This work reveals a function for autophagy in Arabidopsis hypocotyls after germination. By delivering peroxisomes and other cytoplasmic components to the lytic vacuole for degradation, autophagic machinery may help plant cells clean up old organelles for cell remodeling.
Abstract
Plant peroxisomes play a pivotal role during postgerminative growth by breaking down fatty acids to provide fixed carbons for seedlings before the onset of photosynthesis. The enzyme composition of peroxisomes changes during the transition of the seedling from a heterotrophic to an autotrophic state; however, the mechanisms for the degradation of obsolete peroxisomal proteins remain elusive. One candidate mechanism is autophagy, a bulk degradation pathway targeting cytoplasmic constituents to the lytic vacuole. We present evidence supporting the autophagy of peroxisomes in Arabidopsis thaliana hypocotyls during seedling growth. Mutants defective in autophagy appeared to accumulate excess peroxisomes in hypocotyl cells. When degradation in the vacuole was pharmacologically compromised, both autophagic bodies and peroxisomal markers were detected in the wild-type vacuole but not in that of the autophagy-incompetent mutants. On the basis of the genetic and cell biological data we obtained, we propose that autophagy is important for the maintenance of peroxisome number and cell remodeling in Arabidopsis hypocotyls.
INTRODUCTION
The early growth of seedlings and their survival under adverse environments depend on nutrients remobilized from seed storage reserves. Oil is a major seed storage reserve for many plant species including oil crops and the oilseed model species Arabidopsis thaliana. Upon germination of Arabidopsis seeds, triacylglycerol stored in seed oil bodies is hydrolyzed by lipases (Kelly et al., 2011) to generate fatty acids, which are transferred to peroxisomes for β-oxidation (reviewed by Theodoulou and Eastmond, 2012). Acetyl-CoA, produced from the peroxisomal β-oxidation of fatty acids, enters the glyoxylate cycle, which controls the carbon flow from fatty acid catabolism to organic acids. The organic acids are used as sources of carbon for gluconeogenesis and the citric acid cycle to provide sugars and energy for seedling growth.
Two enzymes involved in the glyoxylate cycle—isocitrate lyase (ICL) and malate synthase (MLS)—are located in the matrix of peroxisomes. These two enzymes, which are found in various tissues in addition to the oil-storing tissues of germinating seeds (reviewed by Pracharoenwattana and Smith, 2008), rapidly disappear during postgerminative growth (Nishimura et al., 1986; Lingard et al., 2009). The degradation of the enzymes of the glyoxylate cycle is concomitant with the transition of ICL-positive seedling peroxisomes (formerly called glyoxysomes) to ICL-negative leaf peroxisomes, which participate in photorespiration. Thus, ICL and MLS have been used as model polypeptides to study the degradation of peroxisomal matrix proteins (Zolman et al., 2005; Lingard et al., 2009; Burkhart et al., 2013).
The mechanism of degradation of obsolete peroxisomal proteins is not well understood in plant cells (reviewed by Hu et al., 2012). Matrix proteins like ICL and MLS may be degraded within peroxisomes, but the peroxisomal proteases responsible for their degradation are yet to be identified. An excess of peroxisomal proteins such as PEROXIN5 (PEX5), the receptor of peroxisomal targeting signal type 1, could be polyubiquitylated and degraded by proteasomes, as in budding yeast (Saccharomyces cerevisiae)(Platta et al., 2004). The degradation of peroxisomal proteins by proteasomes is supported by the recent identification of Arabidopsis ICL as a ubiquitylation target (Kim et al., 2013). A third possible pathway for peroxisomal degradation is pexophagy, autophagy that is selective for the degradation of peroxisomes (reviewed by Till et al., 2012). Autophagy directs various cytoplasmic constituents to be degraded in lytic compartments: vacuoles in yeast and plant cells, and lysosomes in mammalian cells. Macroautophagy is the best characterized form of autophagy (macroautophagy is hereafter referred to as autophagy), wherein cytoplasmic cargoes for degradation are initially sequestered by a membrane cisterna called the phagophore. The phagophore expands, forms a sac-like structure, and matures into an autophagosome, a cytoplasmic compartment with a limiting double membrane. Autophagosomes in plant cells are targeted to the vacuole, possibly by fusion of the outer limiting membrane with the tonoplast. In the vacuole, the inner membrane of the autophagosome and its cargoes inside the membrane, called autophagic bodies, are rapidly degraded by acid hydrolases in the vacuolar lumen.
Although pexophagy was first described in mammalian cells (De Duve and Baudhuin, 1966), the mechanisms of pexophagy have been better characterized in the methylotrophic yeast Pichia pastoris. In both P. pastoris and Mus musculus (mouse), a set of Autophagy-related (Atg) genes is required for nonselective autophagy and pexophagy. For example, ~70 to 80% of peroxisomes in mouse liver appear to be degraded by autophagy, which was estimated from an analysis of Atg7 conditional knockout mice (Iwata et al., 2006). Whether pexophagy occurs in plant cells is not clear (Hu et al., 2012), despite evidence supporting it. First, electron microscopic observation suggested pexophagy in castor bean (Ricinus communis) endosperm (Vigil, 1970), although similar observations have not been reported. Second, Atg genes encoding core autophagic machineries are highly conserved in various eukaryotes, including dicots (Hanaoka et al., 2002; Doelling et al., 2002) and monocots (Chung et al., 2009), and reverse genetic studies using Arabidopsis demonstrated their functions in plant autophagy (reviewed by Kim et al., 2012). Of four conserved core autophagic complexes, the ATG8 conjugation system has been the most extensively studied. ATG8, a ubiquitin-like protein, is an important marker for autophagy in yeast, metazoans, and plants. For targeting to the autophagic membrane, ATG8 is conjugated to the membrane phospholipid phosphatidylethanolamine (PE) by the E1 (ubiquitin-activating enzyme)–like ATG7 and the E2 (ubiquitin-conjugating enzyme)–like ATG3. ATG12, another ubiquitin-like protein, forms a conjugate with ATG5, and this conjugate is required for ATG8-PE conjugation in vitro (Fujioka et al., 2008) and in planta (Chung et al., 2010).
In this study, we tested the hypothesis that autophagy plays a role in eliminating obsolete peroxisomal proteins. We showed that atg7 and atg5 mutants accumulated markers for peroxisomal matrix proteins. Consistent with our hypothesis, peroxisomal fluorescent markers were stabilized in the vacuole when vacuolar degradation was pharmacologically inhibited. Furthermore, we observed puncta formed by the autophagic marker green fluorescent protein (GFP)–ATG8a, which partially overlapped with peroxisomal markers. Our data suggest a role for autophagy in the degradation of peroxisomes during postgerminative growth.
RESULTS
Autophagy-Defective Mutants Accumulate Peroxisomal Markers in Hypocotyl Cells
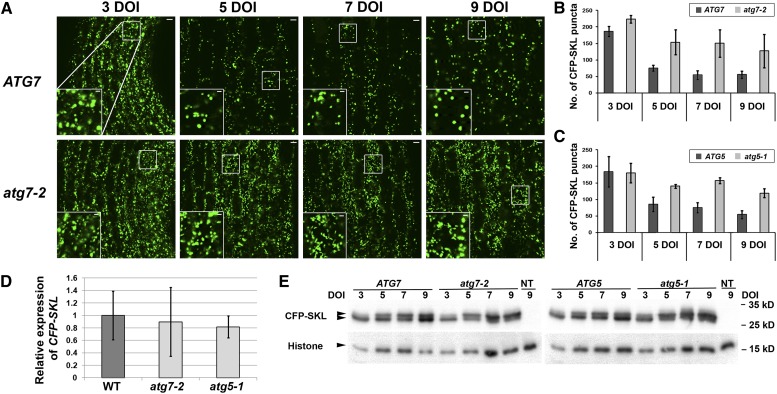
To investigate the potential role of core ATG genes in peroxisome degradation, we crossed an Arabidopsis atg7-2 mutant (Chung et al., 2010) with a transgenic line overproducing a cyan fluorescent protein containing a canonical peroxisomal targeting signal type 1, Ser-Lys-Leu, at its C terminus (CFP-SKL) (Nelson et al., 2007). GFP-SKL, the green fluorescent variant of CFP-SKL, has been widely used as a peroxisomal marker (Mano et al., 2002; Zolman and Bartel, 2004). Arabidopsis ICL and MLS contain similar tripeptides, Ser-Arg-Met and Ser-Arg-Leu, respectively, at their C termini, and Lingard et al. (2009) observed that the pattern of GFP-ICL fluorescence resembled GFP-SKL puncta in Arabidopsis seedlings. atg7-2 homozygous seedlings expressing the CFP-SKL transgene were isolated by genotyping from a segregating F2 population, and self-fertilized progenies from the homozygotes were analyzed by confocal microscopy. Hypocotyls of atg7-2 seedlings expressing the transgene were compared with those of the homozygous wild-type (ATG7) CFP-SKL transgenic line that was used for the genetic cross. In wild-type hypocotyls, the number of peroxisomes was significantly reduced after 5 days of incubation (DOI) at 21°C (Figures 1A and 1B), consistent with a previous report (Mano et al., 2002). In contrast, atg7-2 hypocotyl cells accumulated more CFP-SKL signal after 5, 7, and 9 DOI than did wild-type cells (Figures 1A and 1B). This phenotype was evident also in atg5-1 hypocotyls expressing the CFP-SKL transgene (Figure 1C; see Supplemental Figure 1 online), indicating that defective ATG8 conjugation is responsible for the accumulation of CFP-SKL puncta. After 20 DOI, the accumulation of CFP-SKL puncta in the mutant hypocotyls was still observed (see Supplemental Figure 2A online). Furthermore, the mutant cotyledons after 20 DOI appeared to accumulate slightly more CFP-SKL puncta than did wild-type cotyledons (see Supplemental Figure 2B online); however, less obvious phenotypic differences were observed in cotyledons than in hypocotyls (compared with Supplemental Figures 2C and 2D online).
