This work provides evidence into how inhibition of the methylerythritol phosphate pathway causes plant death. Chlorophyll is composed of a chlorin ring, generated by the tetrapyrrole pathway, and an isoprenoid tail, made from the methylerythritol phosphate pathway. The data herein demonstrate that stoichiometric balance between these pathways is essential for viability.
Abstract
Chlorophyll, essential for photosynthesis, is composed of a chlorin ring and a geranylgeranyl diphosphate (GGPP)–derived isoprenoid, which are generated by the tetrapyrrole and methylerythritol phosphate (MEP) biosynthesis pathways, respectively. Although a functional MEP pathway is essential for plant viability, the underlying basis of the requirement has been unclear. We hypothesized that MEP pathway inhibition is lethal because a reduction in GGPP availability results in a stoichiometric imbalance in tetrapyrrolic chlorophyll precursors, which can cause deadly photooxidative stress. Consistent with this hypothesis, lethality of MEP pathway inhibition in Arabidopsis thaliana by fosmidomycin (FSM) is light dependent, and toxicity of MEP pathway inhibition is reduced by genetic and chemical impairment of the tetrapyrrole pathway. In addition, FSM treatment causes a transient accumulation of chlorophyllide and transcripts associated with singlet oxygen-induced stress. Furthermore, exogenous provision of the phytol molecule reduces FSM toxicity when the phytol can be modified for chlorophyll incorporation. These data provide an explanation for FSM toxicity and thereby provide enhanced understanding of the mechanisms of FSM resistance. This insight into MEP pathway inhibition consequences underlines the risk plants undertake to synthesize chlorophyll and suggests the existence of regulation, possibly involving chloroplast-to-nucleus retrograde signaling, that may monitor and maintain balance of chlorophyll precursor synthesis.
INTRODUCTION
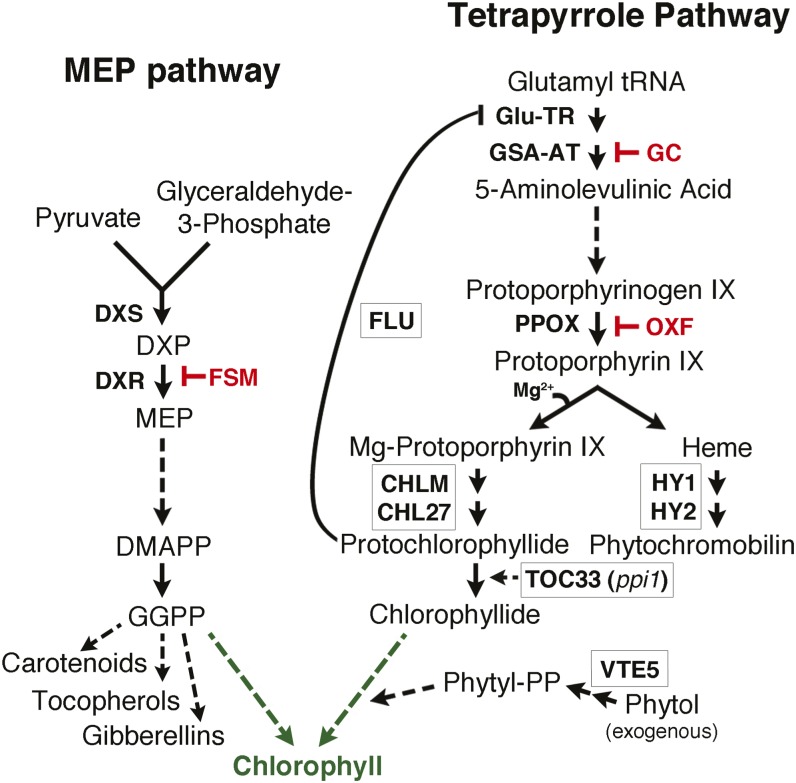
Chlorophyll is one of the most abundant biological molecules on earth and plays an essential role in global carbon cycling through plant solar energy capture and photosynthetic carbon fixation, yet the regulation of chlorophyll biosynthesis remains incompletely understood. Chlorophyll is generated by two distinct biochemical pathways (Figure 1). The tetrapyrrole biosynthetic pathway produces chlorophyllide, a chlorin moiety synthesized from protoporphyrin after Mg2+ incorporation. The methylerythritol phosphate (MEP) metabolic pathway is responsible for the isoprenoid phytol tail of chlorophyll, derived from geranylgeranyl diphosphate (GGPP). Chlorophyll synthase esterifies the hydrophobic hydrocarbon chain of phytyl diphosphate to chlorophyllide (Shalygo et al., 2009). A second, nonplastidic, isoprenoid synthesis pathway, the mevalonate pathway, also exists in plants. Although pathway intermediates may be exchanged between the MEP and mevalonate pathways, the majority of isoprenoids are thought to be derived primarily from one pathway or the other (Bick and Lange, 2003; Hemmerlin et al., 2003; Laule et al., 2003; Schuhr et al., 2003; Bouvier et al., 2005; Dudareva et al., 2005; Hampel et al., 2005).
Figure 1.
The MEP and Tetrapyrrole Pathways Collaborate in Chlorophyll Biosynthesis.
Abbreviated skeletons of the pathways are shown, with steps of particular relevance to this study labeled. Note that only a subset of the intermediates and products are shown for each pathway, and dotted lines indicate several biochemical steps. Of relevance to chlorophyll synthesis, the MEP pathway generates GGPP isoprenoid and the tetrapyrrole pathway produces chlorophyllide. Chemical inhibitors used in this study are shown: FSM inhibits DXR; gabaculine (GC) inhibits glutamate semialdehyde aminotransferase (GSA-AT); and oxyfluorfen (OXF) inhibits protoporphyrinogen oxidase (PPOX). The names of proteins analyzed in this study through use of mutants are in boxes located near pathway step in which the encoded proteins work. ppi1 is the name of the mutant defective in TOC33. The figure also illustrates that exogenous phytol can be incorporated into chlorophyll after phosphorylation.
[See online article for color version of this figure.]
It is well documented that chemical inhibition or genetic impairment of the MEP pathway results in lethality (Mandel et al., 1996; Zeidler et al., 1998; Budziszewski et al., 2001; Gutiérrez-Nava et al., 2004; Rodríguez-Concepción et al., 2004; Hsieh and Goodman, 2005, 2006; Sauret-Güeto et al., 2006; Flores-Pérez et al., 2008, 2010; Hsieh et al., 2008; Phillips et al., 2008). For example, fosmidomycin (FSM), a specific competitive inhibitor of deoxyxylulose-5-P reductoisomerase (DXR), blocks production of MEP pathway products (Laule et al., 2003; Steinbacher et al., 2003; Rodríguez-Concepción et al., 2004) and kills seedlings. FSM-induced lethality in Arabidopsis thaliana is characterized by failure of continued growth after germination; the seedlings die, with bleached-white cotyledons and short roots. These results have led to the conclusion that MEP pathway products are required for seedling life (Estévez et al., 2000; Gutierrez-Nava et al., 2004; Gil et al., 2005; Hsieh and Goodman, 2005, 2006; Hsieh et al., 2008; Phillips et al., 2008). Overexpression of either DXR or DXS, the gene encoding deoxyxylulose 5-P synthase acting one step prior to DXR, results in FSM resistance in Arabidopsis because enhanced MEP pathway flux can overcome the inhibition by FSM and result in successful synthesis of MEP pathway products (Sauret-Güeto et al., 2006). However, several Arabidopsis mutants, identified through screens for FSM resistance, grow in the presence of FSM without apparent upregulation of the MEP pathway or generation of MEP pathway products (Sauret-Güeto et al., 2006; Flores-Pérez et al., 2008, 2010; Van Ree et al., 2011). The ability of plants to survive even when FSM successfully impairs MEP pathway flux indicates that the requirement for MEP pathway product accumulation is conditional; that is, there exist physiological conditions under which abundant MEP pathway products are not required for life. To elucidate the physiological basis for plant survival even with greatly reduced levels of MEP pathway products, we sought to more fully understand the basis for FSM-induced lethality.
