Abstract
Small bowel adenocarcinoma is an uncommon gastrointestinal malignancy with limited data on effective chemotherapy in the adjuvant setting, as well as for advanced disease. We present a case report of a patient with recurrent duodenal adenocarcinoma after resection and adjuvant chemotherapy who experienced a complete response to bevacizumab with oxaliplatin and 5FU (FOLFOX) followed by bevacizumab/capecitabine maintenance therapy for 2 years. The patient continues to be disease-free 8 years after his recurrence. This case highlights the potential of vascular endothelial growth factor (VEGF) inhibitors to enhance chemotherapeutic regimens for advanced small bowel adenocarcinoma.
Keywords: Small bowel adenocarcinoma, bevacizumab, oxaliplatin, capecitabine
Background
Adenocarcinoma of the small bowel is relatively rare malignancy which carries a poor prognosis. The American Cancer Society estimated 6,960 new cases of small intestinal cancer diagnosed in the year 2010 (1). According to the National Cancer Data Base, adenocarcinoma represented 36.9% of all patients with small bowel malignancies (2). Despite recent technical advances in imaging and endoscopy (3), delays in diagnosis is common and the majority (58%) of patients present with advanced Stage III or IV disease (2). Small bowel adenocarcinoma (SBA) also has the poorest prognosis among small bowel cancer histologies, with relative five-year observed survival rate of 32.5%, compared to 39.9% for stromal tumors, 49.6% for lymphomas and 64.6% for carcinoids. The median survival and 5-year disease specific survival (DSS) rates of patients with stage IV or recurrent disease are 9 months and 4.2%, respectively. For patients with stage III disease, median survival is 29.8 months and 5-year survival rate is only 35.4% (4). In addition, 5-year DSS is worse for patients with duodenal adenocarcinoma (28.2%) compared to those with jejunal (37.6%) or ileal (37.8%) primaries. Poorly differentiated tumors also carry a worse prognosis with median survival times of 11.1 months compared to 28.6 months for those with well-differentiated tumors. Literature regarding treatment options in the adjuvant and metastatic setting for small bowel adenocarcinoma is limited. There is an urgent need to evaluate novel strategies to treat these rare malignancies. We hereby report the case of our patient with recurrent duodenal adenocarcinoma with a disease-free survival of more than five years after treatment with chemotherapy and an antiangiogenic agent, bevacizumab.
Case report
An otherwise healthy 45-year-old man presented with significant abdominal bloating and tarry stools and was found to have a mass in the third portion of the duodenum. Computed tomography (CT) scan revealed concentric wall thickening of the distal duodenum and a mildly enlarged aortocaval lymph node. A Whipple procedure was performed and identified a tumor in the third portion of the duodenum. Pathologic examination of the 2.5 cm duodenal mass revealed a moderately to poorly differentiated duodenal adenocarcinoma with focal signet ring features. Metastatic carcinoma was found in three of five periduodenal lymph nodes and one omental implant.
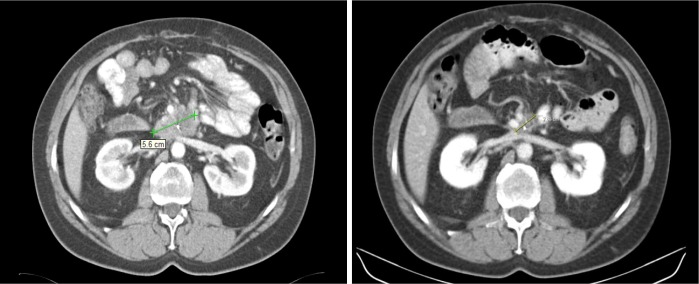
Six weeks following surgery, the patient was started on adjuvant chemotherapy with modified FOLFOX6 for eight doses followed by consolidative chemoradiation. Thirty months following surgery, he developed a local recurrence. CT imaging revealed a soft tissue mass, measuring 4.2 cm × 2.4 cm anterior to the left renal vein and immediately posterior to the superior mesenteric artery. His CEA level was elevated at 16.3 and treatment was started with FOLFOX6 and Bevacizumab with subsequent reduction of the tumor size to 2.4 cm × 1.8 cm after three months (Figure 1). After 12 doses of FOLFOX6, positron emission tomography (PET) showed a residual area without increased FDG uptake, corresponding to the tumor seen on imaging. The patient’s chemotherapy was switched to capecitabine and bevacizumab due to oxaliplatin-related neuropathy. Maintenance chemotherapy was given over a duration of two years after he achieved a complete radiologic and PET response to therapy. The patient continues to be disease-free 8 years since his recurrence.