Figure 1.
Autophagy-Defective Mutant Seedlings Accumulated the Peroxisomal Marker CFP-SKL in Hypocotyl Cells.
(A) Confocal microscopy images of wild-type (ATG7, top panels) and atg7-2 (bottom panels) homozygous hypocotyl tissues expressing the Pro35S-CFP-SKL transgene. Transgenic seedlings were grown on MS agar medium for the indicated number of days. Insets show higher magnification images of the areas marked with white squares. Bars = 10 μm (insets, Bars = 2 μm). (B) and (C) Quantification of peroxisomes from confocal images of wild-type, atg7-2 (B), and atg5-1 (C) hypocotyl cells after 3, 5, 7, or 9 DOI at 21°C. A cytoplasm-rich area (50 × 200 μm) was selected from confocal images, and CFP-SKL dots were quantified with ImageJ using the Cell Counter Plugin. Error bars represent standard deviation (n = 4 seedlings or more). (D) Quantification of CFP-SKL transgene expression in wild-type (WT) and atg7-2 and atg5-1 mutant seedlings by real-time RT-PCR. RNA was extracted from whole seedlings grown on MS agar medium for 9 days. The amount of CFP-SKL transcript was normalized to that of UBC9 as a reference transcript, and relative expression was calculated using the average of normalized values of wild-type samples. Error bars represent standard deviation (n = 3 seedling populations). (E) Immunoblot analysis of wild-type, atg7-2, and atg5-1 hypocotyl extracts after various DOI. Each lane contains protein extract from 15 hypocotyl segments. Anti-GFP antibodies react with two polypeptide species of CFP-SKL with different mobility (arrowheads to top images). In the bottom images, histone H3 protein bands are shown as a loading control. Representative images were chosen from at least three independent experiments. The molecular weight indicated on the right was estimated from protein size markers. NT, nontransgenic Col-0 extract.
[See online article for color version of this figure.]
The abundance of CFP-SKL puncta in the mutants likely results from a difference in posttranscriptional processes because CFP-SKL transgene expression is driven by the constitutive cauliflower mosaic virus 35S promoter (Pro35S) (Nelson et al., 2007). Quantitative RT-PCR analysis indicated that the levels of CFP-SKL transcripts in atg7-2 and atg5-1 mutants did not significantly differ from those in the wild type (Figure 1D). Immunoblot analysis of atg7-2 and atg5-1 hypocotyl extracts revealed two protein bands with slightly different mobility, neither of which was detected in the nontransgenic control (Figure 1E). We could not determine which band represented the unmodified, full-length CFP-SKL polypeptide. If CFP-SKL was covalently modified (e.g., phosphorylated), the bottom band would be the CFP-SKL. Alternatively, the top band might represent the full-length CFP-SKL, whereas the lower band might correspond to a shorter form of CFP-SKL that is partially degraded. When the intensities of both bands were considered, the mutant hypocotyls contained similar levels of CFP-SKL proteins when compared with the wild-type extract (Figure 1E). Together, these data do not support the possibility that increased transcription of the CFP-SKL transgene in the mutants leads to accumulation of CFP-SKL puncta in the atg7-2 hypocotyls.
We asked whether light is required for the ATG7-dependent reduction in peroxisome number. When seedlings were grown in the dark, the number of peroxisomes gradually decreased in wild-type and atg7-2 and atg5-1 hypocotyls (see Supplemental Figure 3 online). The accumulation of CFP-SKL puncta was not evident in the mutant hypocotyls of etiolated seedlings, implying that light triggers ATG7-dependent reduction in peroxisome number in Arabidopsis hypocotyls.
Endogenous Peroxisomal Proteins Accumulate in Autophagy-Defective Hypocotyls
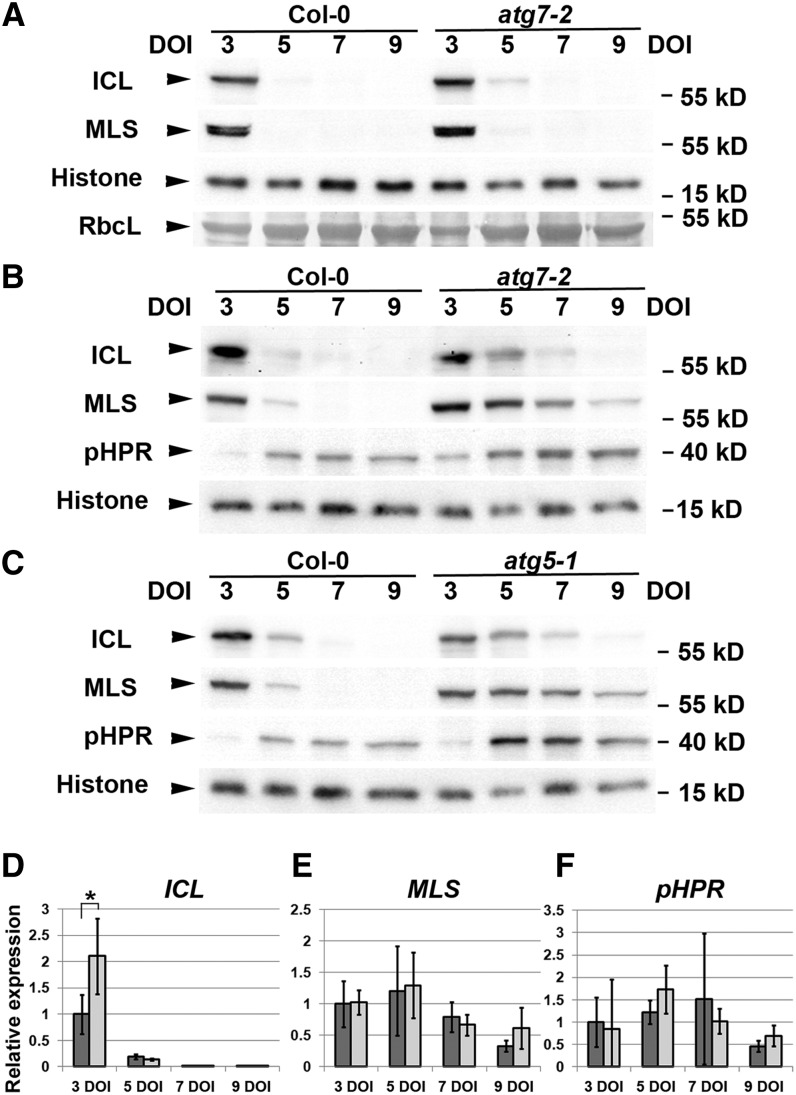
The accumulation of CFP-SKL puncta in autophagy-defective mutants suggests a role for autophagy in the degradation of peroxisomal proteins. To test this hypothesis, we performed immunoblot analysis of nontransgenic hypocotyls, using antisera against ICL and MLS, peroxisomal glyoxylate cycle enzymes. Stabilization of ICL and MLS was used to demonstrate the delayed degradation of peroxisomes in various mutants (Lingard et al., 2009; Burkhart et al., 2013). As expected, ICL and MLS rapidly disappeared in the wild-type seedlings when Rubisco large subunits accumulated (Figure 2A). Time-dependent ICL and MLS degradation was observed also in dissected wild-type hypocotyls (Figures 2B and 2C). After 5 and 7 DOI, ICL was barely detectable in wild-type hypocotyls, and a higher level of ICL was detectable in atg7-2 and atg5-1 hypocotyls (Figures 2B and 2C). Delayed degradation of glyoxylate cycle enzymes in atg7-2 and atg5-1 hypocotyls was confirmed in the immunoblots of MLS (Figures 2B and 2C). The apparent stabilization in the mutants was only transient; ICL and MLS gradually disappeared in mutant hypocotyls.
Figure 2.
Endogenous Peroxisomal Matrix Proteins Accumulated in Autophagy-Defective Mutant Hypocotyls.