The bleached-white cotyledons of FSM-treated seedlings provide a clue as to how FSM treatment may kill seedlings. Bleaching of cotyledons also occurs in the Arabidopsis fluorescent1-1 (flu1-1) mutant (Meskauskiene et al., 2001). flu1-1 dies upon transition from darkness to light because the mutation is thought to cause aberrant protochlorophyllide accumulation in the dark; once moved to light, singlet oxygen (1O2) is generated from the accumulated protochlorophyllide, causing lethal photooxidative stress (Meskauskiene et al., 2001; op den Camp et al., 2003; Kim et al., 2012). Given that aberrant accumulation of chlorophyll porphyrin precursors is sufficient to result in deadly photooxidative stress (Aravind Menon et al., 1989; Kruse et al., 1995; Arakane et al., 1996; Mock and Grimm, 1997), we hypothesized that basal flux through the tetrapyrrole pathway may become toxic if there is a failure to supply stoichiometrically balanced levels of MEP pathway-derived GGPP that are necessary in abundance for conversion of chlorophyllide into chlorophyll. In addition, carotenoids are also synthesized from GGPP and play vital roles, for example, in photooxidative stress protection in plants. Therefore, reductions in carotenoid synthesis by FSM may also contribute to the FSM-induced phenotypes.
In this work, we hypothesize that chlorophyll synthesis, function, and integrity depend upon a balanced accumulation of GGPP and chlorophyll precursors derived from the MEP and tetrapyrrole biosynthesis pathways, respectively. Under conditions of MEP pathway inhibition, the loss of stoichiometric influx of GGPP for esterification to chlorophyllide may be expected to result in free tetrapyrrolic intermediates that can become photoactivated, generate reactive oxygen species, and cause lethality. Furthermore, conditions that reduce tetrapyrrole pathway flux would be predicted to alleviate FSM toxicity. Our findings support these hypotheses and suggest that metabolic flux through the tetrapyrrole and MEP pathways may share coordinated regulation, such that tetrapyrrole intermediate production is counterbalanced with GGPP synthesis. This proposed metabolic balance between the synthesis of both moieties of chlorophyll, chlorophyllide and the terpenoid tail, has significant implications not only for MEP pathway regulation and interpretation of underlying bases for FSM resistance but also for predicted roles of retrograde chloroplast-to-nucleus signaling.
RESULTS
Lethality Attributable to FSM Inhibition of the MEP Pathway Is Light Dependent
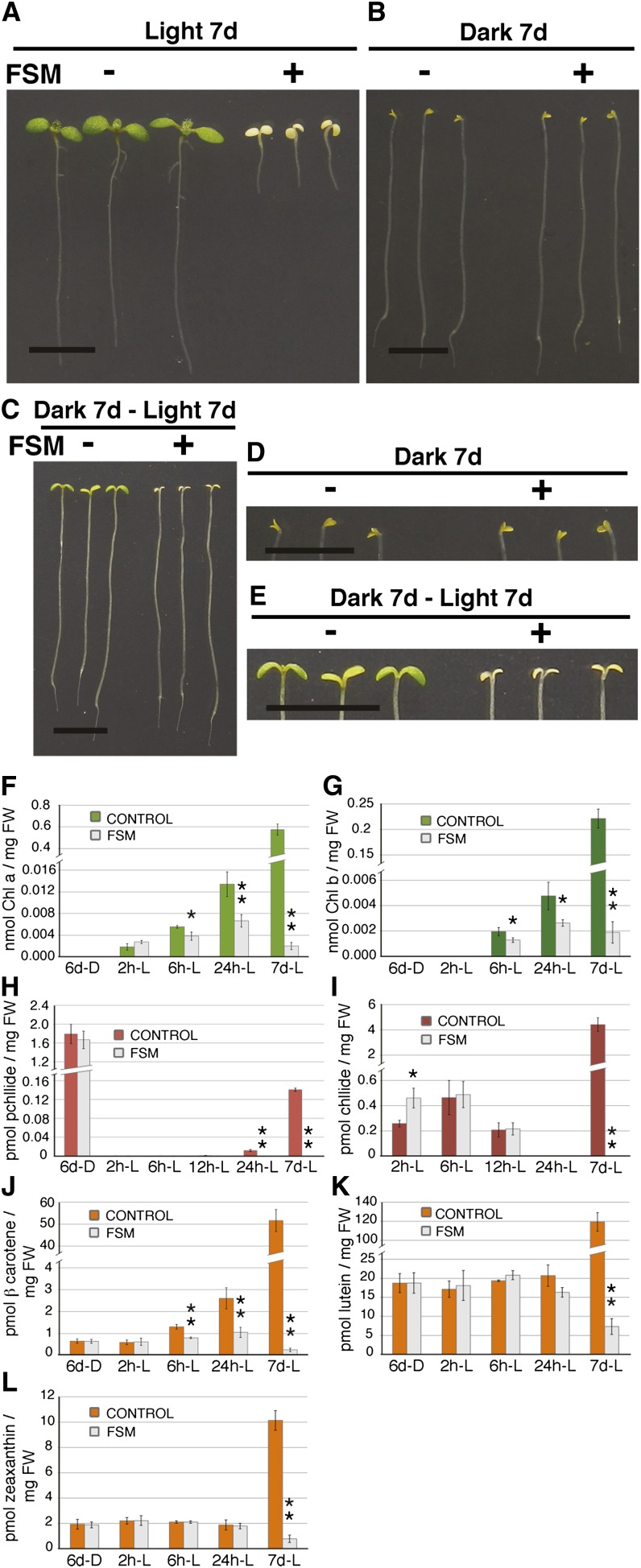
If the hypothesis that FSM-induced lethality is attributable to photoactivation of tetrapyrrole intermediates is true, then FSM toxicity should be light dependent. Figure 2 shows that in contrast with seedlings grown on FSM and exposed to light, which died with the characteristic bleached seedling leaves (Figure 2A), seedlings grown on FSM in the dark showed no detectable phenotypic distinctions from etiolated seedlings grown on medium lacking FSM (Figures 2B and 2D). The lack of requirement for a functional MEP pathway for etiolated seedling growth in the dark was also reported for the cloroplastos alterados1-1 (cla1-1) mutant (Nagata et al., 2002), which is defective in the first step of the MEP pathway (Mandel et al., 1996). By contrast, FSM-exposed, dark-grown seedlings show negative phenotypic consequences when transferred to the light; unlike seedlings on medium lacking FSM, FSM-treated seedlings are defective in developing green pigmentation and become bleached (Figures 2C and 2E). Therefore, the MEP pathway is essential only for light-exposed plants, and the bleached-white phenotype is consistent with the interpretation that death may be a consequence of light-dependent stress, possibly similar to that which occurs in the protochlorophyllide overaccumulation mutant flu1-1 (Meskauskiene et al., 2001; op den Camp et al., 2003).
Figure 2.
Toxicity and Levels of Chlorophyll, Carotenoid, and Tetrapyrrole Porphyrin Intermediates in FSM-Treated Plants Are Light Dependent.
(A) to (C) Representative wild-type Col-0 seedlings grown in light for 7 d (A), in dark for 7 d (B), or in dark for 7 d and then light for 7 d (C), either without (−) or with (+) 50 μM FSM.
(D) Magnified cotyledons of seedlings shown in (B).
(E) Magnified cotyledons of seedlings shown in (C). Bar = 5 mm in (A) to (E).