Figure 1.
Significant radiologic response of the recurrent duodenal adenocarcinoma following 2 cycles of bevacizumab and FOLFOX.
Discussion
Currently, there is no consensus as to the benefit of, and the optimal regimen for, adjuvant therapy for patients with small bowel adenocarcinoma. The rarity of the disease has limited the ability to carry out prospective clinical trials and the optimal regimen remains undefined. Retrospective studies reported no significant survival advantage for patients who received adjuvant chemotherapy after resection of their primary tumors (5-7). In fact, patients who received adjuvant radiotherapy had shorter median survival times at 21.6 months compared to 49.9 months for those who did not (6). However, a multivariate analysis of one of these retrospective studies demonstrated that the use of adjuvant chemotherapy improved disease-free survival, and in patients considered “high risk” (lymph node ratio ≥10%), adjuvant therapy appear to improve survival (7). Despite a lack of clear evidence supporting its use, the National Cancer Data Base [1985-2005] reported an increase in the use of adjuvant chemotherapy from 8.1% in 1985 to 22.5% in 2005 (2). Chemotherapeutic regimens have included 5-FU or capecitabine with or without a platinum compound, such as oxaliplatin (7). Some of these retrospective data are summarized in Table 1.
Table 1. Selected retrospective data regarding adjuvant treatment of small bowel adenocarcinoma.
| Author/Publication | Patient | Number of patients | Treatment | Outcomes | Conclusions |
|---|---|---|---|---|---|
| Bakaeen et al./Arch Surg 2000 | Curative resection of duodenal adenocarcinoma | 17 (total number of patients underwent surgery 68) | Concurrent chemoradiation with 5-FU | 5-year actuarial survival rate 54% | No impact of chemoradiation on survival or cancer recurrence |
| Overman et al./Acta Oncol 2010 | Margin negative surgical resection of small bowel adenocarcinoma (duodenum 67%, Jejunum 20%, Ileum 13%) | 30 (total patients undergoing surgery 54) | Systemic chemotherapy with or without radiation [28] and radiation only [2] | In multivariate analysis, use of adjuvant therapy was associated with improved DFS not OS. In high risk patients (LN ratio ≥10%) adjuvant therapy improved OS but not DFS (P=0.04) |
5-year OS and DFS did not differ between treatment groups. Suggest use of adjuvant therapy for curatively resected small bowel adenocarcinoma |
| Swartz et al./Arch Surg 2007 | Pancreticoduodenectomy for node positive duodenal adenocarcinoma | 14 | Concurrent chemoradiation with 5-FU-based chemotherapy followed by maintainence chemotherapy | 5-year survival rate was 44% and median survival for all patients was 41 months | Chemoradiation compared to historical controls improves median survival (better local control) does not improve overall survival at 5 years |
| Kelsey et al./Int J Radiat Oncol Biol Phys 2007 | Non random. 16= Surgery alone 16= pre or post operative chemoradiotherapy |
16 Preoperative (N=11) Postoperative (N=5) |
Concurrent chemoradiation with 5-FU-based chemotherapy | 5-year survival 57% (vs. 44% with surgery only. P=0.42, NS). Patients with R0 resection, 5 yr survival 83% (vs. 53% in the no chemoradiation arm, P=0.07) | Local failure rates high with surgery alone. Favorable outcomes in patients undergoing complete resection with chemoradiation |
Two years after his last adjuvant chemotherapy, our patient had a radiographic recurrence of duodenal adenocarcinoma with a concurrent rise in his CEA. He then displayed a complete radiographic response to systemic chemotherapy using FOLFOX6 and bevacizumab, followed by maintenance capecitabine and bevacizumab for a period of two years. Remarkably, he continues to be disease-free eight years after his recurrence.
