Abstract
Background:
Saline nasal irrigation (SNI) is often recommended as additional nonpharmacologic treatment, having proven its efficacy in acute and chronic rhinosinusitis and for therapy after sinonasal surgery. To date, however, no systematic review or meta-analysis exists showing the influence of SNI on allergic rhinitis (AR). This study aimed to establish the impact of SNI on symptoms of AR in different patient groups.
Methods:
We conducted a systematic search of Medline, Embase, Cochrane Central Register of Controlled Trials, and ISI Web of Science databases for literature published from 1994 to 2010 on SNI in AR. Prospective, randomized, controlled trials that assessed the effects of SNI on four different outcome parameters were included. The evaluation focused on primary (symptom score) and secondary parameters (medicine consumption, mucociliary clearance, and quality of life).
Results:
Three independent reviewers chose 10 originals that satisfied the inclusion criteria (>400 participants total) from 50 relevant trials. SNI performed regularly over a limited period of up to 7 weeks was observed to have a positive effect on all investigated outcome parameters in adults and children with AR. SNI produced a 27.66% improvement in nasal symptoms, a 62.1% reduction in medicine consumption, a 31.19% acceleration of mucociliary clearance time, and a 27.88% improvement in quality of life.
Conclusion:
SNI using isotonic solution can be recommended as complementary therapy in AR. It is well tolerated, inexpensive, easy to use, and there is no evidence showing that regular, daily SNI adversely affects the patient's health or causes unexpected side effects.
Keywords: Adjunctive treatment, allergic rhinitis, hypertonic saline solution, isotonic saline solution, nasal douche, nasal lavage, nasal obstruction, pollinosis, saline nasal irrigation, sneezing
Allergic rhinitis (AR) is a global health problem. The prevalence of AR is increasing in areas with low and medium levels of frequency and may be plateauing or even decreasing in high prevalence regions.1 It ranges up to 40% for seasonal AR (SAR) and up to 13% for perennial AR.1–3 Medical spending to treat AR almost doubled from $6.1 billion in 2000 to $11.2 billion in 2005.4 Mean annual out-of-pocket expenses related to AR were $520/person in 2005.4 AR affects social life, sleep, school, and work, making treatment imperative. The management of AR encompasses patient education on avoidance of allergens as well as the use of pharmacotherapy and allergen-specific immunotherapy.1 The administration of intranasal glucocorticosteroids is the most effective pharmacologic treatment in AR.5 A pronounced fear of cortisone, however, exists among patients and prescribing physicians.6 The majority of patients with AR has not been treated adequately and, most notably, not according to current guidelines.7
In light of this, nonpharmacologic therapy approaches are of great importance. One such approach is nasal irrigation using saline solutions, which in international guidelines and reviews is recommended as complementary treatment of AR without its efficacy ever having been established conclusively.1,2,5–7
The objective of the present study, therefore, was to verify the effectiveness of nasal irrigation in AR based on the criteria of evidence-based medicine. To this purpose, a systematic literature analysis and a meta-analysis of relevant publications were conducted.
MATERIALS AND METHODS
A search for the literature intended for this review was performed using the comprehensive databases MEDLINE (Medical Literature Analysis and Retrieval System Online), CENTRAL (Cochrane Central Register of Controlled Trials), EMBASE (Excerpta Medica Database), and Web Of Science (ISI Web of Knowledge).