(F) to (L) Etiolated wild-type Col-0 seedlings were treated without (color bars) or with (gray bars) 50 μM FSM and grown in the dark for 6 d, in dark for 6 d (6d-D) and then exposed to light for various lengths of time: 2 h (2 h-L), 6 h (6 h-L), 12 h (12 h-L), 24 h (24 h-L), or for 7 d (7d-L). These tissues were measured for levels of chlorophyll a (F), chlorophyll b (G), protochlorophyllide (H), chlorophyllide (I), β-carotene (J), lutein (K), and zeaxanthin (L). Data in (F) to (L) were collected from at least three biological replicates; each replicate was a pool of ∼50 seedlings. Error bars denote standard deviations. *P < 0.05; **P < 0.005.
FSM Affects Chlorophyll, Chlorophyll Precursor, and Carotenoid Accumulation in Light-Grown but Not Dark-Grown Seedlings
To determine whether the phenotypic effects of FSM treatment on Arabidopsis correlate with MEP product accumulation, we quantified chlorophyll and carotenoid levels in etiolated seedlings and etiolated seedlings exposed to short-term and longer-term light. Analysis of dark-grown seedlings was important to understand the role of the MEP pathway in isoprenoid production prior to photomorphogenesis and to monitor changes in isoprenoid accumulation upon light exposure. Because the formation of the chlorophyll precursor chlorophyllide is light dependent (Castelfranco and Beale, 1983), no chlorophyll can accumulate in dark-grown seedlings regardless of the presence of FSM (Figures 2F and 2G). In response to light, chlorophyll a and chlorophyll b levels increased within 2 to 6 h (Figures 2F and 2G). This light-induced accumulation of chlorophyll a and chlorophyll b, however, was significantly inhibited in seedlings exposed to FSM, as expected from inhibition in GGPP production. After 24 h of light, FSM-treated seedlings had approximately half the chlorophyll a and chlorophyll b levels of control seedlings (Figures 2F and 2G). More profound effects of FSM were apparent on seedlings with multiday exposure to light; 7 d of light exposure in the presence of FSM resulted in at least 100-fold reduction in chlorophyll species and was accompanied by seedling death (Figures 2F and 2G). The low levels of chlorophyll that did accumulate in FSM-treated seedlings (Figures 2F and 2G) suggest that either this concentration of FSM did not completely block MEP pathway flux or that a portion of GGPP for chlorophyll synthesis was generated from isoprenoid precursors from the mevalonate pathway. These results demonstrate that FSM treatment is effective in reducing chlorophyll formation during early seedling development.
FSM inhibition of GGPP availability may be expected to result in free chlorophyllide upon light exposure. Indeed, although FSM has no effect on etiolated seedling protochlorophyllide levels (Figure 2H), FSM-treated Arabidopsis seedlings have nearly twofold more free chlorophyllide relative to untreated seedlings after 2 h of light exposure (Figure 2I). However, this FSM treatment-enhanced chlorophyllide accumulation is transient; chlorophyllide levels in untreated and FSM-treated seedlings are similar after 6 or 12 h of light exposure (Figure 2I). Free tetrapyrrole pathway intermediates, such as protochlorophyllide and chlorophyllide, may activate feedback inhibition of tetrapyrrole pathway flux (Shalygo et al., 2009; Richter et al., 2010). By 7 d of growth in light, the FSM-treated seedlings lack all detectable protochlorophyllide and chlorophyllide (Figures 2H and 2I), likely because the seedlings were dead or dying. These data are consistent with the hypothesis that reduced levels of GGPP can promote the transient availability of potentially dangerous free chlorophyll precursors, which may underlie FSM-induced toxicity.
To test whether there is also a correlation between carotenoid accumulation and FSM sensitivity, we assessed carotenoid levels in FSM-treated Arabidopsis seedlings. β-carotene accumulation in dark-grown seedlings was unaffected by the presence of FSM (Figure 2J). However, whereas β-carotene levels increased ∼5-fold in untreated seedlings exposed to light for 24 h, this light-induced β-carotene accumulation was largely lost in seedlings exposed to FSM (Figure 2J). FSM had only a limited effect on the accumulation of other carotenoid species in dark-grown seedlings exposed to light for less than 24 h. That is, FSM-treated Arabidopsis seedlings showed only modest changes in lutein, neoxanthin, and violaxanthin levels during the first 24 h of light exposure (Figure 2K; see Supplemental Figure 1 online); zeaxanthin and antheraxanthin levels were unaffected by the presence of FSM (Figure 2L; see Supplemental Figure 1 online). These data demonstrate that carotenoid synthesis in etiolated Arabidopsis seedlings is somewhat insensitive to the presence of FSM during early exposure, providing evidence that isoprenoid precursors for carotenoid synthesis are generated by the mevalonate pathway in etiolated seedlings, consistent with previous reports (Park et al., 2002; Rodríguez-Concepción, 2006). As was seen with chlorophyll (Figures 2F and 2G), carotenoid levels were profoundly reduced in seedlings grown on FSM after 1 week in the light (Figures 2J to 2L; see Supplemental Figure 1 online). These results reveal that FSM treatment also results in decreased carotenoid accumulation in light-grown seedlings; therefore, the reduction in carotenoids may contribute to FSM-induced bleaching of seedlings in the light.
FSM Treatment Results in Elevated Transcript Levels of Singlet Oxygen-Responsive Genes
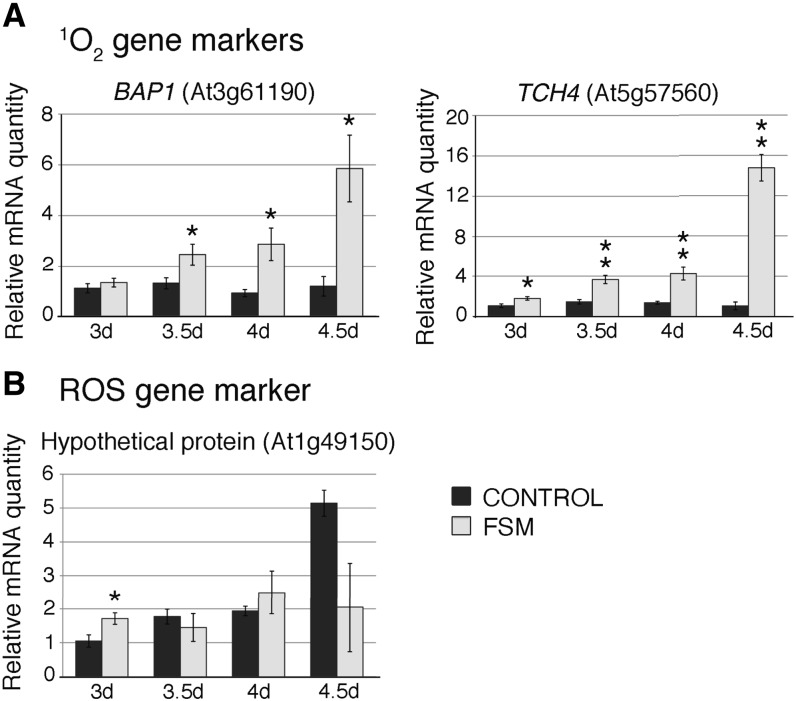
When not properly converted into chlorophyll, the photosensitivity of tetrapyrrolic intermediates generates 1O2 (op den Camp et al., 2003; Triantaphylidès and Havaux, 2009). To further test the hypothesis that FSM toxicity may be caused by 1O2 generated by tetrapyrrolic intermediates that fail to be incorporated with GGPP to form chlorophyll, we monitored transcript levels of BON2-ASSOCIATED PROTEIN1 (BAP1) and TOUCH (TCH4), genes identified previously to be responsive to 1O2, but not to general reactive oxygen stress (op den Camp et al., 2003; Ramel et al., 2012). To avoid the rapid lethality demonstrated by etiolated seedlings exposed to sudden light (Figure 2), seeds were germinated on FSM-containing medium under light. Under these conditions, FSM-treated seedlings continue to enlarge for up to a week, indicating that lethality is more delayed and thus enabling us to monitor gene expression changes over longer time periods. Both BAP1 and TCH4 transcript levels are highly elevated (nearly 6- and 14-fold, respectively) in the light-grown seedlings exposed to FSM compared with control (non-FSM–treated) seedlings (Figure 3A). As a control, we examined expression of a gene encoding a hypothetical protein (HP, At1g49150), which was identified to be responsive to general reactive oxygen stress (op den Camp et al., 2003; Ramel et al., 2012). HP transcript levels were transiently increased but this increase was not sustained in FSM-treated plants (Figure 3B). These transcript data suggest that FSM treatment may result in 1O2 formation possibly because of photooxidizing tetrapyrrole intermediates that fail to be incorporated into chlorophyll. Such photooxidative stress may be responsible for FSM toxicity of light-grown seedlings.