(A) to (C) Immunoblot analysis of whole-seedling (A) and hypocotyl (B) and (C) extracts after various DOI was performed using antisera against ICL, MLS, pHPR, or histone H3 (Histone) as a loading control. The protein band corresponding to Rubisco large subunit (RbcL) was identified in membrane stained with Ponceau S. Each lane contains 30 μg of total proteins (A) or protein extract from 15 hypocotyl segments (B) and (C). Lanes on the left contain protein extracts from the wild type (Col-0), and lanes on the right contain protein extracts from atg7-2 (A) and (B) or atg5-1 (C). Molecular weight, indicated on the right, was estimated from protein size markers. Representative images were chosen from at least three independent experiments. (D) to (F) Quantitative RT-PCR analysis of wild-type (dark gray bars) and atg7-2 (light gray bars) hypocotyls to detect transcripts of ICL (D), MLS (E), and pHPR (F) genes. Hypocotyls were dissected from seedlings grown on MS agar medium for the indicated number of days. The amount of target transcript was normalized to that of UBC9 as a reference transcript, and relative expression was calculated using the average of normalized values of wild-type samples after 3 DOI. Error bars represent standard deviation (n = 4 seedling populations). Asterisk indicates a significant difference (0.01 < P < 0.05; Student’s t test).
The increased number of peroxisomes and stabilization of peroxisomal proteins may result from the developmental retardation of the mutants. This possibility was excluded by immunoblot analysis with antibodies against peroxisomal hydroxypyruvate reductase (pHPR), another peroxisomal protein involved in photorespiration. Because pHPR was shown to accumulate when ICL starts to be degraded (Lingard et al., 2009), delayed transition of seedling peroxisomes to leaf peroxisomes would be observed in developmentally delayed mutants. This was not the case in atg7-2 and atg5-1 mutants, in which pHPR started to accumulate after 3 to 5 DOI, similarly to the wild type (Figures 2B and 2C). In fact, the pHPR levels in the mutants appeared to be higher than those in the wild-type hypocotyl extracts (Figures 2B and 2C). As an internal control, we performed anti–histone H3 immunoblot analysis, which did not show overaccumulation of the nuclear proteins in the mutant hypocotyls (Figures 2B and 2C).
To examine whether transcriptional regulation is responsible for the differential accumulation of peroxisomal proteins in the mutant hypocotyls, we performed quantitative RT-PCR analysis using wild-type and atg7-2 hypocotyls (Figures 2D to 2F). The atg7-2 hypocotyls after 3 DOI contained a higher level of ICL transcripts than did the wild-type hypocotyls. After 5 DOI, ICL expression was markedly decreased in both wild-type and atg7-2 hypocotyls (Figure 2D). However, we detected no significant difference in MLS and pHPR transcript levels between wild-type and atg7-2 hypocotyls at any time point (Figures 2E and 2F). Thus, transcriptional regulation is an unlikely mechanism for the differential accumulation of MLS and pHPR in the atg7-2 hypocotyls (Figure 2B). We cannot exclude the possibility that the increase (twofold) in ICL transcripts in atg7-2 hypocotyls after 3 DOI resulted in a higher level of ICL proteins, which remained until after 7 DOI.
Expression of ATG7 Is Induced in Hypocotyls but Not in Cotyledons
In contrast with the results we obtained with hypocotyl extracts, only slight stabilization of ICL and MLS was observed in whole-seedling extracts of atg7-2 and atg5-1 after 5 DOI (Figure 2A; see Supplemental Figure 4A online). We also noticed that the atg7-2 mutation exerted a greater effect on peroxisome number in hypocotyls than in cotyledons (compared with Supplemental Figures 2C and 2D online). These data imply that the ATG8 conjugation system has at least a partial role in peroxisomal degradation, mainly in hypocotyls. Thus, we hypothesized that autophagy is induced in wild-type hypocotyls after ~5 DOI when the number of CFP-SKL puncta and the levels of ICL protein decreased. We performed quantitative RT-PCR to analyze the time course of core ATG expression in hypocotyls. As a control, we compared the expression pattern of ATG in cotyledons (see Supplemental Figure 5 online). We focused on genes encoding the ATG8 conjugation system because the expression of these genes is often regulated developmentally (van der Graaff et al., 2006; Chung et al., 2009). A preliminary search in the Genevestigator database (http://www.genevestigator.com) revealed that ATG3, ATG8b, ATG8g, and ATG8i showed higher expression levels in hypocotyls than in cotyledons. Our RT-PCR results for selected ATG genes indicated that ATG7 and, to a lesser extent, ATG8b transcripts in hypocotyls increased after 5 DOI and then decreased to basal levels (see Supplemental Figures 5A and 5D online, dark gray bars), whereas transcripts encoding other ATG8 isoforms and ATG5 did not significantly change. Although ATG3 transcripts gradually decreased in hypocotyls (see Supplemental Figure 5B online, dark gray bar), it is possible that an induction of ATG3 peaks before 4 DOI. It was impractical to analyze the expression pattern before 2 DOI because the 2-day-old seedlings were very short, which made it difficult to define their hypocotyl region. Because ATG8-PE conjugation requires both ATG7 and ATG3 activities, the data suggest that ATG8 conjugation is activated in hypocotyls at ~5 DOI. In contrast, this induction was not observed in cotyledon samples, and no ATG3 transcript was detected after 5 DOI (see Supplemental Figure 5 online, light gray bars), consistent with our idea that the phenotypes of ATG7 and ATG5 were more highly associated with hypocotyls than with other organs.
Autophagy-Defective Seedlings Show Normal Peroxisome Functions
The mutant phenotypes may be explained by a previous observation that atg7-2 and atg5-1 mutants are hypersensitive to oxidative stress (Xiong et al., 2007), which could result in stabilization of peroxisomes with defective functions via unknown feedback regulation. To determine whether peroxisomes of atg7-2 and atg5-1 mutants are functional, we investigated their sensitivity to indole-3-butyric acid (IBA), which is converted to auxin by β-oxidation. Mutants defective in peroxisomal β-oxidation are less sensitive to exogenously applied IBA (Zolman et al., 2000). The atg7-2 and atg5-1 mutant roots showed normal sensitivity to IBA when tested by a root growth assay (see Supplemental Figure 6 online), indicating that β-oxidation in peroxisomes is functional in the mutants. Our analysis of peroxisomal proteins also suggested normal peroxisomal import in atg7-2 and atg5-1 seedlings because we detected no precursor form of peroxisomal malate dehydrogenase proteins in these mutants (see Supplemental Figure 4B online). Furthermore, we found no difference in diaminobenzidine staining between wild-type and mutant hypocotyls between 3 and 9 DOI (see Supplemental Figure 7 online), suggesting that hydrogen peroxide levels were not altered, in contrast with a previous report that the hydrogen peroxide level is higher in 8-week-old atg5 mutant leaves than in those of the wild type (Yoshimoto et al., 2009). Taken together, these data support the idea that atg7-2 and atg5-1 mutants retain normal peroxisomal functions at least until 9 DOI.
Peroxisomal Proteins Are Targeted to the Central Vacuole for Degradation
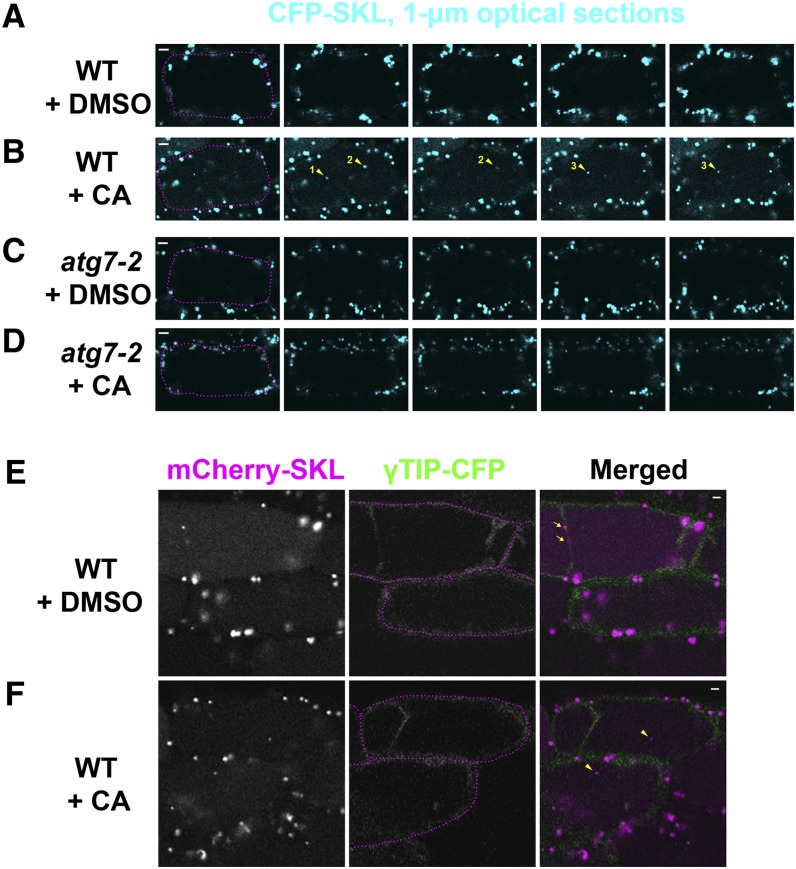
To test the hypothesis that the mutant phenotypes are due to defective autophagy of peroxisomes, we examined the effects of concanamycin A (CA), an inhibitor of the vacuolar proton pump (Dröse et al., 1993; Matsuoka et al., 1997). CA inhibits degradation in the vacuole by blocking the acidification of the vacuole and has been used to stabilize autophagic cargoes in the vacuole (Yoshimoto et al., 2004; Wada et al., 2009; Liu et al., 2012). If peroxisomes are autophagic cargoes targeted into the vacuole for degradation, CA may stabilize peroxisomes that would be degraded otherwise. To study the effect of CA on peroxisome degradation, we germinated CFP-SKL transgenic seeds in liquid medium and found that hypocotyls of seedlings hydroponically grown for 5 to 7 days also exhibited a reduction in the number of CFP-SKL puncta. At day 5 of incubation, CFP-SKL transgenic seedlings in either the wild-type or atg7-2 mutant background in liquid medium were treated with either CA or DMSO. Confocal microscopy of a series of 1 μm optical sections of hypocotyl cells (Figures 3A to 3D) revealed occasional CFP-SKL bodies in the central portion of cells treated with CA (Figure 3B, yellow arrowheads). Note that typical hypocotyl cells, like mature root cells, have a large central vacuole, which causes chloroplasts, peroxisomes, and most of the cytoplasm to be positioned in the peripheral region of cells. These CFP-SKL bodies, which were typically less than 1 μm in diameter, may represent partially degraded peroxisomes in the vacuole. The CFP-SKL bodies were not observed in the center of wild-type hypocotyl cells treated with DMSO (Figure 3A) or in atg7-2 cells treated with DMSO (Figure 3C) or CA (Figure 3D), except in cells where cytoplasmic peroxisomes were found in transvacuolar strands (see below).