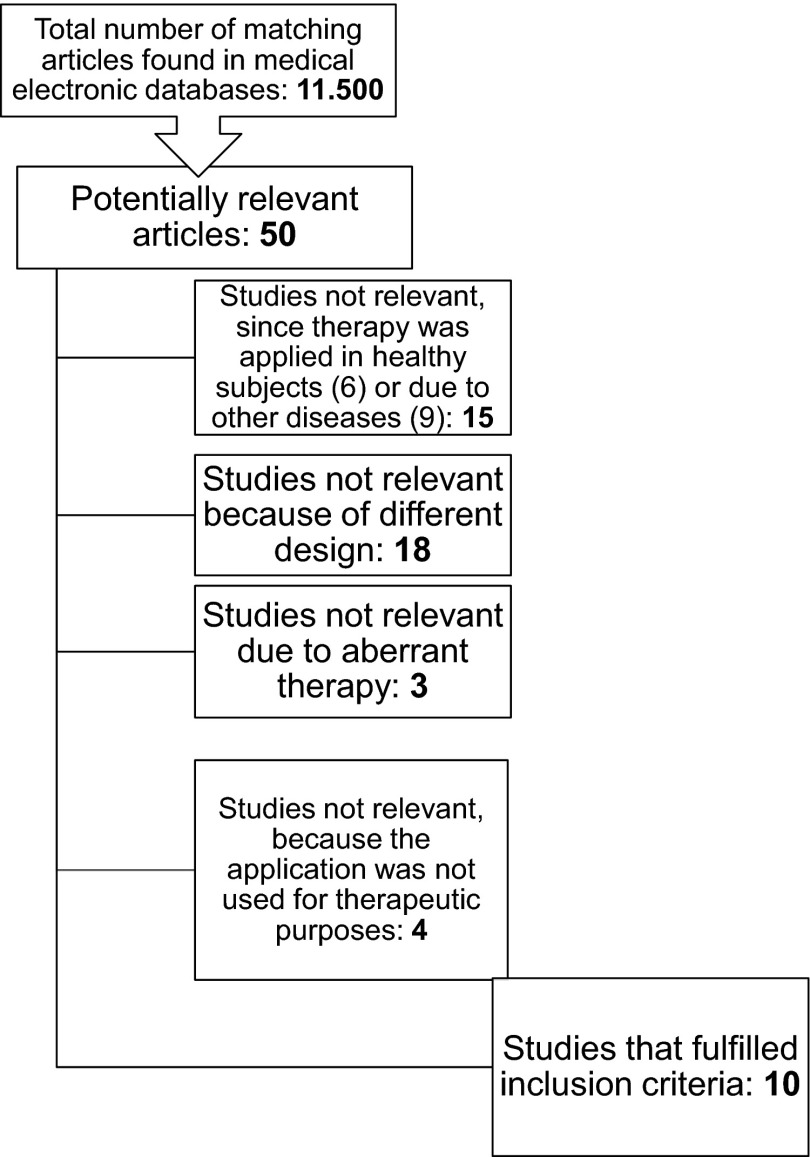
The systematic search for relevant original articles was based on the topic areas “allergic rhinitis,” “nasal irrigation,” and “treatment.” The corresponding key words (allergic rhinitis, saline irrigation, intranasal lavage, saline solutions, nasal douche, nasal rinsing, nasal saline, nasal saline wash, treatment, saline treatment, and alternative treatment) were used in the search in alternating combinations and linked via the operators “AND” and “OR.” The defined limitations only allowed for randomized, controlled studies. No restrictions were made in terms of period of publication and study duration. Furthermore, only studies published in English and German and only those having human subjects of investigation were incorporated in the search. Additional literature was found while reviewing the reference lists of selected articles and, particularly, reviews. After excluding duplicate articles, 50 clinical studies remained in which their titles harmonized with the defined topic. Another 40 studies were excluded based on the information about study design, subjects, intervention/application, control group, outcome parameters, and diagnosis. Decisions about exclusions were made by two independent reviewers (KH, RM). Any discrepancies were discussed by three of the authors (KH, RW, RM). Only articles that fulfilled the following criteria were included:
Study design—Prospective studies having at least evidence level IIa (German Cochrane Center—Cochrane Classification).8
Study content—Local, nasal applications with saline solution for treating seasonal or perennial AR.
Subjects—Adults, pregnant women and children as patients. Confirmation of diagnosis by means of positive patient history or allergy testing using skin tests (prick test) or blood tests (e.g., radioallergosorbent test).
Intervention—Nasal irrigation in liquid or nebulized form.
Primary results—Improvement of cardinal allergy symptoms–sneezing, itching, obstruction, and secretion.