Figure 3.
Transcript Levels of Singlet Oxygen-Responsive Genes Are Increased with FSM Treatment.
Wild-type seedlings were grown in the light for 3 d (3d), 3.5 d (3.5d), 4 d (4d), or 4.5 d (4.5d) without (black bars) and with (gray bars) 50 μM FSM and assayed for transcript levels using quantitative RT-PCR. (A) Transcript levels of singlet oxygen-associated genes BAP1 and TCH4.
(B) Transcript levels of a general reactive oxygen stress–associated gene encoding a hypothetical protein.
Data were compiled from three independent experiments, each with three to five biological samples consisting of ∼50 pooled seedlings. Error bars denote standard error of the mean. *P < 0.05; **P < 0.0005.
Chemical Inhibition of the Tetrapyrrole Pathway Enhances FSM Resistance
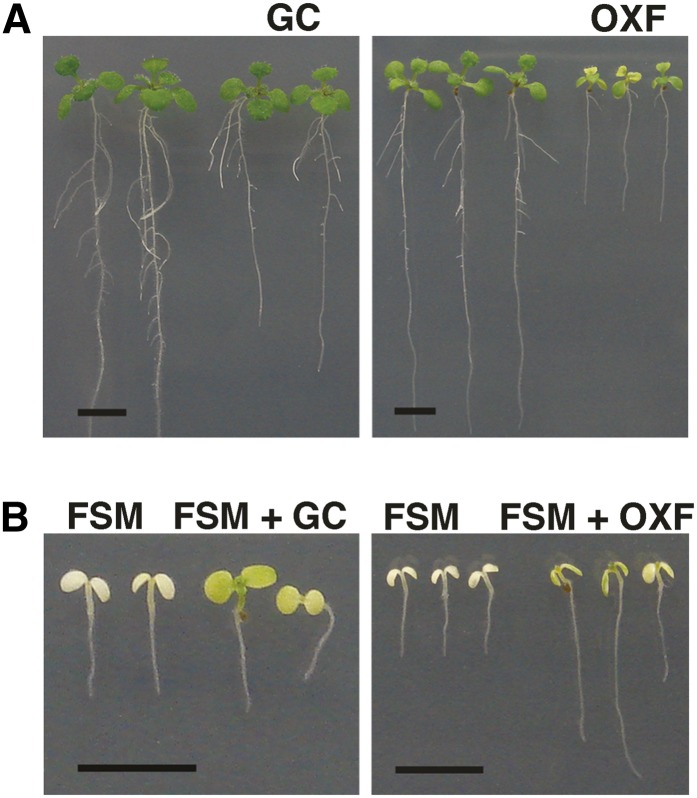
If aberrant tetrapyrrole product accumulation owing to GGPP unavailability were responsible for FSM toxicity, then reduction of tetrapyrrole pathway flux would be predicted to improve FSM tolerance. Gabaculine and oxyfluorfen inhibit glutamate 1–semialdehyde aminotransferase and protoporphyrinogen oxidase, respectively, and block the tetrapyrrole pathway (Flint, 1984; Jacobs and Jacobs, 1993; Lee et al., 1993; Matsumoto et al., 1999). At low concentrations, gabaculine and oxyfluorfen impaired Arabidopsis seedling growth slightly (Figure 4A); however, gabaculine and oxyfluorfen improved seedling growth on FSM compared with FSM treatment alone (Figure 4B). These data indicate that chemical inhibition of tetrapyrrole pathway flux can reduce FSM toxicity.
Figure 4.
FSM Toxicity Is Reduced in the Presence of Tetrapyrrole Pathway Inhibitors.
(A) Representative seedlings grown without inhibitors, with 1 μM gabaculine (GC), or with 5 nM oxyfluorfen (OXF).
(B) Representative seedlings grown with 30 μM FSM alone, 30 μM FSM with 1 μM gabaculine (GC), or 50 μM FSM with 5 nM OXF. Bar = 5 mm.
Mutants Defective in the Tetrapyrrole Pathway Display Enhanced FSM Resistance
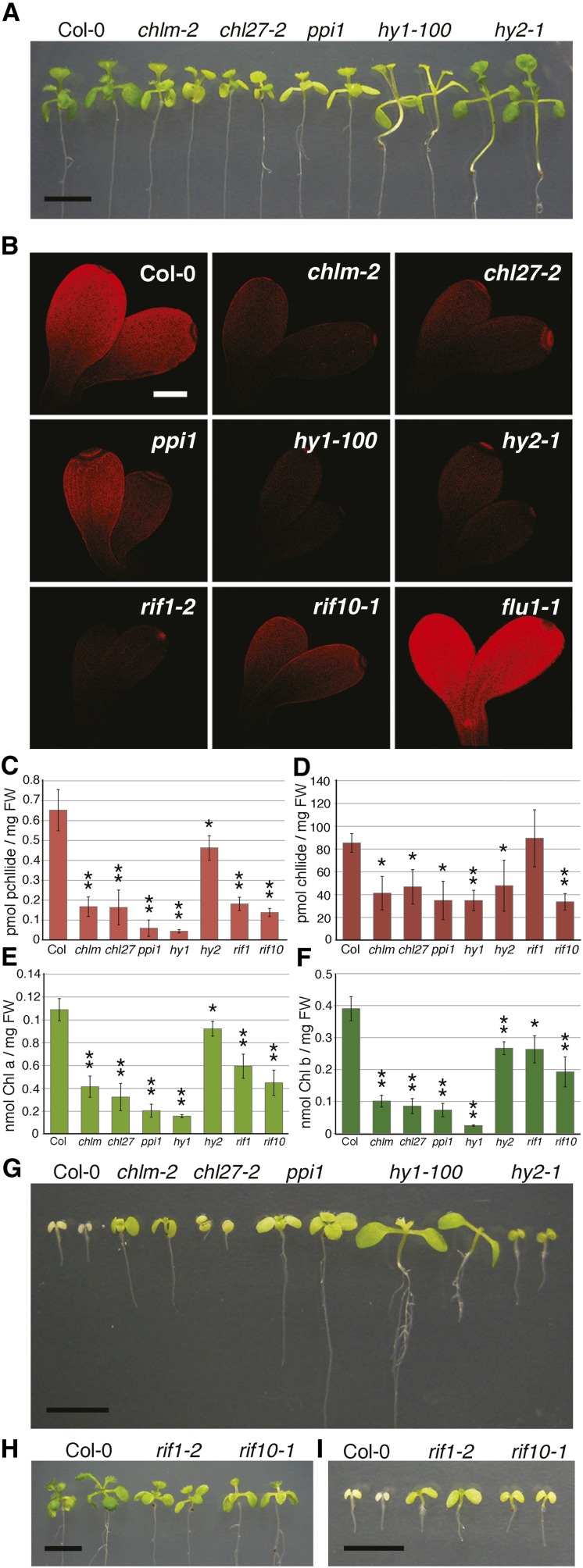
To verify that reduction in the tetrapyrrole pathway can promote FSM resistance, we tested a series of Arabidopsis mutants with defects in proteins critical for tetrapyrrole pathway function. Mutants chlm-2 and chl27-2 have defective enzymes, Mg-protoporphyrin IX methyltransferase (Pontier et al., 2007) and Mg-protoporphyrin IX monomethylester cyclase (Bang et al., 2008), respectively, which act in the Mg branch of the tetrapyrrole biosynthetic pathway (Figure 1), and plastid protein import1 (ppi1) is defective in TOC33, a outer chloroplast membrane transport protein critical for chloroplast protein import, including uptake of NADPH:protochlorophyllide oxidoreductase proteins (Jarvis et al., 1998), which convert protochlorophyllide to chlorophyllide (Figure 1). hy1-100 and hy2-1 are defective in steps downstream of heme production to generate phytochromobilin (Parks and Quail, 1991), the chromophore of phytochrome. Loss of HY1 and HY2 function is thought to lead to reduced tetrapyrrole pathway flux as a result of negative feedback on glutamyl-transfer RNA reductase function mediated through heme accumulation (Figure 1) (Cornah et al., 2003; Rüdiger and Grimm, 2006). As expected for plants with defects in tetrapyrrole synthesis, chlm-2, chl27-2, ppi1, hy1-100, and hy2-1 mutants had varying levels of impaired growth and pigmentation relative to the wild-type (Arabidopsis ecotype Columbia-0 [Col-0]) control seedlings (Figure 5A). The genetic defects in these mutants are predicted to result in reduced tetrapyrrole product synthesis and accumulation; to verify this prediction, we imaged fluorescence emission (610 to 670 nm) of dark-grown etiolated cotyledons exposed to blue light, to detect accumulating amounts of protochlorophyllide in etiolated seedlings (Meskauskiene et al., 2001). The flu1-1 mutant served as a control; etiolated flu1-1 has strong fluorescence as a result of the overaccumulation of protochlorophyllide (Meskauskiene et al., 2001). Whereas Col-0 showed moderate cotyledon fluorescence and flu1-1 had very bright fluorescence, the tetrapyrrole mutants had reduced blue light–induced fluorescence relative to the wild type (Figure 5B), indicating a reduction in accumulation of chlorophyll intermediates. We also directly quantified protochlorophyllide (Figure 5C), chlorophyllide (Figure 5D), chlorophyll a (Figure 5E), and chlorophyll b (Figure 5F) levels in these mutants when grown in the light. Although there were variations in relative levels, all of the mutants had significant reductions in the tetrapyrrole pathway products measured relative to the wild type, verifying that these mutants suffer from defects that affect the tetrapyrrole pathway.
Figure 5.
Tetrapyrrole Pathway Mutants have Defects in Chlorophyll and Porphyrin Accumulation and Increased FSM Resistance.
(A) The representative wild type (Col-0) and mutants with defects in tetrapyrrole pathway steps grown on unsupplemented medium.
(B) Intensity of fluorescence emitted at 610 to 670 nm from 7-d-old dark-grown seedlings excited with 405 nm light. Bar = 0.2 mm.