Figure 3.
Peroxisomal Protein Markers Are Degraded in the Vacuole of Wild-Type Hypocotyl Cells, but Not in the Vacuole of Autophagy-Defective Mutant Cells.
Seedlings were grown in liquid MS medium for 6 days and treated with either DMSO (A), (C), and (E) or 1 μM CA (B, D, and F) for 17 h before image acquisition. (A) to (D) Confocal Z-series of wild-type (A) and (B) or atg7-2 (C) and (D) transgenic hypocotyls expressing the Pro35S-CFP-SKL transgene. Optical sections were acquired at 1-μm intervals. Yellow arrowheads with numbers indicate three vacuolar CFP-SKL puncta stabilized by CA. Magenta dotted lines were added to images in the first column to highlight the boundary of each cell. (E) and (F) Confocal images of wild-type transgenic hypocotyls expressing both the Pro35S-mCherry-SKL and Pro35S-γ-TIP-CFP transgenes. Transvacuolar strands can be seen in the top cells at γ-TIP-CFP images. Magenta dotted lines were added to images in the middle column. In the merged images on the right, the signals of mCherry-SKL and γTIP-CFP are pseudocolored magenta and green, respectively. Two yellow arrows (E) label cytoplasmic peroxisomes in the transvacuolar strand. Two yellow arrowheads (F) indicate vacuolar mCherry-SKL bodies lacking γTIP-CFP signal. Representative images were chosen from at least four biological replicates. Bars =5 μm (A) to (D) or 2 μm (E) and (F).
To verify the subcellular location of the partially degraded peroxisomes, we performed a double-labeling experiment in which a tonoplast marker, gamma tonoplast intrinsic protein (γTIP)-CFP, was compared with mCherry-SKL (Nelson et al., 2007). We generated transgenic plants expressing both γTIP-CFP and mCherry-SKL, hydroponically grew the transgenic seedlings, and treated them with either CA or DMSO. We searched for any stabilized mCherry-SKL bodies in the central portion of the cells (Figures 3E and 3F) and indeed found mCherry-SKL bodies in the center of hypocotyl cells treated with CA (Figure 3F, yellow arrowheads). These mCherry-SKL signals in the center were distinct from the vacuolar boundary decorated by γTIP-CFP. In both the DMSO control and CA-treated cells, simultaneous labeling of mCherry-SKL and γTIP-CFP signals was occasionally detected in the center of hypocotyl cells (Figure 3E, yellow arrows), indicating that these signals were derived from cytoplasmic peroxisomes in transvacuolar strands. On the basis of the data shown in Figure 3, we conclude that CA stabilizes peroxisomal proteins in the vacuole and that small CFP-SKL and mCherry-SKL bodies in the center of the cells represent peroxisomal proteins stabilized in the vacuole.
Vacuolar Targeting of Peroxisomal Proteins Requires ATG7
Our data indicated that ATG7 and ATG5 are required for the degradation of peroxisomes in the vacuole of hypocotyl cells during postgerminative growth (Figures 1 and 3). Because these two genes are essential for the conjugation of ATG8 to PE, we speculated that ATG8-PE plays a role in targeting peroxisomes to the vacuole. Specifically, ATG8-positive autophagic vesicles may sequester and deliver peroxisomes to the vacuole. To examine this possibility, we designed double-labeling experiments in which GFP-ATG8a, a marker for various autophagic structures (Thompson et al., 2005), was monitored with mCherry-SKL bodies used as reference (Figure 4). GFP-ATG8a fluorescence in plant cells displays either in a diffuse or in a punctate pattern. The former is presumed to represent a nonconjugated, soluble form of GFP-ATG8a, whereas the latter is likely a membrane-associated form of GFP-ATG8a-PE (Chung et al., 2010).
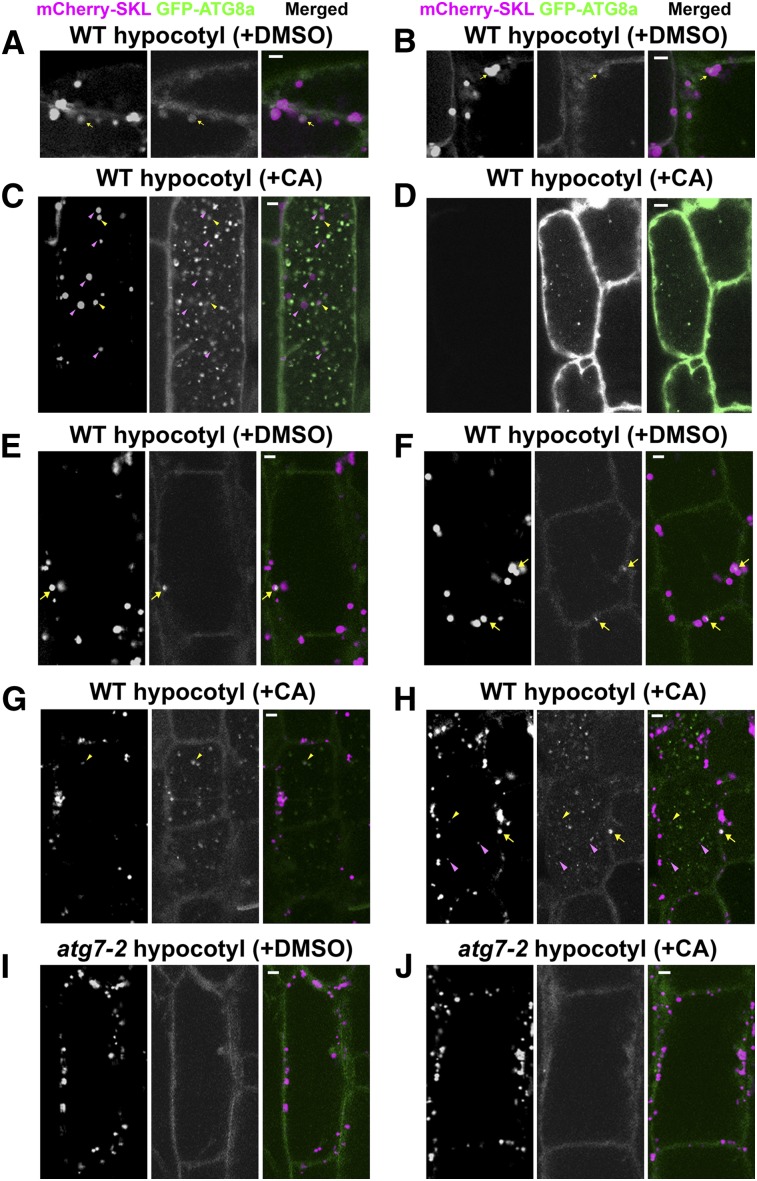
Figure 4.
Peroxisomal Markers Partially Overlap with Autophagic Markers in Wild-Type Hypocotyls, but Not in Autophagy-Defective Mutant Hypocotyls.