Secondary results—Medicine consumption, mucociliary clearance time (MCT), and quality of life.
In the end, excluded were:
Fifteen studies in which another respiratory disorder was (also) treated.
Eighteen studies that had a divergent design.
Three studies because of their different treatment methods.
Four studies in which saline nasal irrigation (SNI) was not applied for therapeutic purposes.
Ten studies (Fig. 1) fulfilled the aforementioned inclusion criteria.9–18
Figure 1.
Flowchart of study selection.
Quality assessment was conducted thereafter by two independent reviewers according to Jadad.19 The Jadad scale is currently the only validated scale for assessing methodological study quality.20 During the assessment process, the parameters randomization, blinding, dropouts, and supplementary questions were evaluated. Only the study by Barbieri et al.9 was assessed as qualitatively low at <3 points. All other relevant studies achieved a minimum of 3 to a maximum of 5 points. No randomization was conducted in only 1 of the 10 studies.10 All studies had a control group in which either SNI was not applied or, alternatively, saline nasal spray, oily drops, steroids, or cetirizine was administered. In one study, two active control groups with isotonic and hypertonic SNI were compared.18 A meta-analysis was prepared thereafter to be able to compare the respective improvement in the symptom scores of the subjects after SNI and the relative improvement to the control groups. The symptom score was evaluated in 8 of the 10 studies.9,10,12–17 Further meta-analyses were based on the secondary parameters and their improvement after applying SNI. The consumption of medicine was examined in three studies,12–14 MCT in four,9,10,16,18 and quality of life in two trials.11,17
Statistical Methods
The present meta-analysis was performed using Comprehensive Meta-Analysis Version 2.2.057 software (Biostat, Englewood, NJ). First, the relative improvements of the individual variables were defined, either with regard to the maximal scale value (symptom score and quality of life) or the baseline value (medicine consumption and MCT). Moreover, the standard deviation of the change before as opposed to following therapy was estimated using either the standard deviation of the baseline value (more exact information on the variability of the change could unfortunately not be gathered from the individual articles) or 25% of the scale range (with normally distributed values the range mean ± [i.e., a width of four standard deviations] covers ∼95% of the measured values). The relative improvements were then weighted using a random effects model and combined.
RESULTS
Analysis of the Literature
In all, 10 original articles published from 1994 to 2010 were included in the closer analysis. They vary considerably with regard to study design, number of subjects, study duration, and in terms of the saline solutions used, mode of application, and the parameters assessed. Despite the heterogeneity of the studies involved, a congruent trend in the results could be established:
In the prospective, randomized, and controlled study by Barbieri et al.9 in 2002, iodine bromide thermal water from Salsomaggiore was applied in spray form in 40 patients with AR seven times daily over a period of 30 days. The control group used oil drops over the same period of time and at the same frequency. After treatment, a symptom score improvement of 45% and an acceleration of MCT by 29% was observed after assessing the nasal obstruction on a scale of 0–10 in the active group compared with prestudy values.9
Cingi et al.10 conducted a prospective study with 100 AR patients in 2010. Before and after the 10-day application of seawater gel spray (2 sprays/nostril at 4-hour intervals), the four cardinal symptoms were evaluated on a scale of 0 to 3. An improvement of 31% in the symptom scores could be established. MCT was quickened by 12%.10
In a prospective, randomized, single-blinded, placebo-controlled study, Cordray et al.11 compared the effectiveness of hypertonic seawater spray used by 15 study participants with SAR for 1 week (2 sprays/nostril three times daily). The evaluation, based on the “Rhinoconjunctivitis Quality of Life Score,” showed clinically and statistically significant improvements (p < 0.0001) of symptoms. Answers were given to 28 questions on quality of life and assessed on a scale of 0 to 6, based on which an improvement in quality of life of 23% was calculated.11
