Sydney, Australia). Over 50,000 total joint arthroplasties are performed yearly within these institutions.
Guideline Review
In order to create a survey of infection-prevention strategies, a review of consensus guidelines was undertaken as described by Merollini et al. [25]. Selection criteria included clinical guidelines on SSI providing evidence-based recommendations on multiple infection prevention strategies, published within the past 15 years, and published in the English language. Four clinical guidelines met this criteria: Centers for Disease Control and Prevention's “Guideline for Prevention of Surgical Site Infection” (1999) [23], British Orthopedic Association “Primary Total Hip Replacement: A Good Guide to Practice” [5], National Institute of Clinical Excellence’s “Surgical Site Infection: Prevention and Treatment of Surgical Site Infection” (2008) [31], and the National Health and Medical Research Council's “Australian Guidelines for the Prevention and Control of Infection in Health Care”(2010) [30]. Selection of infection prevention strategies was based on a review of these guidelines. Inclusion criteria included all strategies relevant for orthopedic surgery. Exclusion criteria included strategies specific to other surgical disciplines (e.g., cardiac, gastrointestinal) or those relevant to routine hospital hygiene procedures (e.g., instrument sterilization).
Survey
Twenty-eight recommendations from American, Australian, and British evidence-based guidelines for infection prevention strategies were used to develop our questionnaire [25]. An additional three questions from a Swedish national project were also included (“Removal of piercings,” “Preoperative showering with clorhexidine,” and “Timing of prophylactic antibiotics”) as well as a question on the importance of the WHO surgical safety checklist. Twenty-eight orthopedic surgeons participated in the survey with the distribution of countries shown in Fig. 1. Participants were not informed about existing guidelines prior to the survey, but were informed on the aim of the survey. The participants were asked to rank each of the strategies according to the importance in preventing SSIs after primary arthroplasty surgery. Five answer options (scores 1–5) were given: “no knowledge/comment,” score 1; “little or no importance,” score 2; “limited importance,” score 3; “significant importance,” score 4; and “critical importance,” score 5. The proportions of strategies considered important by orthopedic surgeons were calculated using descriptive analyses of frequencies and percentages [25]. These scores are a mean value and indicate the overall perception of importance [25]. An SSI prevention strategy was considered as highly important if it was classified as significant or critically important by ≥75% the orthopedic surgeons surveyed and reached a mean response score of ≥3.5 [25]. Statistical analyses were performed using SPSS software.
Fig. 1.
Distribution of countries for participating orthopedic surgeons in the ISOC survey.
Results
A summary of evidence-based guideline recommendations are presented in Table 1. Preventive strategies recommended across most guidelines include prophylactic antibiotics, preoperative shower, antiseptic skin preparation for patients and surgical staff, sterile gowns and gloves, postoperative infection surveillance, and sterile dressing for 24–48 h (see Table 1). Prevention strategies not recommended by each of the evidence-based guidelines include routine hair removal from patients and use of hand jewelry and nail cosmetics by staff (see Table 1). Other measures not recommended by more than one of the guidelines include routine nasal decontamination, routine wound irrigation and intracavity lavage to reduce infection rates, and routine topical antibiotic administration on the incision (see Table 1).
Table 1.