(A) to (C) Confocal images of wild-type transgenic hypocotyls expressing both the Pro35S-mCherry-SKL and Pro35S-GFP-ATG8a transgenes. (D) Confocal image of hypocotyls expressing only the Pro35S-GFP-ATG8a transgene. (E) to (J) Confocal images of wild-type (E) to (H) or atg7-2 (I) and (J) transgenic hypocotyls expressing both the Pro35S-mCherry-SKL and ProUBQ10-GFP-ATG8a transgenes. The transgenic seedlings were grown in liquid MS medium for 6 days and treated with either DMSO (A), (B), (E), (F), and (I) or 1 μM CA (C), (D), (G), (H), and (J) for 17 h before analysis. Representative images were chosen from at least five biological replicates. In the merged images, signals of mCherry-SKL and GFP-ATG8a are pseudocolored magenta and green, respectively. Bars =5 μm. (A) Yellow arrows indicate an autophagosome-like signal sequestering a peroxisome. (B), (E), (F), and (H) Yellow arrows mark phagophore-like signals partially overlapping or juxtaposed with a peroxisome. (C), (G), and (H) Yellow arrowheads label autophagic bodies overlapping a peroxisomal signal, and magenta arrowheads indicate vacuolar peroxisomal puncta lacking GFP-ATG8a signal.
Round GFP-ATG8a puncta in the cytoplasm, possibly representing autophagosomes (Figure 4A), were detected after 6 DOI in the hypocotyl cells of wild-type seedlings expressing both the GFP-ATG8a and mCherry-SKL transgenes. The GFP-ATG8a puncta were 2 μm in diameter or smaller, with one overlapping with cytoplasmic mCherry-SKL signal (Figure 4A, yellow arrow). Similar results were obtained from mCherry-SKL transgenic plants coexpressing a GFP-ATG8a transgene under the control of the polyubiquitin gene promoter (ProUBQ10) (Suttangkakul et al., 2011) (see Supplemental Figure 8A online, yellow arrows). In addition to the putative autophagosomes sequestering peroxisomes, we detected phagophore-like GFP-ATG8a puncta partially overlapping with a cytoplasmic mCherry-SKL structure (Figure 4B, yellow arrow).
We also performed double-labeling experiments using atg7-2 mutant seedlings expressing both the GFP-ATG8a and mCherry-SKL transgenes (see Supplemental Figure 8B online). As previously reported in autophagy-defective mutants (Yoshimoto et al., 2004; Suttangkakul et al., 2011), atg7-2 mutant cells accumulate diffuse GFP-ATG8a signal in the cytoplasm, indicating its lack of delivery to the vacuole. In addition, the mutant cells occasionally contained cytoplasmic GFP-ATG8a foci that were typically brighter than GFP-ATG8a puncta in wild-type cells (see Supplemental Figure 8B online, green arrows) (Yoshimoto et al., 2004; Kim et al., 2012). Unlike in wild-type cells, GFP-ATG8a foci in the mutant cells barely overlap with mCherry-SKL puncta.
CA stabilizes GFP-ATG8a puncta in the vacuole of hypocotyl cells (Thompson et al., 2005), and GFP-ATG8, in combination with CA, has also been used as a marker for autophagic bodies in the vacuole. To determine whether mCherry-SKL bodies that are stabilized in the vacuole (Figure 3) overlap with GFP-ATG8a–positive autophagic bodies, we incubated the transgenic seedlings expressing both Pro35S-mCherry-SKL and Pro35S-GFP-ATG8a in medium containing CA (Figure 4C). Analysis of confocal optical sections revealed fewer vacuolar mCherry-SKL bodies (Figure 4C, magenta and yellow arrowheads) than GFP-ATG8a autophagic bodies. We observed colocalization of the two signals in the vacuole (Figure 4C, yellow arrowheads), although typical mCherry-SKL bodies in the vacuole did not exhibit GFP-ATG8a signals (Figure 4C, magenta arrowheads). As a negative control, we observed hypocotyls of transgenic plants expressing only Pro35S-GFP-ATG8a (Figure 4D) and did not detect any mCherry signal.
To investigate whether ATG7 is needed for colocalization, we analyzed the effect of DMSO and CA on wild-type and atg7-2 hypocotyls expressing both the ProUBQ10-GFP-ATG8a and Pro35S-mCherry-SKL transgenes (Figures 4E to 4J). Compared with the strong induction by Pro35S, the ProUBQ10 only moderately induced GFP-ATG8a transgene expression. Nevertheless, two fluorescent proteins showing similar localization patterns were observed among the wild-type hypocotyl cells (Figures 4E to 4H). In addition to globular GFP-ATG8a signals, we also observed GFP-ATG8a puncta that apparently represent phagophores that are either overlapping or juxtaposed with cytoplasmic peroxisomes in wild-type hypocotyl cells (yellow arrows in Figures 4E and 4F). The effects of CA treatment were reproducible in the ProUBQ10-GFP-ATG8a transgenic line in the wild-type background; we observed the colocalization of autophagic bodies with a peroxisomal signal (Figures 4G and 4H; yellow arrowheads) and, more frequently, GFP-ATG8a–negative mCherry-SKL signal in the vacuole (Figure 4H, magenta arrowheads). In contrast, DMSO-treated atg7-2 mutant hypocotyl cells had numerous mCherry-SKL puncta and diffuse GFP-ATG8a signals in the cytoplasm (Figure 4I), consistent with the data shown above (Figure 1A; see Supplemental Figure 8B online). Treatment of the mutant with CA had little effect on the localization of mCherry-SKL and GFP-ATG8a, and the mutant cells lacked autophagic bodies and vacuolar mCherry-SKL bodies (Figure 4J). Taken together, these data indicate that GFP-ATG8a–positive phagophores and autophagosomes partially colocalized with cytoplasmic peroxisomes, and that ATG7 is required for the degradation of peroxisomal proteins in the vacuole.
DISCUSSION
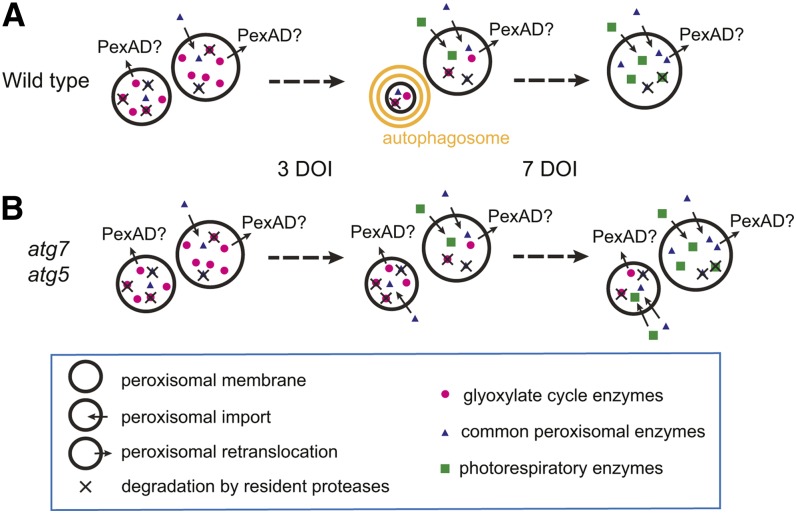
Recent studies have shown that a few types of organelles in plant cells are degraded by autophagy. ATG5 and other core ATG genes are involved in the degradation of chloroplasts (Ishida et al., 2008; Wada et al., 2009), amyloplasts (Nakayama et al., 2012), and endoplasmic reticulum (ER) (Liu et al., 2012). However, whether the core ATG genes are also required for the degradation of other types of organelles, such as peroxisomes, mitochondria, and oil bodies, has not been clear. Here, we presented evidence supporting ATG7- and ATG5-dependent autophagy of peroxisomes in Arabidopsis hypocotyls. Two independent mutants, atg7-2 and atg5-1, both defective in ATG8 conjugation to PE (Chung et al., 2010), accumulated peroxisomal markers in hypocotyls—CFP-SKL puncta, ICL, and MLS (Figures 1 and 2). The accumulation of CFP-SKL puncta in the mutants is unlikely owing to ectopic transgene overexpression because endogenous ICL and MLS levels were also affected by the mutations. The inhibition of ICL and MLS degradation by the mutations was only transient (Figures 2B and 2C), implying an ATG7- and ATG5-independent mechanism for the degradation of peroxisomal proteins. ICL and MLS proteins in Arabidopsis hypocotyls may be degraded either by peroxisomal matrix proteases or by other autophagy-independent degradation mechanisms, possibly facilitating the autophagic degradation of peroxisomes (Figure 5).
Figure 5.
A Model for the Degradation of Peroxisomal Proteins in Wild-Type and Autophagy-Defective Hypocotyls.
Three possible mechanisms of peroxisomal degradation are shown in the model: degradation by resident proteases, PexAD, and autophagy. Autophagy appeared to be activated in wild-type hypocotyls (A) after ~5 DOI but was not functional in autophagy-defective atg5 and atg7 (B). Three types of peroxisomal proteins are depicted. (i) Glyoxylate cycle enzymes such as ICL and MLS were found in seedling peroxisomes at day 3 of incubation but were rapidly degraded at approximately day 5 and not detected after 7 DOI. (ii) Photorespiratory enzymes like pHPR were imported after 3 DOI and accumulated in peroxisomes afterward. (iii) Common peroxisomal enzymes, such as those involved in oxidation, were present in peroxisomes throughout all stages. In this study, CFP-SKL and mCherry-SKL may be considered common peroxisomal proteins. The types of peroxisomal proteins selected by each possible mechanism of degradation are not clear.
