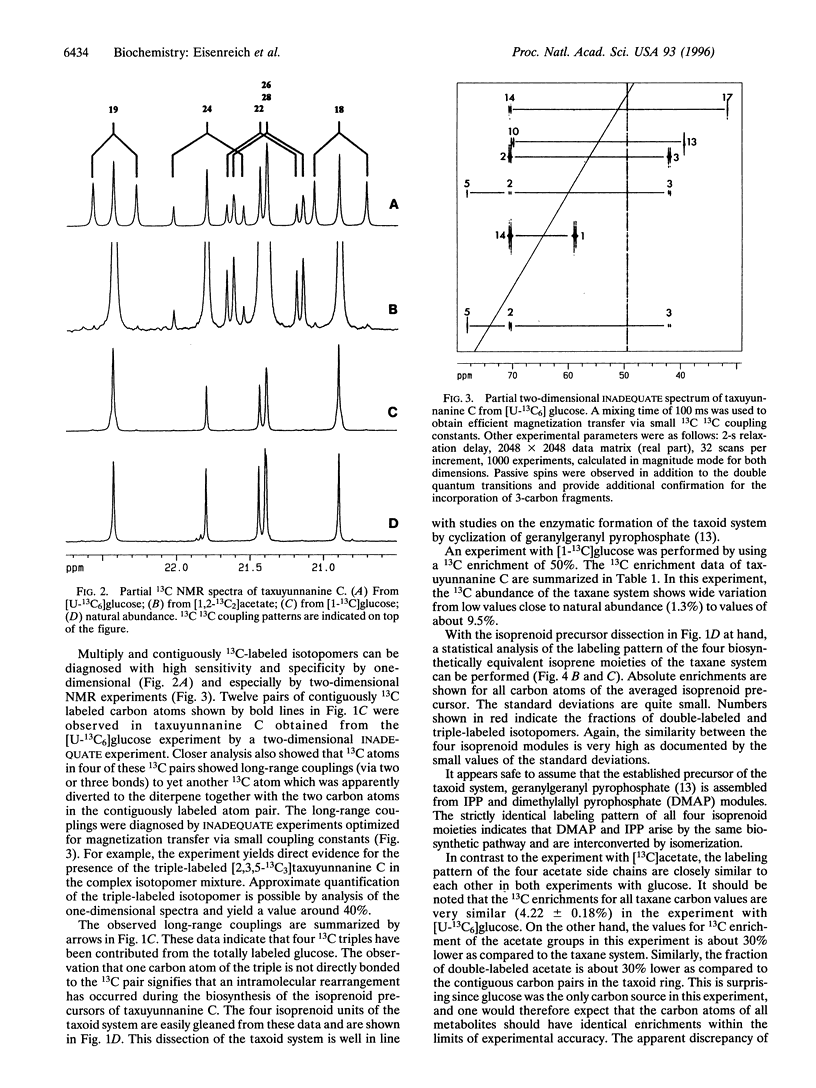
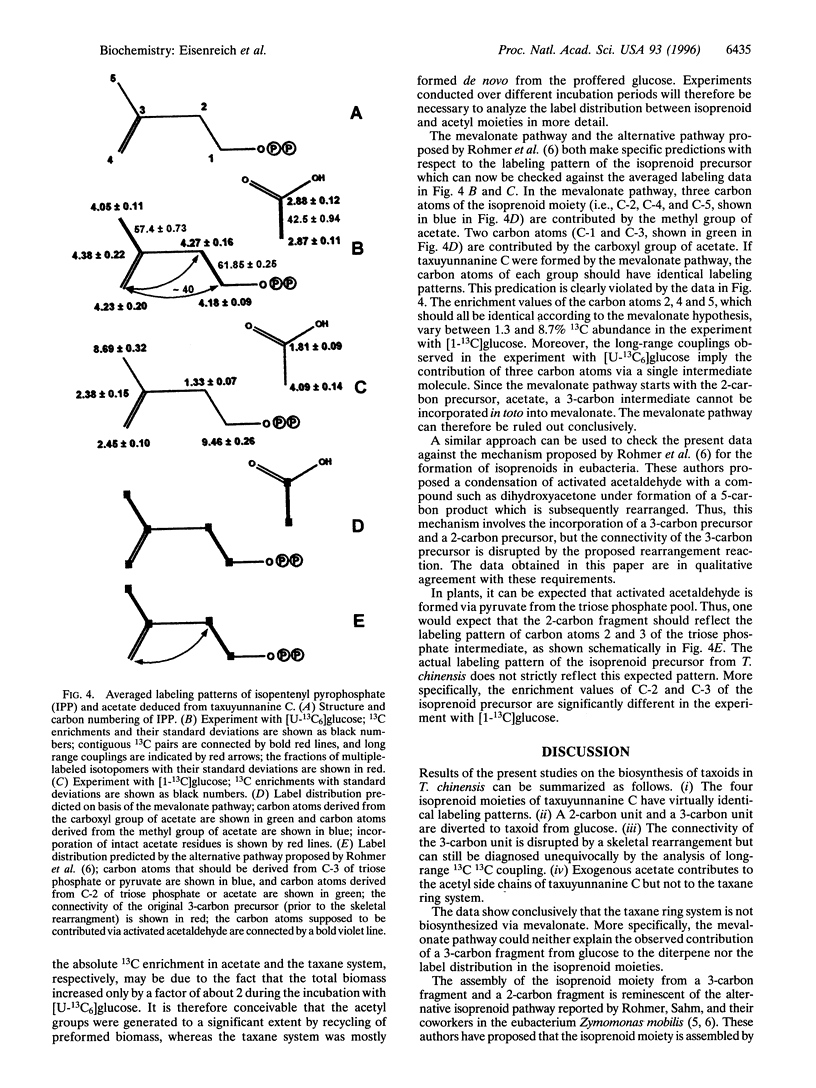
Abstract
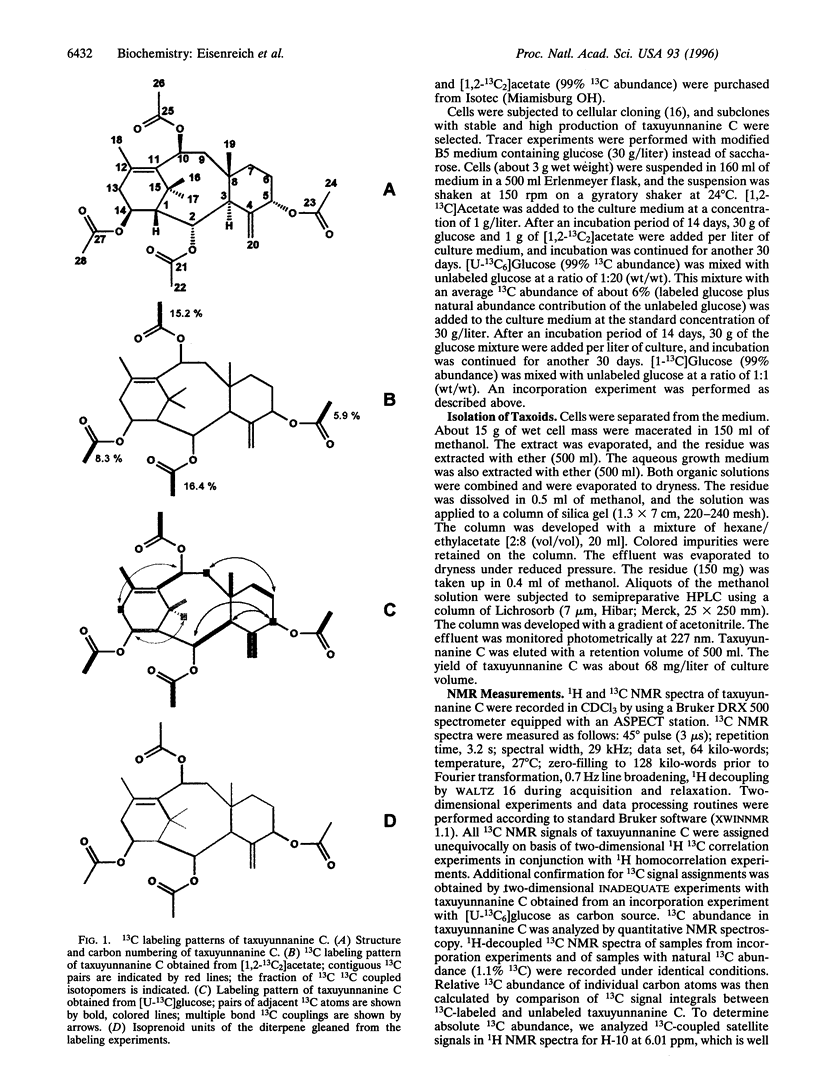
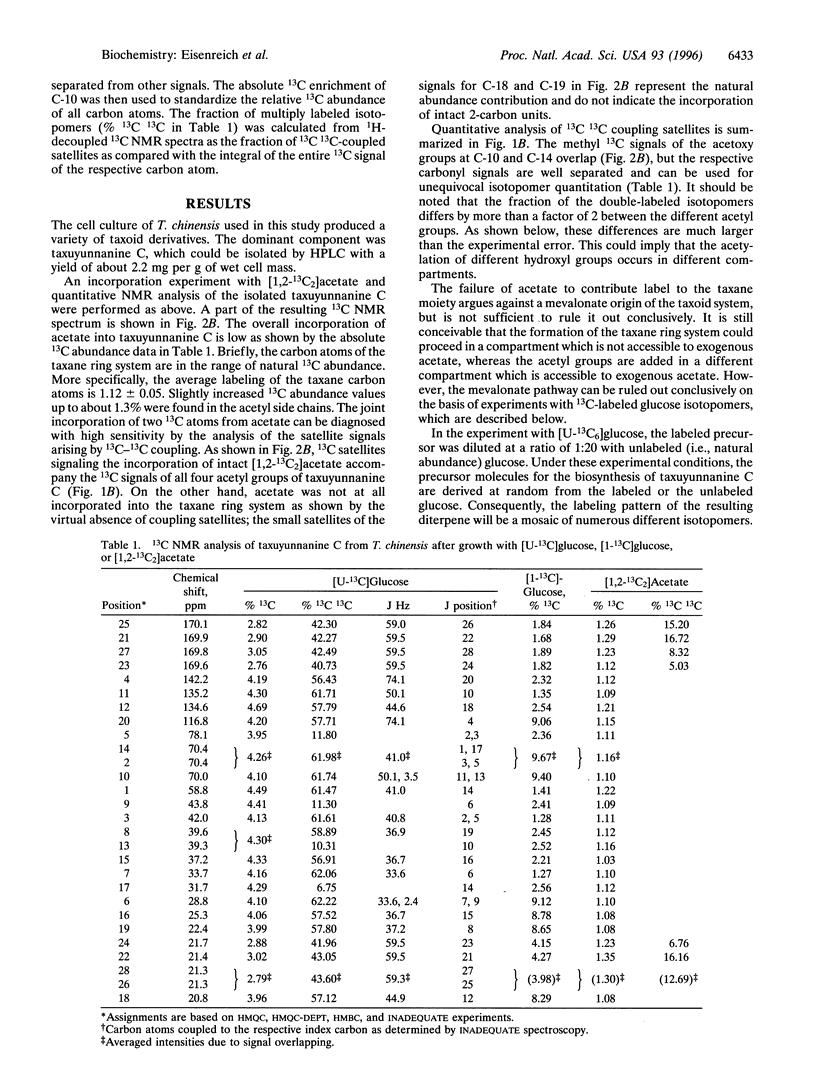
A cell culture of Taxus chinensis was established to produce the diterpene 2alpha,5alpha,10beta,14beta-tetra-acetoxy4 ++ +(20),11-taxadiene (taxuyunnanine C) in 2.6% (dry weight) yield. The incorporation of [U-13C6]glucose, [1-13C]glucose, and [1,2-13C2]acetate into this diterpene was analyzed by NMR spectroscopy. Label from [1,2-13C2]acetate was diverted to the four acetyl groups of taxuyunnanine C, but not to the taxane ring system. Label from [1-13C]glucose and [U-13C6]glucose was efficiently incorporated into both the taxane ring system and the acetyl groups. The four isoprenoid moieties of the diterpene showed identical labeling patterns. The analysis of long-range 13C13C couplings in taxuyunnanine C