Summary of recommendations on the prevention of SSI from evidence-based guidelines
| CDC (1999) | BOA (2006) | NICE (2008) | NHMRC (2010) | |
|---|---|---|---|---|
| Preoperative | ||||
| Antibiotic prophylaxis | For (IA) | For | For (1+) | For |
| Preoperative shower | For (IB) | NA | For (1+) | For |
| Antiseptic skin preparation of patient | For (IB) | NA | For (1+) | For |
| Hand/forearm antisepsis by surgical staff | For (IB) | NA | For | For (B) |
| Tobacco cessation > 30 days preoperatively | For (IB) | NA | NA | NA |
| Blood glucose control in diabetics | For (IB) | NA | NA | NA |
| Nutrition intervention for immune improvement | No recom | NA | NA | NA |
| Hair removal | Against (IA) | NA | Against (1+) | Against |
| Hand jewelry, artificial nails/nail polish | Against (II) | NA | For | Against |
| Nasal decontamination | No recom | NA | Against (1+) | Consider |
| Intraoperative | ||||
| Sterile gowns | For (IB) | For | For (4) | For (C) |
| Sterile gloves | For (IB) | NA | For (1-) | For (GPP) |
| Surgical mask | For (IB) | NA | NA | For (C) |
| Ultraclean air | For (II) | For | NA | NA |
| Antibiotic impregnated cement | NA | For | NA | NA |
| Administration of oxygen | No recom | NA | For (1+) | NA |
| Closed suction drain | For (IB) | NA | NA | NA |
| Shoe cover | Against (IB) | NA | NA | No recom |
| Patient warming | NA | NA | NA | NA |
| UV radiation | Against (IB) | NA | NA | NA |
| Wound irrigation | NA | NA | Against (1+) | Against |
| Intracavity lavage | NA | NA | Against (1+) | Against |
| Incision drape | NA | NA | Against non-iodophor (1+) | Against non-iodophor (1+) |
| Electrocautery for surgical incision | NA | NA | Against (1+) | NA |
| Postoperative | ||||
| Surveillance | For (IB) | For | NA | For |
| Sterile wound dressing for 24-48 hr | For (IB) | NA | For (4) | For |
| Administration of antibiotics up to 24 hours | NA | For | NA (only as SSI treatment) | NA (only as SSI treatment) |
| Topical antimicrobial agents over surgical incision | NA | NA | Against (1+) | Against |
NHMRC Guidelines
A = Body of evidence that can be trusted to guide practice
B = Body of evidence that can be trusted to guide practice in most situations
C = Body of evidence provides some support for recommendations but care should be taken in its application
D = Body of evidence is weak and recommendation must be applied with caution
GPP = Weak body of evidence but recommended based on expert opinion or clinical experience
NICE Guidelines
1++ High quality meta-analysis or systematic review of randomized controlled trials (RCTs)
1+ Well conducted meta-analyses or systematic review of RCTs
1- Meta-analysis or systematic review of RCTs with high risk of bias
2++ High quality case control or cohort study with very low risk of confounding or bias
2+ Well conducted case control or cohort study with low risk of bias or confounding
2- Case control or cohort study with high risk of confounding or bias
3 Non-analytical studies (case report, case series)
4 Expert opinion, formal consensus
CDC Guidelines
IA = strongly recommended for implementation, supported by well-designed studies
IB = strongly recommended for implementation and supported by some evidence and strong theoretical rationale