[See online article for color version of this figure.]
It has been proposed that degradation of peroxisomal proteins in plant cells depends on proteolytic activities in three subcellular locations—peroxisome matrix, cytosol, and the vacuole (Zolman et al., 2005; Lingard et al., 2009; Burkhart et al., 2013; Figure 5). One model for extraperoxisomal degradation of matrix proteins by the 26S proteasome is peroxisome-associated protein degradation (PexAD) (Lingard et al., 2009; Burkhart et al., 2013), which is analogous to the well-defined process of ER–associated protein degradation. Prerequisites for PexAD are polyubiquitylation and retranslocation of matrix proteins, whereas pexophagy is assumed to deliver peroxisomes to vacuoles for degradation. To examine the possible degradation of ICL and MLS in the peroxisomes, Lingard and Bartel (2009) performed a genetic analysis of genes encoding three putative peroxisomal proteases in Arabidopsis, namely, Lon-related protease 2 (LON2), Deg/HtrA protease 15 (DEG15), and peroxisomal M16 protease (PXM16), but found that mutations in these genes and double mutations of lon2 deg15 and lon2 pxm16 did not result in the stabilization of ICL and MLS. Instead, lon2 mutants are defective in the import of peroxisomal proteins and the processing of peroxisomal targeting signal type 2 (Lingard and Bartel, 2009).
While this work was being revised, Farmer et al. (2013) reported that atg7, atg3, and atg2 mutations were isolated from a forward-genetic screen for lon2 suppressors, providing independent evidence for pexophagy in young Arabidopsis seedlings. Our observation of pexophagy in Arabidopsis seedlings supports their model that defective autophagy in the absence of LON2 proteases leads to the persistence of small peroxisomes, which enables the suppression of various lon2 phenotypes.
Several mutations have been shown to stabilize the enzymes of the glyoxylate cycle: a pex4 pex22 double mutation (Zolman et al., 2005) and single mutations of pex14, pex6, and ped1 (Burkhart et al., 2013). PEX4 is a ubiquitin-conjugating enzyme and PEX22 is a peroxisomal membrane protein that interacts with PEX4. Although the ubiquitylation targets of PEX4 have yet to be identified in Arabidopsis, PEX4 was proposed to be involved in PEX5 recycling and/or PexAD (Hu et al., 2012; Lingard et al., 2009). PEX14, presumed to form a docking point for PEX5, is another peroxisomal membrane protein that is important for the import of proteins containing a peroxisomal targeting signal (Hayashi et al., 2000). A yeast homolog of PEX6 is an ATPase tethered to the peroxisomal membrane and mediates the recycling of PEX5 back to the cytosol. Finally, PED1 is a peroxisomal thiolase involved in β-oxidation of fatty acids.
Given the potential role of autophagy in peroxisome degradation, the involvement of PEX14 and PEX4 in peroxisomal protein degradation is intriguing. Pex14p in a methylotrophic yeast Hansenula polymorpha was implicated in the selective autophagy of peroxisomes (Bellu et al., 2001). Mammalian Pex14p interacts with LC3, a mammalian homolog of ATG8, which is required for peroxisomal protein degradation (Hara-Kuge and Fujiki, 2008). The involvement of PEX4 in peroxisomal degradation underscored the role of ubiquitylation, which induces the autophagy of mammalian peroxisomes (Kim et al., 2008). Thus, our report of autophagy in Arabidopsis hypocotyls provides an explanation for the genetic data published previously (Zolman et al., 2005; Burkhart et al., 2013). Whether PEX4-dependent ubiquitylation mediates PexAD, pexophagy, or both, needs further investigation.
Our cell biological data indicated that peroxisomes in Arabidopsis hypocotyl cells are delivered to the vacuole via the ATG7-dependent autophagic pathway. CA stabilized peroxisomal markers in the vacuole (Figure 3), and ATG7 was required for vacuolar targeting of mCherry-SKL bodies (Figure 4). We detected colocalization of autophagosomes and phagophores with peroxisomes in the wild-type cytoplasm (Figures 4,A, 4B, 4E, 4F, and 4H). In addition, we observed two pools of mCherry-SKL bodies in the vacuole when wild-type hypocotyls were treated with CA. Some of the mCherry-SKL bodies emitted GFP-ATG8a signals (i.e., autophagic bodies) (Figure 4, yellow arrowheads in 4C, 4G, and 4H), whereas others did not (Figures 4C and 4H; magenta arrowheads). It appears that plant organelles delivered to the vacuole by autophagy either contain or lack ATG8 markers. We compared our colocalization images with similar data that other research groups recently published to investigate the autophagy of plant organelles (Ishida et al., 2008; Liu et al., 2012). When plant cells undergo ER stress, a punctate pattern of the ER marker GFP-HDEL was found in the vacuole of leaf protoplasts treated with CA (Liu et al., 2012). Some of the GFP-HDEL puncta were colocalized with an autophagic marker, Cerulean-ATG8e, but others were not (Liu et al., 2012), similar to our data (Figures 4C and 4H). Likewise, a small fraction of Rubisco-containing bodies lacking GFP-ATG8 signals were evident when mesophyll cells were treated with CA (Ishida et al., 2008).
The reason for the presence of the vacuolar mCherry-SKL bodies lacking GFP-ATG8a signals is not clear. Peroxisomes may be released from autophagic bodies in the vacuole, and/or GFP-ATG8, attached to autophagic bodies sequestering peroxisomes, may be removed prior to the degradation of peroxisomes that contains mCherry-SKL proteins in the matrix. Alternatively, peroxisomes may be delivered to the vacuole also via unknown autophagic routes that are not associated with ATG8 but still require ATG7 (Figures 3D and 4J). One of the possible routes is protrusion microautophagy involving a direct engulfment of whole organelles by the tonoplast (Bassham et al., 2006). If protrusion microautophagy of peroxisomes is a major autophagic route for peroxisomal degradation, peroxisomes stabilized by CA should be enclosed by the tonoplast. However, we failed to observe γTIP-CFP signals enclosing mCherry-SKL bodies in the vacuole (Figure 3F).
We did not investigate the possibility that ATG8 isoforms other than ATG8a have a higher affinity to peroxisomes. Nine Arabidopsis genes encode ATG8 isoforms, all of which can be conjugated to PE in vitro (Fujioka et al., 2008). No specific biochemical and genetic roles have been assigned to any of these ATG8 genes, although their gene expression pattern varies (Yoshimoto et al., 2004; Sláviková et al., 2005; Thompson et al., 2005). It is not known whether ATG8 isoforms show specific subcellular localizations. To test the possibility of differential affinities to peroxisomes, fluorescent markers fused to various ATG8 isoforms may be used for colocalization with peroxisomal markers.
The transition of seedling peroxisomes to leaf peroxisomes appears to be a gradual process, as several reports described a transitional form of peroxisomes containing both glyoxylate cycle and photorespiratory enzymes (Titus and Becker, 1985; Nishimura et al., 1986; Sautter, 1986). We do not know which of these three types of peroxisomes is subjected to autophagy (Figure 5). However, our data suggest that autophagy regulated by ATG7 and ATG5 generally affects the homeostasis of multiple types of peroxisomes. We observed higher levels of ICL, MLS, and pHPR proteins in the atg7 and atg5 mutants (Figure 2). Interestingly, Guiboileau et al. (2013) reported that catalase, another peroxisomal protein, overaccumulated in 30-day-old and 60-day-old atg5 mutants, suggesting that peroxisomal proteins are degraded by autophagy in mature plants. Future experiments using a quantitative assay are necessary to assess the selectivity of autophagy in Arabidopsis hypocotyls.
What are the biological functions of autophagy during hypocotyl development? During postgerminative growth, seedling peroxisomes containing glyoxylate cycle enzymes are converted to leaf peroxisomes containing photorespiratory enzymes. ICL-positive peroxisomes become obsolete organelles and are rapidly eliminated by autophagy. In addition, autophagy may play a role in the quality control of damaged and potentially toxic components of cells. Glyoxylate cycle enzymes like ICL and MLS were found to be highly sensitive to oxidative damage (Yanik and Donaldson, 2005; Anand et al., 2009). Autophagy of peroxisomes containing damaged enzymes would allow hypocotyl cells to carry out normal functions. Supporting this view, our results revealed that ATG7 was induced in hypocotyls after ~5 DOI, but not in cotyledons (see Supplemental Figure 5 online). This organ-specific induction is also consistent with the observation that ICL and MLS stabilization is more clearly detected in hypocotyls than in whole seedlings (Figure 2), which consist of cotyledons, true leaves, hypocotyls, and roots. Autophagy is spatiotemporally fine tuned during cell remodeling and differentiation of multicellular organisms (reviewed by Mizushima and Komatsu, 2011), and the local induction of autophagy in Arabidopsis hypocotyls can be a good model to study the regulation of autophagy by a developmental program in plants.