For patients with unresected or metastatic SBA, there was a significant improvement in overall survival with systemic therapy compared to those who received no therapy (12 vs. 2 months; P=0.02) based on the MD Anderson retrospective study (5). Older fluoropyrimidine-based regimens, such as 5FU, doxorubicin with mitomycin yielded a disappointing 18% response rate and an 8-month median survival (8). Two of the 38 evaluable patients had complete radiologic responses. More recently, oxaliplatin plus capecitabine produced a 50% response rate (3 complete responses) with a 20.4-month median survival among 31 patients with small bowel and ampullary adenocarcinomas (9). Excluding the patients with ampullary tumors, response rate was 61% for the 18 patients with SBA. Further support for the use of oxaliplatin-based regimen in SBA arose from a retrospective French multicenter study (10). FOLFOX was associated with a 34% response rate, median progression-free survival of 6.9 months and median OS of 17.8 months. Thus, oxaliplatin-based chemotherapy has been suggested as a new standard for the treatment of metastatic or recurrent SBA (Table 2).
Table 2. Prospective studies in metastatic small bowel adenocarcinoma.
| Author/Publication | Study | Number of patients | Patient characteristics | Chemotherapy | Outcomes: response rates | Outcomes: median survival |
|---|---|---|---|---|---|---|
| Gibson et al./Oncologist 2005 (8) | Phase II, ECOG multi-institutional study | 39 | Locally advanced or metastatic SBA or ampullary adenocarcinoma | 5-FU 600 mg/m2 (days1, 8, 29, 36), doxorubicin 30 mg/m2 (days 1, 29) & Mitomycin C 10 mg/m2 (day 1 only) | 18% (n=7/39, 2 CR) | 8 months |
| Overman et al./JCO 2009 | Phase II MDACC single institution study | 31 | Advanced, unresectable or metastatic SBA or ampullary adenocarcinoma | Capecitabine 750 mg/m2 BID (Days 1-14) and Oxaliplatin 130 mg/m2 (day 1) every 21 days | 52% (3 CR) in the 25 metastatic patients. 61% in SBA vs. 33% in ampullary adenocarcinoma |
20.4 months |
This is the first case report of bevacizumab used both with first-line FOLFOX, and with maintenance capecitabine, in a patient with SBA resulting in a complete radiologic response and prolonged progression-free survival 8 years after his recurrence. Vascular endothelial growth factor A (VEGF-A) overexpression was observed in 91% of SBA (11). Bevacizumab is an anti-VEGF monoclonal antibody with proven efficacy in the treatment of metastatic colorectal cancer (12). Although the mechanism of its efficacy has not been elucidated, results indicate that it renders cancer cells more sensitive to cytotoxic chemotherapy (13). Malignant epithelial cells of the gastrointestinal tract including small bowel adenocarcinoma express VEGF mRNA strongly, in contrast to normal epithelium, hyperplastic polyps, and adenomas (14). A recent study of 54 patients with small bowel adenocarcinoma confirmed this finding. 50 (91%) of these patients’ tissue displayed expression of VEGF-A, with high levels of its expression observed in 44 (81%) patients (11). Thus, there is basic science evidence to suggest that bevacizumab may be effective in SBA. Clinical success with bevacizumab in SBA was reported in 2008 by Tsang et al. (15). A 68-year-old with advanced adenocarcinoma of the jejunum received 8 cycles of gemcitabine and bevacizumab with regression of disease as measured by PET one year after presentation.
In treating this patient’s recurrence, we hypothesized that bevacizumab would give added efficacy to the standard cytotoxic chemotherapy regimen. As noted above, MDACC’s prospective study’s success with capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel included 10% of patients who achieved a complete radiographic response. Because the chemotherapeutic regimens used for this patient’s recurrence also included FOLFOX (which includes oxaliplatin) and, later, capecitabine, it is difficult to ascertain the contribution of bevacizumab to his excellent response. Nonetheless, we present these findings to propose that bevacizumab does warrant further study for treatment of SBA. Table 3 lists current studies for patients with SBA, including one study from MD Anderson utilizing bevacizumab with chemotherapy.