(C) to (F) Protochlorophyllide (C), chlorophyllide (D), chlorophyll a (E), and chlorophyll b (F) levels in light-grown seedlings. Data were collected from at least three biological replicates; each replicate was a pool of ∼50 seedlings. Error bars denote standard deviation. *P < 0.05; **P < 0.005, respectively.
(G) Representative Col-0 and mutants grown on 30 μM FSM.
(H) Col-0 and two FSM-resistant mutants, rif1-2 and rif10-1, grown on unsupplemented medium.
(I) Col-0, rif1-2, and rif10-1 grown on 30 μM FSM.
Bar = 5 mm in (A) and (G) to (I).
Remarkably, the defects in tetrapyrrole product accumulation in these mutants correlate with growth advantages in the presence of the MEP pathway inhibitor FSM (Figure 5G). The degree of FSM rescue was variable among mutants. Whereas chl27-2 and hy2-1, like Col-0, failed to develop true leaves on FSM, chl27-2 and hy2-1 cotyledons developed more green coloration when grown on FSM than Col-0 (Figure 5G). chlm-2, ppi1, and hy1-100 shoots not only developed green coloration when grown on FSM-containing medium, but the FSM-grown seedlings also elongated their roots, expanded their cotyledons, and showed differing abilities to develop true leaves (Figure 5G). The enhanced FSM resistance of these tetrapyrrole pathway mutants was at least comparable to that of mutants previously identified specifically because of their resistant-in-fosmidomycin phenotypes, rif1-2 (also known as noa1) and rif10-1 (Sauret-Güeto et al., 2006; Flores-Pérez et al., 2008) (Figure 5I). Interestingly, on medium lacking FSM, these rif mutants were also pale green (Figure 5H) and accumulated less protochlorophyllide, chlorophyll a, and chlorophyll b (Figures 5B, 5C, 5E, and 5F), suggesting that they may also be characterized by a reduced tetrapyrrole pathway flux through as yet undefined indirect mechanisms. The one exception to this trend is that only rif10-1 and not rif1-2 had significantly reduced chlorophyllide levels relative to the wild type (Figure 5D).
Although there is a relationship between reduced chlorophyll accumulation in these mutants and an increased tolerance to FSM, the relationship is not perfectly correlative. For example, ppi1 showed more apparent resistance than chl27-2, even though the two mutants both had similar reductions in chlorophyll accumulation relative to the wild type (Figures 5E to 5G). The relationship is likely complex because the gene defects may result in different porphyrin species and accumulation levels depending on the biosynthetic step affected and there may also be feedback regulation as a consequence. Furthermore, the mutations alone cause differing degrees of growth impairment (Figure 5A) that also likely contributed to the seedling phenotypes on FSM.
Together, the data in Figures 4 and 5 indicate that chemical inhibition or genetic defects in the tetrapyrrole pathway can confer enhanced FSM resistance and thus provide additional strong evidence for a mechanistic positive link between the toxicity of FSM inhibition of the MEP pathway and the propensity for accumulation of intermediates from the tetrapyrrole biosynthetic pathway.
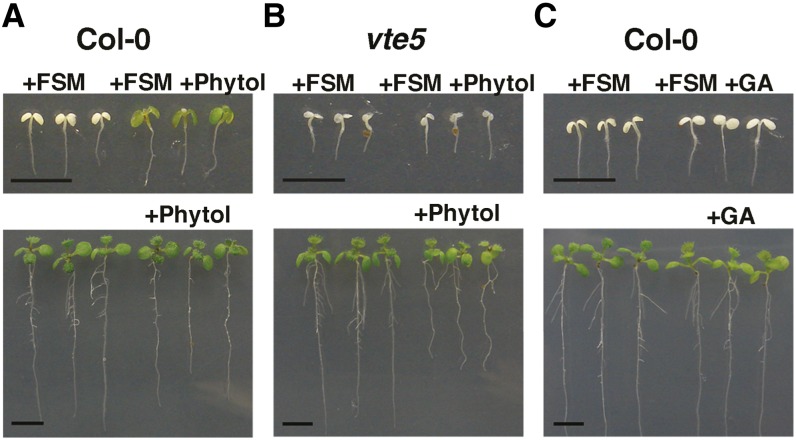
Exogenous Phytol Confers Enhanced FSM Resistance
FSM inhibits flux through the MEP pathway and affects both chlorophyll and carotenoid accumulation, at least in seedlings grown in the light for several days (Figure 2). If the deficiency of GGPP moieties for chlorophyll incorporation is a critical consequence that leads to FSM-induced seedling death, then exogenous provision of phytol should confer FSM resistance. Exogenous phytol can be taken up, phosphorylated, and incorporated into chlorophyll and, to a lesser extent, tocopherol in Arabidopsis (Ischebeck et al., 2006). Figure 6A shows that exogenous phytol enhanced FSM resistance. This phytol-induced FSM resistance was dependent on VITAMIN E PATHWAY GENE5 (VTE5), the enzyme required for phytol phosphorylation prior to chlorophyll incorporation (Valentin et al., 2006) (Figure 6B). Finally, exogenous availability of gibberellin A3, a different MEP-derived isoprenoid similarly converted from GGPP, fails to confer FSM resistance (Figure 6C), demonstrating that rescue by phytol is consistent with the ability of phytol to be incorporated into chlorophyll. To rule out a role for tocopherol in FSM resistance, we analyzed whether phytol can confer FSM resistance to vitamin E pathway gene1 (vte1) and vitamin E pathway gene1 (vte2), mutants defective in tocopherol synthesis. These two mutants show increased growth and green cotyledon coloration in the presence of FSM when supplemented with phytol compared with FSM-treated seedlings lacking phytol treatment (see Supplemental Figure 2 online). Therefore, tocopherol production is not required for phytol-induced FSM resistance, which is consistent with the interpretation that phytol provides enhanced resistance to FSM because of the incorporation of phytol into chlorophyll and thus a reduction in porphyrin intermediate accumulation.