II = suggested for implementation and supported by suggestive studies or theoretical rationale
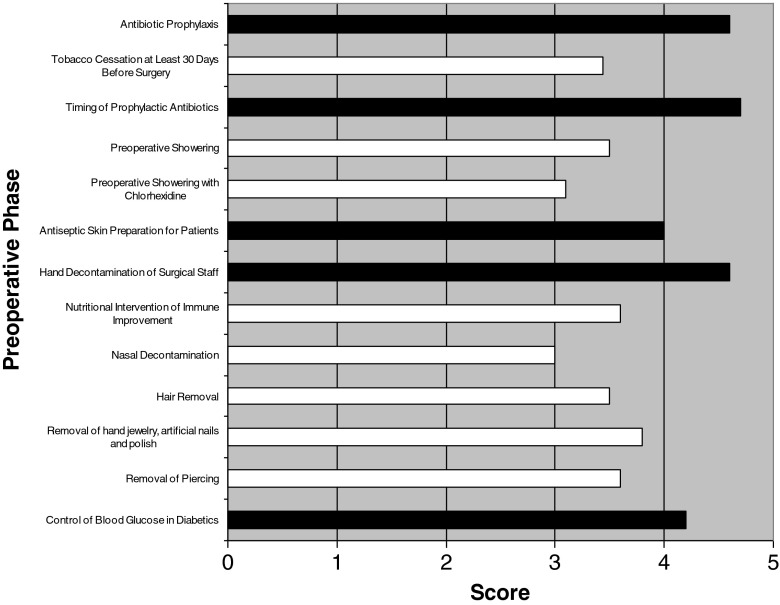
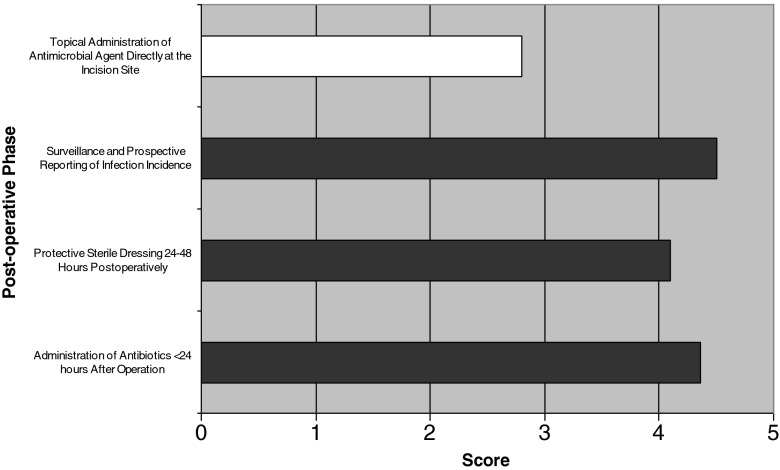
A summary of the ISOC survey are presented in Figs. 2, 3, and 4. Preoperative prevention strategies described as “significant” or “critically” important by >75% of orthopedic surgeons surveyed include control of blood glucose in diabetic patients (mean, 4.2; range, 3–5), hand decontamination by surgical staff (mean, 4.6; range, 3–5), antiseptic skin preparation for patients (mean, 4.0; range, 1–5), and timing and administration of prophylactic antibiotics (mean, 4.7 and 4.6, respectively; range, 3–5 and 3–5, respectively; see Fig. 2). Intraoperative prevention strategies described as “significant” or “critically” important by >75% of orthopedic surgeons surveyed included use of surgical mask (mean, 4.5; range, 3–5), double surgical gloves (mean, 4; range, 2–5), sterile surgical gown (mean, 4.8; range, 4–5), antibiotic-impregnated cement (mean, 4.1; range, 2–5), ultra-clean air/laminar airflow (mean, 4.1; range, 2–5), intracavity lavage (mean, 3.9; range, 2–5), and routine wound irrigation (mean, 4; range, 2–5; see Fig. 3). Postoperative prevention strategies described as “significant” or “critically” important by >75% of orthopedic surgeon's surveyed included infection surveillance and prospective reporting (mean, 4.5; range, 3–5), protective dressing 24–48 h postoperative (mean, 4.1; range, 3–5), and use of antibiotics <24 h postoperative (mean, 4.4; range, 2–5; see Fig. 4). The WHO safety checklist was deemed “significant” or “critical” importance in 85.7% of cases.
Fig. 2.
Considerations on preoperative prevention strategies determined by participating surgeons. Black Considered “significant” or “critical” importance by >75% of the surveyed clinicians. White Considered “significant” or critical” importance by <75% of the surveyed clinicians.
Fig. 3.
Considerations on intraoperative prevention strategies determined by participating surgeons. Black Considered “significant” or “critical” importance by >75% of the surveyed clinicians. White Considered “significant” or critical” importance by <75% of the surveyed clinicians.
Fig. 4.
Considerations on postoperative prevention strategies determined by participating surgeons. Black Considered “significant” or “critical” importance by >75% of the surveyed clinicians. White Considered “significant” or critical” importance by <75% of the surveyed clinicians.
Discussion
The use of standardized, evidence-based guidelines should theoretically improve surgical outcomes and decrease patient morbidity across institutions. In the past 15 years, a number of guidelines on the prevention of surgical site infection have been developed, and these were reviewed in this paper. We hypothesized that existing evidence-based guidelines on SSI prevention in TJA would not fully represent the views of clinicians who routinely treat these patients. Additionally, we wanted to provide areas of deficiency that should be addressed by future level I studies with the goal of including them in future guidelines.