METHODS
Mutants and Transgenic Plants
Arabidopsis thaliana T-DNA insertional mutants atg5-1 and atg7-2 were previously described (Thompson et al., 2005; Chung et al., 2010). The pex5-1 mutant seeds (Zolman et al., 2000), transgenic seeds expressing Pro35S-CFP-SKL and Pro35S-γTIP-CFP, and a binary vector for expressing Pro35S-mCherry-SKL (Nelson et al., 2007) were obtained from The Arabidopsis Information Resource. To generate transgenic Arabidopsis plants expressing GFP-ATG8a under the control of ProUBQ10, Gateway LR Clonase II (Invitrogen) reaction was used, in which an entry clone of the full-length AtATG8a cDNA was inserted into the destination vector pMDC99-AtUBQ10p-GFP (Suttangkakul et al., 2011). The resulting expression clone pMDC99-AtUBQ10p-GFP-AtATG8a was introduced into Agrobacterium tumefaciens strain GV3010, and an Agrobacterium transformant was used to infect Col-0 plants by the floral-dip method (Clough and Bent, 1998). After selection by hygromycin resistance, multiple T1 transgenic lines were analyzed by confocal microscopy to confirm a common pattern of subcellular GFP-ATG8a distribution, which was similar to that of the Pro35S-GFP-ATG8a marker line described previously (Thompson et al., 2005). We chose a ProUBQ10-GFP-ATG8a transgenic line showing a 3:1 segregation ratio for hygromycin resistance in the T2 population. The transgenic line exhibited normal plant morphology in T3 homozygous populations. For colocalization with mCherry-SKL, the representative ProUBQ10-GFP-ATG8a transgenic line was crossed with atg7-2 mutants, and the resulting F1 hybrids were transformed with the binary vector containing Pro35S-mCherry-SKL. Kanamycin-resistant seedlings were selected and genotyped for the mCherry-SKL and GFP-ATG8a transgenes and for atg7-2.
Plant Growth Conditions
Arabidopsis seeds were surface sterilized in 50% (v/v) bleach for 20 min and washed three times with sterile water. The seeds were sown on solid Murashige and Skoog (MS) medium (1× MS macronutrient salt with vitamins, 1% [w/v] Suc, 0.7% [w/v] agar, pH 5.7) and stored at 4°C for 2 days. The plates containing the seeds were incubated at 20 to 22°C under a long-day photoperiod (16 h light and 8 h dark). Uniform germination was monitored after 2 DOI at 21°C. The IBA resistance assay and in vivo detection of hydrogen peroxide were performed as described by Zolman et al. (2000) and Thordal-Christensen et al. (1997), respectively. For liquid culture, the surface-sterilized seeds were placed in 24-well plates containing MS liquid medium (1× MS macronutrient salt with vitamins, 1% [w/v] Suc, pH 5.7). Ten seeds per well were added to 1 mL of MS liquid medium. The 24-well plates were stored at 4°C for 2 days, and incubated with shaking (100 rpm) at 20 to 22°C under a long-day photoperiod (16 h light and 8 h dark). When needed, CA (Wako Chemicals) or an equal volume of DMSO was added to the liquid medium and the samples were further incubated for 17 h before analysis.
RNA Analysis
Total RNA was extracted from whole seedlings, hypocotyls, or cotyledons. For cDNA synthesis, 1 μg of total RNA was added to the reverse transcriptase reaction using oligo-dT primers (Thermo Scientific). Quantitative real-time PCR was performed using SYBR Green (Qiagen) and the gene-specific primers listed in Supplemental Table 1 online. Primers were designed using PrimerQuest (Integrated DNA Technologies), with a typical amplicon size of ~100 bp. Primer efficiencies were 98 to 100%. With UBIQUITIN CONJUGATING ENZYME9 (UBC9) cDNA as an internal reference (Czechowski et al., 2005), relative quantification of transgene expression was calculated from threshold cycle values (Livak and Schmittgen, 2001).
Immunoblot Analysis
Whole seedlings or dissected hypocotyls were homogenized in Laemmli buffer. Protein samples were separated by SDS-PAGE and transferred onto Immobilon-P polyvinylidene fluoride membrane (Millipore). Immunoblots were incubated in a blocking solution (PBS containing 0.1% [v/v] Triton X-100 and either 5% [w/v] nonfat dry milk or 3% [w/v] BSA). Primary antibodies were diluted in blocking solution as follows: 1:2000 for anti-ICL (Ettinger and Harada, 1990) and 1:1000 for anti-GFP (Roche Applied Science), anti-MLS (Ettinger and Harada, 1990), anti-pHPR (Agrisera) (Kleczkowski et al., 1986), anti–peroxisomal malate dehydrogenase (Pracharoenwattana et al., 2007), and anti–histone H3 (Abcam). Chemiluminescence was detected using horseradish peroxidase–conjugated secondary antibodies and SuperSignal West Pico Chemiluminescent Kit (Thermo Scientific).
Microscopy
Images of hypocotyl cells were acquired using a Zeiss 510 laser scanning confocal microscope (Carl Zeiss). Emission filters BP480-520IR, BP500-530IR, and BP565-615IR were used to detect signals of CFP, GFP, and mCherry, respectively. Raw images were processed using an LSM Image Viewer (Carl Zeiss) and Adobe Photoshop CS5 (Adobe Systems). Peroxisome number was estimated from CFP-SKL fluorescence images with ImageJ (National Institutes of Health) using the Cell Counter Plugin.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ATG3 (At5g61500), ATG5 (At5g17290), ATG7 (At5g45900), ATG8a (At4g21980), ATG8b (At4g04620), ATG8d (At2g05630), ATG8g (At3g60640), ATG8i (At3g15580), ICL (At3g21720), MLS (At5g03860), pHPR/HPR1 (At1g68010), and UBC9 (At4g27960).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Confocal Microscopy Images of Wild-Type and atg5-1 Hypocotyl Tissues Expressing the Pro35S-CFP-SKL Transgene.
Supplemental Figure 2. Peroxisome Number after 20 DOI in Wild-Type, atg7-2, and atg5-1 Seedlings Expressing the Pro35S-CFP-SKL Transgene.
Supplemental Figure 3. Peroxisome Number in the Hypocotyls of Etiolated Wild-Type, atg7-2, and atg5-1 Seedlings Expressing the Pro35S-CFP-SKL Transgene.
Supplemental Figure 4. Determination of Peroxisomal Protein Levels in Whole Seedlings of Autophagy-Defective Mutants.
Supplemental Figure 5. Expression of ATG7 Is Induced in Hypocotyls during PostGerminative Growth.
Supplemental Figure 6. Sensitivity of Col-0, atg7-2, and atg5-1 Roots to IBA.
Supplemental Figure 7. In Situ Detection of Hydrogen Peroxide in Wild-Type and Autophagy-Defective Hypocotyls.
Supplemental Figure 8. ATG7 Is Required for the Formation of Autophagic Vesicles Overlapping with Peroxisomal Markers.
Supplemental Table 1. Primers Used in this Study.
Acknowledgments
We thank John Harada (anti-ICL and anti-MLS), Steven Smith, and John Bussell (anti–peroxisomal malate dehydrogenase) for kindly providing antisera. We thank Richard Vierstra for advice on the preparation of the article and for generously sharing genetic stocks, atg7-2, atg5-1, and transgenic lines expressing GFP-ATG8a. We appreciate technical support by Sang Sook Lee and Hye Sun Cho. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2011-0010683) and by a grant from the Next-Generation BioGreen 21 Program (No. PJ009004), Rural Development Administration, Republic of Korea.
AUTHOR CONTRIBUTIONS
J.K., H.L., H.N.L., S.K., and T.C. designed the research. J.K., H.L., H.N.L., S.K., K.D.S., and T.C. performed the research. J.K., H.L., H.N.L., S.K., K.D.S., and T.C. analyzed the data. T.C. wrote the article. J.K., H.L., H.N.L., S.K., and T.C. revised and edited the article.