Figure 6.
Exogenous Phytol Confers FSM Resistance.
(A) Col-0 seedlings grown in the presence of 50 μM FSM or FSM and 21 mM phytol (top), or unsupplemented medium or 21 mM phytol (bottom).
(B) vte5 seedlings grown in the presence of 50 μM FSM or FSM and 21 mM phytol (top) or unsupplemented media or 21 mM phytol (bottom).
(C) Col-0 seedlings grown in the presence of 50 μM FSM or FSM, and 10 μM gibberellin (GA) (top), or unsupplemented medium or 10 μM GA (bottom). Bar = 5 mm.
DISCUSSION
These results provide evidence that maintenance of MEP pathway flux is critical for seedling life because the provision of GGPP is needed in balance with tetrapyrrole pathway products for chlorophyll biosynthesis. Chlorophyll is highly abundant in plants and thus in the biosphere; therefore, GGPP for chlorophyll biogenesis is undoubtedly a major output of the MEP pathway. We found that MEP pathway inhibition causes increased expression of singlet oxygen-marker genes and seedling death in a light-dependent manner, data that are consistent with the hypothesis that FSM lethality is caused by photooxidative stress from tetrapyrrole pathway intermediates that fail to be metabolized into chlorophyll molecules. Indeed, reduced MEP pathway flux can be tolerated by reducing the function of the tetrapyrrole pathway, providing evidence that proportionality of MEP pathway flux to tetrapyrrole pathway flux, rather than overall productivity, is the key attribute responsible for seedling viability. Although carotenoid production through the MEP pathway is also undoubtedly important for photooxidative stress protection in plants, FSM-induced carotenoid reduction may not be the primary cause of FSM lethality. Indeed, we find that provision of exogenous phytol leads to enhanced FSM tolerance, consistent with the interpretation that an elevated level of isoprenoid precursor that can be incorporated into chlorophyll, but not carotenoids (Ischebeck et al., 2006), is sufficient to reduce the toxic effects of FSM. Furthermore, we find that the phytol rescue requires the function of VTE5, but not VTE1 or VTE2, indicating that a modification required for chlorophyll incorporation is essential, whereas tocopherol synthesis activities are not essential for reducing FSM toxicity.
This insight into the consequences of MEP pathway inhibition may help to inform new interpretations of the mechanistic bases by which single mutations, such as the rif mutations, confer FSM resistance. FSM resistance can arise not only from increased MEP pathway flux (Sauret-Güeto et al., 2006), but also from decreased tetrapyrrole pathway flux (Figures 4B and 5G). The pathways may be quantitatively synchronized to elevate tolerance to FSM inhibition. Indeed, the FSM resistance phenotypes of rif1-2 and rif10-1 (Sauret-Güeto et al., 2006; Flores-Pérez et al., 2008) correlate with reduced chlorophyll and protochlorophyllide accumulation (Figure 5C, 5E, 5F, and 5I); therefore, these gene products may possibly affect tetrapyrrole pathway flux.
The recognition of the importance of metabolic balance between the MEP and tetrapyrrole pathways leads to the prediction that there may be a regulated coordination between these chloroplastic biosynthetic pathways. Recent work has implicated an MEP isoprenoid intermediate, methylerythritol cyclodiphosphate, as a plastid-derived signaling metabolite that can trigger the expression of photosynthesis-associated nuclear genes (Xiao et al., 2012). Our work leads us to hypothesize that the tetrapyrrole pathway acts as an important signaling pathway whereby tetrapyrrole pathway flux is monitored and relayed into signals that may then proportionally regulate MEP pathway flux. MEP pathway enzymes may be regulated posttranslationally or, alternatively, at the transcriptional level. Because all of the enzymes functioning in the MEP pathway are nuclear encoded, transcriptional regulation would require tetrapyrrole pathway–derived retrograde signals, exiting the chloroplast to somehow regulate transcriptional activity in the nucleus. Revealing the mechanism of coordination of the MEP and tetrapyrrole pathways, two metabolic pathways critical for chlorophyll production and photosynthesis, is a critical next step toward a full elucidation of chloroplast biogenesis and maintenance in plants.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Col-0 wild-type and mutant line seeds were surface-sterilized, stratified, and sown on one-half-strength Murashige and Skoog agar medium supplemented with or without the inhibitors FSM (Invitrogen), oxyfluorfen PESTANAL (Sigma-Aldrich), and gabaculine (Toronto Research Chemicals), and with or without gibberellic acid 3 (MP Biomedical) and phytol (Sigma-Aldrich), at the indicated concentrations. Plants were grown in incubators with 22°C under constant light at ∼80 μmol m−2s−1. Seeds were provided by the following: flu1-1 by Klaus Apel (Boyce Thompson Institute), vte5 by Nick Wagner (Monsanto Company), ppi1 by Danny Schnell (University of Massachusetts Amherst), vte1-1 and vte2-1 by Dean Dellapenna (Michigan State University), and rif1-2 and rif10-1 by Manuel Rodriguez-Concepcion (Centre for Research on Agricultural Genomics). Mutant lines chlm-2 (At4g25080), chl27-2 (At3g56940), hy1-100 (At2g26670), and hy2-1 (At3g09150) were obtained from the ABRC.
HPLC Analysis of Pigments and Chlorophyll Precursors
Wild-type and mutant seedlings grown in the dark for 6 d, and then exposed to light for 0, 2, 6, 12, or 24 h or for 7 d were freeze-dried, homogenized under frozen conditions, and subsequently suspended in ice-cold acetone/0.1 M NH4OH (9/1, v/v) and centrifuged. The supernatants were analyzed by the Agilent 1100 or 1290 HPLC system using a diode array and fluorescence detectors. Chlorophyll and carotenoids were separated on a Prontosil 200-3-C30 (Bischoff-Chromatography) column (3 μm; 250 × 4.6 mm; 21°C) at a flow rate of 1 mL min−1, eluted with a gradient of solvent A (90% acetonitrile; 10% water; 0.1% triethylamine) and solvent B (100% ethyl acetate), and detected at an absorption wavelength of 440 nm. Protochlorophyllide and chlorophyllide were separated on a Nova-Pak C18 (Waters) column (4 µm; 150 × 3.9 mm; 21°C) at a flow rate of 1 mL min−1, eluted with a gradient of solvent A (90% methanol; 10% 1 M ammonium acetate pH 7.0) and solvent B (80% methanol; 20% acetone), and detected at λex 435 nm and λem 644 nm, respectively, as well as absorbance at 430 nm. Identification and quantification of pigments and precursors were performed with authentic standards. Statistical analysis was performed with the Student’s t test.