Many of the recommended preventive strategies of the guidelines such as preoperative prophylactic antibiotics, preoperative skin preparation of both the patients and surgical staff, and sterile surgical attire were also considered important by the participating surgeons presented in our ISOC cohort. Additional strategies such as ultraclean air, antibiotic cement, wound irrigation, and preoperative blood glucose control were also considered as highly important by the participating surgeons, but were not recommended or not uniformly addressed in existing guidelines on SSI prevention.
Preoperative optimization of risk continues to be a major focus of research in preventing SSI in TJA, and many different risk factors have been described. Some of the most important include diabetes mellitus (DM), obesity, inflammatory arthritis, preoperative anemia, congestive heart failure, and renal insufficiency [1, 4, 13, 17, 19, 24, 28, 33]. In recent years, the role of diabetes and risk of SSI in TJA has received significant attention. Jämson et al. [19] found that a preoperative diagnosis of DM increased the risk of periprosthetic infection within 1 year of surgery in TJA. Morbid obesity with body mass index >40 also increased risk of SSI in this same study, with preoperative DM further increasing the risk of SSI in this cohort of patients [19]. Recent studies have questioned the role of preoperative glycemic control as a risk factor for SSI, and hemoglobin A1C values necessary for surgery have not been established [1, 17, 19]. Obesity and DM continues to increase in the US population receiving elective TJA, and further studies need to be performed to determine the role of preoperative optimization of these high risk patients.
The use of antibiotic cement in primary TJA was considered highly important by the surveyed surgeons and remains controversial internationally. Early registry data from Norway suggested that routine use of antibiotic cement, in combination with prophylactic antibiotics, for primary total hip arthroplasty reduced the rates of revision arthroplasty [12]. Additionally, studies identified subsets of patients, particularly those with preoperative DM, who may benefit from antibiotic cement in primary TJA. Chiu et al. [6] found that routine use of antibiotic cement in TKA for patients with preoperative DM reduced infection rates from 13.5% without antibiotic cement to 0% with antibiotic cement. The use of antibiotic cement for primary TJA remains controversial, however, as some studies have not found reductions in infection rates with its usage [14, 27, 29]. Additionally, cost effectiveness is critical to decision making, and Cummins et al. [7] found that, with revision due to infection as primary outcome, cement costs <$650 or patient age <71 would be needed to keep costs below $50,000 quality-adjusted life years. Similarly, Merollini et al. [26] found that, in the Australian context, the use of antibiotic cement is cost effective; however, in combination with ultraclean air systems, costs increased by AUD/$4.59 million and health outcomes decreased (127 QALYs lost). The cost effectiveness of antibiotic cement may vary in different settings depending on patient characteristics such as age and the cost of cement. Use of antibiotic cement in primary arthroplasty is a subject that needs to be evaluated and incorporated into evidence-based guidelines.
Routine testing and decontamination of carriers of resistant organisms is another area of interest in infection prevention in TJA. Neither the evidence-based guidelines nor the participating surgeons found this to be critically important in infection prevention, and long-term effects of antibiotic resistance are not well established. While indiscriminate nasal decontamination may not be cost effective, identifying carriers of resistant organisms, particularly methicillin-resistant Staphylococcus aureus, and treating them preoperatively with nasal decontamination may be effective in reducing rates of SSI in the elective orthopedic surgery population [30, 32, 36]. Kim et al. [20] found that a preoperative screening program for S. aureus carriage with preoperative treatment of positive patients resulted in decreased SSI rates in patients undergoing elective orthopedic surgery from 0.45% prior to implementation of these protocols to 0.19% in the study period. Further research should aid recommendations on the importance of nasal decontamination and long-term effects on antibiotic resistance in elective TJA patients.