obtained from an experiment with [U-13C6]glucose documents the involvement of an intramolecular rearrangement in the biosynthesis of the isoprenoid precursor. The labeling patterns are inconsistent with the mevalonate pathway. The taxoid data share important features with the alternative pathway of isoprenoid biosynthesis operating in certain eubacteria Rohmer, M., Knani, M., Simonin, P., Sutter, B. & Sahm, H. (1993) Biochem. J. 295, 517-524].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTAILE J., LOOMIS W. D. Biosynthesis of terpenes. II. The site and sequence of terpene formation in peppermint. Biochim Biophys Acta. 1961 Aug 19;51:545–552. doi: 10.1016/0006-3002(61)90612-6. [DOI] [PubMed] [Google Scholar]
- Bach T. J. Some new aspects of isoprenoid biosynthesis in plants--a review. Lipids. 1995 Mar;30(3):191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- Banthorpe D. V., Charlwood B. V., Francis M. J. The biosynthesis of monoterpenes. Chem Rev. 1972 Apr;72(2):115–155. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- Eisenreich W., Strauss G., Werz U., Fuchs G., Bacher A. Retrobiosynthetic analysis of carbon fixation in the phototrophic eubacterium Chloroflexus aurantiacus. Eur J Biochem. 1993 Aug 1;215(3):619–632. doi: 10.1111/j.1432-1033.1993.tb18073.x. [DOI] [PubMed] [Google Scholar]
- Hezari M., Lewis N. G., Croteau R. Purification and characterization of taxa-4(5),11(12)-diene synthase from Pacific yew (Taxus brevifolia) that catalyzes the first committed step of taxol biosynthesis. Arch Biochem Biophys. 1995 Oct 1;322(2):437–444. doi: 10.1006/abbi.1995.1486. [DOI] [PubMed] [Google Scholar]
- Koepp A. E., Hezari M., Zajicek J., Vogel B. S., LaFever R. E., Lewis N. G., Croteau R. Cyclization of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene is the committed step of taxol biosynthesis in Pacific yew. J Biol Chem. 1995 Apr 14;270(15):8686–8690. doi: 10.1074/jbc.270.15.8686. [DOI] [PubMed] [Google Scholar]
- Ma W., Stahlhut R. W., Adams T. L., Park G. L., Evans W. A., Blumenthal S. G., Gomez G. A., Nieder M. H., Hylands P. J. Yunnanxane and its homologous esters from cell cultures of Taxus chinensis var. mairei. J Nat Prod. 1994 Sep;57(9):1320–1324. doi: 10.1021/np50111a027. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Knani M., Simonin P., Sutter B., Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993 Oct 15;295(Pt 2):517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R., Bacher A. Biosynthesis of riboflavin. Studies on the mechanism of L-3,4-dihydroxy-2-butanone 4-phosphate synthase. J Biol Chem. 1991 Nov 5;266(31):20610–20618. [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]