Quantitative Real-Time RT-PCR
Seedlings grown in unsupplemented and FSM-treated media were pooled to at least 50 mg per biological replicate. At least three biological replicates were assayed per condition, and the data were confirmed with repeated experiments. RNA isolation followed by DNase treatment was completed using the ISOLATE II plant kit (Bioline). One milligram of RNA was reverse transcribed into cDNA with poly (dT) reverse primer and Superscript III reverse transcriptase (Invitrogen), according to manufacturer’s instructions. Quantitative real-time PCR was performed with a CFX96 Real-Time PCR Detection System (Bio-Rad) using SYBR Green PCR Master Mix (Eurogentec). Specific primers for each gene were as follows: BAP1 (forward 5′-CGAATCGAGAAGAAGCAATCC-3′, reverse 5′-ACCTTCAGGTGAATACCTTCC-3′), TCH4 as described (Lee et al., 2005) (forward 5′-GAAACTCCGCAGGAACAGTC-3′, reverse 5′- TGTCTCCTTTGCCTTGTGTG-3′), and HP as described (Ramel et al., 2012) (forward 5′-GACACGACGCCTACAGACAA-3′, reverse 5′-CAACATCTCCATCGCATCAG-3′). TUBULIN4 (forward 5′-CTGTTTCCGTACCCTCAAGC-3′, reverse 5′-AGGGAAACGAAGACAGCAAG-3′) was used as a control to normalize gene expression in each sample. Relative transcript levels were quantified as previously reported (Lee et al., 2005). In short, comparison of relative transcript was calculated using the equation: dCt(sample) = Ct(gene of interest) − Ct(TUB4) and relative quantity = 2 – (dCt (sample) – dCt (control). Statistical comparison between untreated and treated tissues within time points was performed with the Student’s t test.
Porphyrin Precursor Fluorescence
Seeds were sown on plates under dim light and immediately placed in complete darkness to grow for 7 d. The etiolated seedlings were examined using confocal microscopy (Zeiss LSM 710 NLO) with an excitation wavelength of 405 nm and the fluorescence was measured within the range from 610 to 670 nm to detect the most prominent emission spectra of protochlorophyllide and chlorophyllide (Myśliwa-Kurdziel et al., 2003; Stadnichuk et al., 2005). Data were compiled from at least six biological replicates for each genotype sample.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: FLU (At3g14110), CHLM (At4g25080), CHL27 (At3g56940), HY1 (At2g26670), HY2 (At3g09150), RIF1/NOA1 (At3g47450), RIF10 (At3g03710), BAP1(At3g61190), TCH4 (At5g57560), HP (At1g49150), VTE5 (At5g04490), VTE1 (At4g32770), and VTE2 (At2g18950).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article.
Supplemental Figure 1. Carotenoid Levels in FSM-Treated Seedlings.
Supplemental Figure 2. FSM Resistance Conferred by Exogenous Phytol Is Independent of Tocopherol Accumulation.
Acknowledgments
This material is based upon work supported by a grant from the National Science Foundation (MCB 0817976 to J.B.) and a FOR804 grant from the Deutsche Forschungsgemeinschaft (GR-936/12-2 to B.G.). Confocal microscopy was performed on equipment obtained through a Shared Instrumentation Grant from the National Institutes of Health (S10RR02639901). The authors thank Kate Hebl for assistance with microscopy. The authors also thank Klaus Apel for flu1-1, Nick Wagner and Monsanto Company for vte5, Danny Schnell for ppi1, Dean Dellapenna for vte1-1 and vte2-1, and Manuel Rodriguez-Concepcion for rif1-2 and rif10-1. Seeds for hy1-100, hy2-1, chlm-2, and chl27-2 were obtained from the ABRC. The authors also thank Dr. Bonnie Bartel and Braam laboratory members for helpful suggestions and discussions.
AUTHOR CONTRIBUTIONS
J.B. conceived the initial approach and hypothesis; S.K., H.S., K.V.R., K.K., A.S. and A.R. performed the research; and S.K, H.S., K.V.R, A.R., B.G., and J.B. designed the experiments, analyzed the data, and wrote the article.
References
- Arakane K., Ryu A., Hayashi C., Masunaga T., Shinmoto K., Mashiko S., Nagano T., Hirobe M. (1996). Singlet oxygen (1 delta g) generation from coproporphyrin in Propionibacterium acnes on irradiation. Biochem. Biophys. Res. Commun. 223: 578–582 [DOI] [PubMed] [Google Scholar]
- Aravind Menon I., Persad S.D., Haberman H.B. (1989). A comparison of the phototoxicity of protoporphyrin, coproporphyrin and uroporphyrin using a cellular system in vitro. Clin. Biochem. 22: 197–200 [DOI] [PubMed] [Google Scholar]
- Bang W.Y., Jeong I.S., Kim D.W., Im C.H., Ji C., Hwang S.M., Kim S.W., Son Y.S., Jeong J., Shiina T., Bahk J.D. (2008). Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant Cell Physiol. 49: 1350–1363 [DOI] [PubMed] [Google Scholar]
- Bick J.A., Lange B.M. (2003). Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 415: 146–154 [DOI] [PubMed] [Google Scholar]
- Bouvier F., Rahier A., Camara B. (2005). Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lipid Res. 44: 357–429 [DOI] [PubMed] [Google Scholar]
- Budziszewski G.J., et al. (2001). Arabidopsis genes essential for seedling viability: Isolation of insertional mutants and molecular cloning. Genetics 159: 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P.A., Beale S.I. (1983). Chlorophyll biosynthesis: Recent advances and areas of current interest. Annu. Rev. Plant Physiol. 34: 241–276 [Google Scholar]
- Cornah J.E., Terry M.J., Smith A.G. (2003). Green or red: What stops the traffic in the tetrapyrrole pathway? Trends Plant Sci. 8: 224–230 [DOI] [PubMed] [Google Scholar]
- Dudareva N., Andersson S., Orlova I., Gatto N., Reichelt M., Rhodes D., Boland W., Gershenzon J. (2005). The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 102: 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez J.M., Cantero A., Romero C., Kawaide H., Jiménez L.F., Kuzuyama T., Seto H., Kamiya Y., León P. (2000). Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol. 124: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D.H. (1984). Gabaculine inhibits delta-ALA synthesis in chloroplasts. Plant Physiol. 75 (suppl.): 17016663565 [Google Scholar]
- Flores-Pérez U., Pérez-Gil J., Closa M., Wright L.P., Botella-Pavía P., Phillips M.A., Ferrer A., Gershenzon J., Rodríguez-Concepción M. (2010). Pleiotropic regulatory locus 1 (PRL1) integrates the regulation of sugar responses with isoprenoid metabolism in Arabidopsis. Mol. Plant 3: 101–112 [DOI] [PubMed] [Google Scholar]
- Flores-Pérez U., Sauret-Güeto S., Gas E., Jarvis P., Rodríguez-Concepción M. (2008). A mutant impaired in the production of plastome-encoded proteins uncovers a mechanism for the homeostasis of isoprenoid biosynthetic enzymes in Arabidopsis plastids. Plant Cell 20: 1303–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M.J., Coego A., Mauch-Mani B., Jordá L., Vera P. (2005). The Arabidopsis csb3 mutant reveals a regulatory link between salicylic acid-mediated disease resistance and the methyl-erythritol 4-phosphate pathway. Plant J. 44: 155–166 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Nava Mde.L., Gillmor C.S., Jiménez L.F., Guevara-García A., León P. (2004). CHLOROPLAST BIOGENESIS genes act cell and noncell autonomously in early chloroplast development. Plant Physiol. 135: 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel D., Mosandl A., Wüst M. (2005). Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): Metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66: 305–311 [DOI] [PubMed] [Google Scholar]
- Hemmerlin A., Hoeffler J.F., Meyer O., Tritsch D., Kagan I.A., Grosdemange-Billiard C., Rohmer M., Bach T.J. (2003). Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 278: 26666–26676 [DOI] [PubMed] [Google Scholar]