Evidence-based guidelines have been shown in both orthopedic and nonorthopedic literature to improve patient outcomes and standardize clinician behavior [11, 15, 16]. Hsu et al. [16] found that introduction of national evidence-based guidelines in Taiwan improved clinician adherence to the administration of preoperative antibiotics in TJA and reduced readmission rates in this subset of patients. Another benefit to guideline development is encouraging multicenter cooperation, which may lead to larger scale level I studies to answer remaining questions. Some limitations in the development of consensus evidence-based guidelines exist. Individual variation between practice settings and across country lines creates considerations that may be difficult to address in a consensus manner. Additionally, guidelines are based on the strength of the data behind them, and significant short comings still exist regarding data for SSI prevention in TJA. We hope that identifying these deficiencies will create the impetus for improved large scale, international studies addressing these issues.
In conclusion, a number of short-comings exist in current evidence-based guidelines on prevention of SSI. Many of these guidelines are not specific to the field of TJA, and while the guidelines do address surgeries involving implants, many other disciplines are included in the analyses. Questions specific to TJA such as the use of antibiotic cement, ultraclean air, and preoperative patient optimization may be different from other surgical disciplines and other subspecialties of orthopedic surgery. In the future, consensus evidence-based recommendations in the prevention of SSI in TJA patients may be more beneficial than existing guidelines in optimizing care of this patient population. This has been successfully applied in the diagnosis of prosthetic joint infection through the American Academy of Orthopedic Surgeons evidence based guidelines. Our international group of orthopedic surgeons agreed with many of the recommendations of existing SSI prevention guidelines, but additional guidelines specific to TJA should be developed in the future to fully address issues and concerns specific to this patient population. Additionally, improved data quality through large scale randomized prospective trials would help improve guideline development.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1224 kb)
Acknowledgments
Acknowledgments
No grant funding was used to support this project. We would like to acknowledge all the International Society of Orthopedic Centers members who participated in the survey.
Disclosures
ᅟ
Conflict of Interest:
Katharina Merollini, PhD, Benjamin F. Ricciardi, MD and Annette W-Dahl, PhD have declared that they have no conflict of interest. Mathias P. Bostrom, MD is a paid consultant to Smith and Nephew, received grant support, is a board member of the Orthopedic Research Society and HSS Journal, outside the work. Lars Lidgren, MD owns stock/stock options in Orthocell and Bone support, is a board member of International Society of Orthopedic Centers (ISOC) and European Bone and Joint Infection Society, outside the work. Jonas Ranstam, MD receives personal fees from Medtronic Inc. for publication committee membership, is a deputy editor for Osteoarthritis Cartilage, and a board member for OARSI, outside the work.
Human/Animal Rights:
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent:
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level I: (Guidelines) and Level V Survey (Expert Consensus). See the Guidelines for Authors for a complete description of levels of evidence.
References
- 1.Adams AL, Paxton EW, Wang JQ, Johnson ES, Bayliss EA, Ferrara A, Nakasato C, Bini SA, Namba RS. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;95(6):481–7. doi: 10.2106/JBJS.L.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, Berry DJ. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794–800. doi: 10.2106/JBJS.K.00072. [DOI] [PubMed] [Google Scholar]
- 5.Primary total hip replacement: a guide to good practice. London: British Orthopaedic Association; 2006. [Google Scholar]
- 6.Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomized study. J Bone Joint Surg Br. 2001;83(5):691–5. doi: 10.1302/0301-620X.83B5.11737. [DOI] [PubMed] [Google Scholar]
- 7.Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesaeter LB, Finlayson SR. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634–41. doi: 10.2106/JBJS.G.01029. [DOI] [PubMed] [Google Scholar]
- 8.Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pedersen AB, Kärrholm J, Garellick G, Pulkkinen P, Eskelinen A, Mäkelä K, Engesæter LB. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83(5):449–58. doi: 10.3109/17453674.2012.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale H, Hallan G, Espehaug B, Havelin LI, Engesæter LB. Increasing risk of revision due to deep infection after hip Arthroplasty. Acta Orthop. 2009;80:6,639–45. doi: 10.3109/17453670903506658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danish Knee Arthroplasty register. Annual report 2010. Available: http://www.knee.dk/groups/dkr/pdf/DKRreportEnglish2010.pdf. Accessed: 4/21/2013.