Glossary
- ICL
isocitrate lyase
- MLS
malate synthase
- PEX5
PEROXIN5
- PE
phosphatidylethanolamine
- GFP
green fluorescent protein
- DOI
days of incubation
- pHPR
peroxisomal hydroxypyruvate reductase
- IBA
indole-3-butyric acid
- CA
concanamycin A
- PexAD
peroxisome-associated protein degradation
- ER
endoplasmic reticulum
- LON2
Lon-related protease 2
- MS
Murashige and Skoog
- UBC9
UBIQUITIN CONJUGATING ENZYME 9
References
- Anand P., Kwak Y., Simha R., Donaldson R.P. (2009). Hydrogen peroxide induced oxidation of peroxisomal malate synthase and catalase. Arch. Biochem. Biophys. 491: 25–31 [DOI] [PubMed] [Google Scholar]
- Bassham D.C., Laporte M., Marty F., Moriyasu Y., Ohsumi Y., Olsen L.J., Yoshimoto K. (2006). Autophagy in development and stress responses of plants. Autophagy 2: 2–11 [DOI] [PubMed] [Google Scholar]
- Bellu A.R., Komori M., van der Klei I.J., Kiel J.A.K.W., Veenhuis M. (2001). Peroxisome biogenesis and selective degradation converge at Pex14p. J. Biol. Chem. 276: 44570–44574 [DOI] [PubMed] [Google Scholar]
- Burkhart S.E., Lingard M.J., Bartel B. (2013). Genetic dissection of peroxisome-associated matrix protein degradation in Arabidopsis thaliana. Genetics 193: 125–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T., Phillips A.R., Vierstra R.D. (2010). ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci. Plant J. 62: 483–493 [DOI] [PubMed] [Google Scholar]
- Chung T., Suttangkakul A., Vierstra R.D. (2009). The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiol. 149: 220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. (1966). Peroxisomes (microbodies and related particles). Physiol. Rev. 46: 323–357 [DOI] [PubMed] [Google Scholar]
- Doelling J.H., Walker J.M., Friedman E.M., Thompson A.R., Vierstra R.D. (2002). The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 277: 33105–33114 [DOI] [PubMed] [Google Scholar]
- Dröse S., Bindseil K.U., Bowman E.J., Siebers A., Zeeck A., Altendorf K. (1993). Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry 32: 3902–3906 [DOI] [PubMed] [Google Scholar]
- Ettinger W.F., Harada J.J. (1990). Translational or post-translational processes affect differentially the accumulation of isocitrate lyase and malate synthase proteins and enzyme activities in embryos and seedlings of Brassica napus. Arch. Biochem. Biophys. 281: 139–143 [DOI] [PubMed] [Google Scholar]
- Farmer L.M., Rinaldi M.A., Young P.G., Danan C.H., Burkhart S.E., Bartel B. (2013). Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell 25: 4085–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Noda N.N., Fujii K., Yoshimoto K., Ohsumi Y., Inagaki F. (2008). In vitro reconstitution of plant Atg8 and Atg12 conjugation systems essential for autophagy. J. Biol. Chem. 283: 1921–1928 [DOI] [PubMed] [Google Scholar]
- Guiboileau A., Avila-Ospina L., Yoshimoto K., Soulay F., Azzopardi M., Marmagne A., Lothier J., Masclaux-Daubresse C. (2013). Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. New Phytol. 199: 683–694 [DOI] [PubMed] [Google Scholar]
- Hanaoka H., Noda T., Shirano Y., Kato T., Hayashi H., Shibata D., Tabata S., Ohsumi Y. (2002). Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 129: 1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge S., Fujiki Y. (2008). The peroxin Pex14p is involved in LC3-dependent degradation of mammalian peroxisomes. Exp. Cell Res. 314: 3531–3541 [DOI] [PubMed] [Google Scholar]
- Hayashi M., Nito K., Toriyama-Kato K., Kondo M., Yamaya T., Nishimura M. (2000). AtPex14p maintains peroxisomal functions by determining protein targeting to three kinds of plant peroxisomes. EMBO J. 19: 5701–5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Baker A., Bartel B., Linka N., Mullen R.T., Reumann S., Zolman B.K. (2012). Plant peroxisomes: Biogenesis and function. Plant Cell 24: 2279–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H., Yoshimoto K., Izumi M., Reisen D., Yano Y., Makino A., Ohsumi Y., Hanson M.R., Mae T. (2008). Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 148: 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J., Ezaki J., Komatsu M., Yokota S., Ueno T., Tanida I., Chiba T., Tanaka K., Kominami E. (2006). Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem. 281: 4035–4041 [DOI] [PubMed] [Google Scholar]
- Kelly A.A., Quettier A.L., Shaw E., Eastmond P.J. (2011). Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol. 157: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Scalf M., Smith L.M., Vierstra R.D. (2013). Advanced proteomic analyses yield a deep catalog of ubiquitylation targets in Arabidopsis. Plant Cell 25: 1523–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.K., Hailey D.W., Mullen R.T., Lippincott-Schwartz J. (2008). Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. USA 105: 20567–20574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kwon C., Lee J.H., Chung T. (2012). Genes for plant autophagy: Functions and interactions. Mol. Cells 34: 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L.A., Randall D.D., Blevins D.G. (1986). Purification and characterization of a novel NADPH(NADH)-dependent glyoxylate reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochem. J. 239: 653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M.J., Bartel B. (2009). Arabidopsis LON2 is necessary for peroxisomal function and sustained matrix protein import. Plant Physiol. 151: 1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M.J., Monroe-Augustus M., Bartel B. (2009). Peroxisome-associated matrix protein degradation in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 4561–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Burgos J.S., Deng Y., Srivastava R., Howell S.H., Bassham D.C. (2012). Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 24: 4635–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Hayashi M., Kato A., Kondo M., Nishimura M. (2002). Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: Dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43: 331–341 [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Higuchi T., Maeshima M., Nakamura K. (1997). A vacuolar-type H+-ATPase in a nonvacuolar organelle is required for the sorting of soluble vacuolar protein precursors in tobacco cells. Plant Cell 9: 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Komatsu M. (2011). Autophagy: Renovation of cells and tissues. Cell 147: 728–741 [DOI] [PubMed] [Google Scholar]
- Nakayama M., Kaneko Y., Miyazawa Y., Fujii N., Higashitani N., Wada S., Ishida H., Yoshimoto K., Shirasu K., Yamada K., Nishimura M., Takahashi H. (2012). A possible involvement of autophagy in amyloplast degradation in columella cells during hydrotropic response of Arabidopsis roots. Planta 236: 999–1012 [DOI] [PubMed] [Google Scholar]
- Nelson B.K., Cai X., Nebenführ A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Nishimura M., Yamaguchi J., Mori H., Akazawa T., Yokota S. (1986). Immunocytochemical analysis shows that glyoxysomes are directly transformed to leaf peroxisomes during greening of pumpkin cotyledons. Plant Physiol. 81: 313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta H.W., Girzalsky W., Erdmann R. (2004). Ubiquitination of the peroxisomal import receptor Pex5p. Biochem. J. 384: 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Smith S.M. (2008). When is a peroxisome not a peroxisome? Trends Plant Sci. 13: 522–525 [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I., Cornah J.E., Smith S.M. (2007). Arabidopsis peroxisomal malate dehydrogenase functions in β-oxidation but not in the glyoxylate cycle. Plant J. 50: 381–390 [DOI] [PubMed] [Google Scholar]
- Sautter C. (1986). Microbody transition in greening watermelon cotyledons Double immunocytochemical labeling of isocitrate lyase and hydroxypyruvate reductase. Planta 167: 491–503 [DOI] [PubMed] [Google Scholar]
- Sláviková S., Shy G., Yao Y., Glozman R., Levanony H., Pietrokovski S., Elazar Z., Galili G. (2005). The autophagy-associated Atg8 gene family operates both under favourable growth conditions and under starvation stresses in Arabidopsis plants. J. Exp. Bot. 56: 2839–2849 [DOI] [PubMed] [Google Scholar]
- Suttangkakul A., Li F., Chung T., Vierstra R.D. (2011). The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 23: 3761–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou F.L., Eastmond P.J. (2012). Seed storage oil catabolism: A story of give and take. Curr. Opin. Plant Biol. 15: 322–328 [DOI] [PubMed] [Google Scholar]
- Thompson A.R., Doelling J.H., Suttangkakul A., Vierstra R.D. (2005). Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138: 2097–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H., Zhang Z., Wei Y., Collinge D. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11: 1187–1194 [Google Scholar]
- Till A., Lakhani R., Burnett S.F., Subramani S. (2012). Pexophagy: The selective degradation of peroxisomes. Int. J. Cell Biol. 2012: 512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus D.E., Becker W.M. (1985). Investigation of the glyoxysome-peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J. Cell Biol. 101: 1288–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff E., Schwacke R., Schneider A., Desimone M., Flügge U.I., Kunze R. (2006). Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol. 141: 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E.L. (1970). Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J. Cell Biol. 46: 435–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S., Ishida H., Izumi M., Yoshimoto K., Ohsumi Y., Mae T., Makino A. (2009). Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 149: 885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Contento A.L., Nguyen P.Q., Bassham D.C. (2007). Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 143: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik T., Donaldson R.P. (2005). A protective association between catalase and isocitrate lyase in peroxisomes. Arch. Biochem. Biophys. 435: 243–252 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K., Hanaoka H., Sato S., Kato T., Tabata S., Noda T., Ohsumi Y. (2004). Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 16: 2967–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K., Jikumaru Y., Kamiya Y., Kusano M., Consonni C., Panstruga R., Ohsumi Y., Shirasu K. (2009). Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21: 2914–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Bartel B. (2004). An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proc. Natl. Acad. Sci. USA 101: 1786–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Monroe-Augustus M., Silva I.D., Bartel B. (2005). Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell 17: 3422–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Yoder A., Bartel B. (2000). Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156: 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]