- Hsieh M.H., Goodman H.M. (2005). The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol. 138: 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.H., Goodman H.M. (2006). Functional evidence for the involvement of Arabidopsis IspF homolog in the nonmevalonate pathway of plastid isoprenoid biosynthesis. Planta 223: 779–784 [DOI] [PubMed] [Google Scholar]
- Hsieh M.H., Chang C.Y., Hsu S.J., Chen J.J. (2008). Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol. Biol. 66: 663–673 [DOI] [PubMed] [Google Scholar]
- Ischebeck T., Zbierzak A.M., Kanwischer M., Dörmann P. (2006). A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 281: 2470–2477 [DOI] [PubMed] [Google Scholar]
- Jacobs J.M., Jacobs N.J. (1993). Porphyrin accumulation and export by isolated barley (Hordeum vulgare) plastids (effect of diphenyl ether herbicides). Plant Physiol. 101: 1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P., Chen L.J., Li H., Peto C.A., Fankhauser C., Chory J. (1998). An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282: 100–103 [DOI] [PubMed] [Google Scholar]
- Kim C., Meskauskiene R., Zhang S., Lee K.P., Lakshmanan Ashok M., Blajecka K., Herrfurth C., Feussner I., Apel K. (2012). Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24: 3026–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse E., Mock H.P., Grimm B. (1995). Reduction of coproporphyrinogen oxidase level by antisense RNA synthesis leads to deregulated gene expression of plastid proteins and affects the oxidative defense system. EMBO J. 14: 3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule O., Fürholz A., Chang H.S., Zhu T., Wang X., Heifetz P.B., Gruissem W., Lange M. (2003). Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Polisensky D.H., Braam J. (2005). Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: A focus on calmodulin-like and XTH genes. New Phytol. 165: 429–444 [DOI] [PubMed] [Google Scholar]
- Lee H.J., Duke M.V., Duke S.O. (1993). Cellular localization of protoporphyrinogen-oxidizing activities of etiolated barley (Hordeum vulgare L.) leaves (relationship to mechanism of action of protoporphyrinogen oxidase-inhibiting herbicides). Plant Physiol. 102: 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M.A., Feldmann K.A., Herrera-Estrella L., Rocha-Sosa M., León P. (1996). CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 9: 649–658 [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Kashimoto Y., Warabi E. (1999). Basis for common chickweed (Stellaria media) tolerance to oxyfluorfen. Pestic. Biochem. Physiol. 64: 47–53 [Google Scholar]
- Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. (2001). FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98: 12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock H.P., Grimm B. (1997). Reduction of uroporphyrinogen decarboxylase by antisense RNA expression affects activities of other enzymes involved in tetrapyrrole biosynthesis and leads to light-dependent necrosis. Plant Physiol. 113: 1101–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myśliwa-Kurdziel B., Amirjani M.R., Strzałka K., Sundqvist C. (2003). Fluorescence lifetimes of protochlorophyllide in plants with different proportions of short-wavelength and long-wavelength protochlorophyllide spectral forms. Photochem. Photobiol. 78: 205–212 [DOI] [PubMed] [Google Scholar]
- Nagata N., Suzuki M., Yoshida S., Muranaka T. (2002). Mevalonic acid partially restores chloroplast and etioplast development in Arabidopsis lacking the non-mevalonate pathway. Planta 216: 345–350 [DOI] [PubMed] [Google Scholar]
- op den Camp R.G.L., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Göbel C., Feussner I., Nater M., Apel K. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Kreunen S.S., Cuttriss A.J., DellaPenna D., Pogson B.J. (2002). Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.M., Quail P.H. (1991). Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell 3: 1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.A., León P., Boronat A., Rodríguez-Concepción M. (2008). The plastidial MEP pathway: Unified nomenclature and resources. Trends Plant Sci. 13: 619–623 [DOI] [PubMed] [Google Scholar]
- Pontier D., Albrieux C., Joyard J., Lagrange T., Block M.A. (2007). Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis. Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 282: 2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylidès C., Havaux M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Peter E., Pörs Y., Lorenzen S., Grimm B., Czarnecki O. (2010). Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol. 51: 670–681 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Concepción M. (2006). Early steps in isoprenoid biosynthesis: Multilevel regulation of the supply of common precursors in plant cells. Phytochem. Rev. 5: 1–15 [Google Scholar]
- Rodríguez-Concepción M., Forés O., Martinez-García J.F., González V., Phillips M.A., Ferrer A., Boronat A. (2004). Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell 16: 144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger, W., and Grimm, B. (2006). Chlorophyll metabolism, an overview. In Chlorophylls and Bacteriophylls. Biochemistry, Biophysics, Functions and Applications, B. Grimm, R.J. Porra, W. Rüdiger, and H. Scheer, eds (Dordrecht, The Netherlands: Springer), pp. 133–146. [Google Scholar]
- Sauret-Güeto S., Botella-Pavía P., Flores-Pérez U., Martínez-García J.F., San Román C., León P., Boronat A., Rodríguez-Concepción M. (2006). Plastid cues posttranscriptionally regulate the accumulation of key enzymes of the methylerythritol phosphate pathway in Arabidopsis. Plant Physiol. 141: 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhr C.A., Radykewicz T., Sagner S., Latzel C., Zenk M.H., Arigoni D., Bacher A., Rohdich F., Eisenreich W. (2003). Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem. Rev. 2: 3–16 [Google Scholar]
- Shalygo N., Czarnecki O., Peter E., Grimm B. (2009). Expression of chlorophyll synthase is also involved in feedback-control of chlorophyll biosynthesis. Plant Mol. Biol. 71: 425–436 [DOI] [PubMed] [Google Scholar]
- Stadnichuk I.N., Amirjani M.R., Sundqvist C. (2005). Identification of spectral forms of protochlorophyllide in the region 670-730 nm. Photochem. Photobiol. Sci. 4: 230–238 [DOI] [PubMed] [Google Scholar]
- Steinbacher S., Kaiser J., Eisenreich W., Huber R., Bacher A., Rohdich F. (2003). Structural basis of fosmidomycin action revealed by the complex with 2-C-methyl-D-erythritol 4-phosphate synthase (IspC). Implications for the catalytic mechanism and anti-malaria drug development. J. Biol. Chem. 278: 18401–18407 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C., Havaux M. (2009). Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 14: 219–228 [DOI] [PubMed] [Google Scholar]
- Valentin H.E., Lincoln K., Moshiri F., Jensen P.K., Qi Q., Venkatesh T.V., Karunanandaa B., Baszis S.R., Norris S.R., Savidge B., Gruys K.J., Last R.L. (2006). The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 18: 212–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree K., Gehl B., Chehab E.W., Tsai Y.C., Braam J. (2011). Nitric oxide accumulation in Arabidopsis is independent of NOA1 in the presence of sucrose. Plant J. 68: 225–233 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Savchenko T., Baidoo E.E.K., Chehab W.E., Hayden D.M., Tolstikov V., Corwin J.A., Kliebenstein D.J., Keasling J.D., Dehesh K. (2012). Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149: 1525–1535 [DOI] [PubMed] [Google Scholar]
- Zeidler J., Schwender J., Müller C., Wiesner J., Weidemeyer C., Beck E., Jomaa H., Lichtenthaler H.K. (1998). Inhibition of the non-mevalonate 1-deoxy-d-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z. Naturforsch. 53c: 980–986 [Google Scholar]