- 11.Dean NC, Bateman KA, Donnelly SM, Silver MP, Snow GL, Hale D. Improved clinical outcomes with utilization of a community-acquired pneumonia guideline. Chest. 2006;130(3):794–9. doi: 10.1378/chest.130.3.794. [DOI] [PubMed] [Google Scholar]
- 12.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian Arthroplasty Register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590–595. doi: 10.1302/0301-620X.79B4.7420. [DOI] [PubMed] [Google Scholar]
- 13.Everhart JS, Altneu E, Calhoun JH. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin Orthop Relat Res 2013;471:3112–3119. [DOI] [PMC free article] [PubMed]
- 14.Gandhi R, Razak F, Pathy R, Davey JR, Syed K, Mahomed NN. Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. J Arthroplasty. 2009;24(7):1015–8. doi: 10.1016/j.arth.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Hepner KA, Rowe M, Rost K, Hickey SC, Sherbourne CD, Ford DE, Meredith LS, Rubenstein LV. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007;147(5):320–9. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hsu C, Cheng SH. Practice guideline adherence and health care outcomes—use of prophylactic antibiotics during surgery in Taiwan. J Eval Clin Pract. 2009;15(6):1091–6. doi: 10.1111/j.1365-2753.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 17.Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726–9.e1. doi: 10.1016/j.arth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Jämsen E, Furnes O, Engesæter LB, Konttinen YT, Odgaard A, Stefánsdóttir A, Lidgren L. Prevention of deep infection in joint replacement surgery a review. Acta Orthop. 2010;81(6):660–666. doi: 10.3109/17453674.2010.537805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Spencer M, Davidson SM, Li L, Shaw JD, Gulczynski D, Hunter DJ, Martha JF, Miley GB, Parazin SJ, Dejoie P, Richmond JC. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820–6. doi: 10.2106/JBJS.I.01050. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8):61–5.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. the Hospital Infection Control Practices Advisory Committee. Guideline for the prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20:247–280. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 24.Marchant MH, Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621–9. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 25.Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221–6. doi: 10.1016/j.ajic.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Merollini KM, Crawford RW, Whitehouse SL, Graves N. Surgical site infection prevention following total hip arthroplasty in Australia: a cost-effectiveness analysis. Am J Infect Control. 2013;41:803–809. doi: 10.1016/j.ajic.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Namba RS, Chen Y, Paxton EW, Slipchenko T, Fithian DC. Outcomes of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty. 2009;24(6 Suppl):44–7. doi: 10.1016/j.arth.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br. 2012;94(10):1330–8. doi: 10.1302/0301-620X.94B10.29184. [DOI] [PubMed] [Google Scholar]
- 29.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95(9):775–82. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 30.National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare. Commonwealth of Australia. 2010. Available: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cd33_infection_control_healthcare_0.pdf. Accessed: 4/19/2013.
- 31.National Institute for Health and Clinical Excellence. Prevention and Treatment of Surgical Site Infection. October 2008. Available: http://www.nice.org.uk/nicemedia/live/11743/42378/42378.pdf. Accessed 4/19/2013.
- 32.Rao N, Cannella B, Crossett LS, Yates AJ, Jr, McGough R., 3rd A preoperative decolonization protocol for Staphylococcus aureus prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466(6):1343–8. doi: 10.1007/s11999-008-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, Fevang BT. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res (Hoboken) 2010;62(4):473–9. doi: 10.1002/acr.20036. [DOI] [PubMed] [Google Scholar]
- 34.Swedish Knee Arthroplasty Register Annual Report 2012. Avaliable online. http://www.knee.nko.se/english/online/uploadedFiles//117_SKAR_2012_Engl_1.0.pdf. Accessed 4/20/2013.
- 35.Tsaras G, Osmon DR, Mabry T, Lahr B, St Sauveur J, Yawn B, Kurland R, Berbari EF. Incidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, Olmsted County, Minnesota, 1969–2007. Infect Control Hosp Epidemiol. 2012;33(12):1207–12. doi: 10.1086/668421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcox MH, Hall J, Pike H, Templeton PA, Fawley WN, Parnell P, Verity P. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196–201. doi: 10.1016/S0195-6701(03)00147-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1224 kb)