Table 3. Selected ongoing clinical trials in small bowel adenocarcinoma. Ref: www.clinicaltrials.gov.
| Clinicaltrials.gov | Title | Treatment | Phase | Primary outcome measure | Sponsor/Collaborators | Anticipated completion date |
|---|---|---|---|---|---|---|
| NCT01202409 | Phase II Study of Panitumumab Combined With Capecitabine and Oxaliplatin (CAPOX) in Patients With KRAS Wild-type Locally Advanced or Metastatic Adenocarcinoma of the Small Bowel or Ampulla of Vater | Capecitabine 750 mg/m2 bid × 14 days, Oxaliplatin 130 mg/m2 day 1 and Panitumumab 9 mg/kg day 1 every 21 day cycle | II | Response Rate | M.D. Anderson Cancer Center/Amgen | Nov-14 |
| NCT00433550 | A Phase II Trial of Pharmacogenetic-Based Dosing of Irinotecan (I), Oxaliplatin (O), and Capecitabine (C) as First-Line Therapy for Advanced Small Bowel Adenocarcinoma | Group 1 (6/6 UGT1A1 genotype): I (d1)+O (d1)+C (d2-15). Group 2 (6/7): I as group 1, Lower doses of O and C. Group 3 (7/7): I, O and C at lower doses |
II | Tumor response after 12 courses of study treatment | North Central Cancer Treatment Group/National Cancer Institute (NCI) | Dec-12 |
| NCT01208103 | Phase II Study of Bevacizumab Combined With Capecitabine and Oxaliplatin (CAPOX) in Patients With Advanced Adenocarcinoma of the Small Bowel or Ampulla of Vater | Capecitabine 750 mg/m2 bid × 14 days, Oxaliplatin 130 mg/m2 day 1 and Bevacizumab 7.5 mg/kg day 1 every 21 day cycle | II | Progression free survival | M.D. Anderson Cancer Center/Genentech | May-15 |
| NCT00987766 | Phase Ib Trial of Gemcitabine and Oxaliplatin (GEMOX) With Erlotinib in Patients With Advanced Biliary Tract Cancer. (includes Pancreatic Cancer, Duodenal Cancer, or Ampullary Cancer) | Erlotinib by mouth daily 6 days every other week, Gemcitabine IV every other week, Oxaliplatin IV every other week | I | MTD and recommended phase II dose of erlotinib in combination with gemcitabine and oxaliplatin | Vanderbilt-Ingram Cancer Center/National Cancer Institute (NCI) | Jan-16 |
Conclusions
This is an unusual case of a patient with prolonged disease-free survival of a recurrent small bowel signet ring adenocarcinoma who experienced a complete radiologic response to bevacizumab with oxaliplatin-based chemotherapy. Further investigation of this regimen is warranted.
Acknowledgements
Disclosure: The authors have no conflict of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300 [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71 [DOI] [PubMed] [Google Scholar]
- 3.Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy 2008;40:488-95 [DOI] [PubMed] [Google Scholar]
- 4.Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer 1999;86:2693-706 [DOI] [PubMed] [Google Scholar]
- 5.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518-26 [DOI] [PubMed] [Google Scholar]
- 6.Chang HK, Yu E, Kim J, et al. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol 2010;41:1087-96 [DOI] [PubMed] [Google Scholar]
- 7.Overman MJ, Kopetz S, Lin E, et al. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 2010;49:474-9 [DOI] [PubMed] [Google Scholar]
- 8.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 2005;10:132-7 [DOI] [PubMed] [Google Scholar]
- 9.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 2009;27:2598-603 [DOI] [PubMed] [Google Scholar]
- 10.Zaanan A, Costes L, Gauthier M, et al. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol 2010;21:1786-93 [DOI] [PubMed] [Google Scholar]
- 11.Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer 2012:144-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42 [DOI] [PubMed] [Google Scholar]
- 13.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science 2006;312:1171-5 [DOI] [PubMed] [Google Scholar]
- 14.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993;53:4727-35 [PubMed] [Google Scholar]
- 15.Tsang H, Yau T, Khong PL, et al. Bevacizumab-based therapy for advanced small bowel adenocarcinoma. Gut 2008;57:1631-2 [DOI] [PubMed] [Google Scholar]