In 2003, Garavello et al.12 investigated the effectiveness of nasal irrigation with a hypertonic (3.0%) saline solution for treating AR in children (aged 6–12 years). In a prospective, nonblinded, randomized controlled study, 10 children received hypertonic SNIs (2.5 mL/nostril using a disposable syringe) three times daily for 6 weeks during the entire pollen season. Another 10 children received no nasal irrigation and constituted the control group. The rhinitis score showed a reduction of 3% in allergy-induced symptoms and an absolute decrease in medicine consumption of 100%.12
Garavello et al.13 used SNI with hypertonic saline solution in a randomized, controlled, unblinded study of 20 children with AR in 2005. Nasal rinsing consisted of 3 sprays/nostril, each containing 50 μL, three times daily. The analysis of the rhinoconjunctivitis score, answered daily, yielded a clinically and statistically significant 13% reduction in symptoms in the active treatment group after the 7th week in comparison with the beginning of the study. Furthermore, an average of 5% less antihistamine was consumed during the period in which nasal irrigation was performed regularly.13
In 2010, Garavello et al.14 evaluated the applicability of nasal irrigation in pregnant women with SAR in a prospective, randomized study. Twenty-two pregnant women with AR underwent nasal irrigation using a hypertonic saline solution (10 mL/ nostril using a disposable syringe) three times a week over a period of 6 weeks. The control group did not receive local therapy. The symptom score worsened, in fact, by 6% from the preseasonal value to the end of the investigation and antihistamine consumption increased by 67%. However, compared with the women who did not apply nasal irrigation, the symptom score and consumption of antihistamines were significantly lower in subjects who used nasal irrigation.14
Klimek et al.15 investigated the effects of nasal irrigation treatment with isoosmotic saline solution in adult patients with SAR in an open-label, randomized, parallel-group study in 2001. In this study, 75 patients were treated over 2–4 weeks with diverse drugs as needed, and one-half of the patients additionally performed SNI, using an Emser saline solution and the Rhinocare nasal douche (250 mL) two to three times daily. In the nasal irrigation group, significant reductions of 42% in the symptom scores resulted. Overall, 15.0 ± 9.7 tablets on average were consumed during the observation period, which in relation to the recommended daily dose during the total time period corresponds to a quotient of 0.51 ± 0.4.15
In the course of a prospective, randomized study published in 2009, Li et al.16 treated 26 children (aged 8–15 years) with AR in three groups over a period of 12 weeks either only with SNI in group 1, or a combination of SNI and steroids in group 2 or only with steroids in group 3. A solution of 500 mL of normal saline (0.9% sodium chloride) was used twice a day for nasal irrigation. The symptom score fell by 19% in group 1 and 30% in group 2. MCT was accelerated by 37% in group 1 and 53% in group 2.16
In their prospective, randomized, controlled study published in 2005, Rogkakou et al.17 investigated the effect of hypertonic saline nasal spray, combined with antihistamines, on symptoms and quality of life in AR. Over a period of 4 weeks, 14 patients took either 10 mg of cetirizine daily or they combined cetirizine with the four-time daily dose of hypertonic saline nasal spray. Based on the daytime- and nighttime-specific symptoms, a Quality of Life Index, and acoustic rhinomanometry, it was shown that local saline application leads to intensified improvement of the parameters.17
Ural et al,18, in their prospective, randomized controlled study in 2009, proved that SNI with isotonic and hypertonic saline solution can shorten MCT in AR. During the 10-day study, 21 patients with AR applied SNI with hypertonic (n = 11) or isotonic (n = 10) saline solution (4 mL/nostril using a disposable syringe twice daily). MCT, ascertained with the aid of the saccharin clearance test, worsened by 1% after the application of hypertonic SNI; isotonic SNI, however, led to significant improvement in MCT by 45%.18
Study Design
Of the 10 relevant studies involved, 7 were randomized and controlled.9,11–15,17 The specific procedure of randomization was described in detail in 4 of these studies.12,14,15,17 Oily drops,9 isotonic saline spray,11 no topical application,12–15 steroids,16 or cetirizine17 served as controls. There were no control groups only in the studies conducted by Cingi et al.10 and Ural et al.18; instead, Ural's study used a parallel-group design to compare the efficacy of isotonic and hypertonic solution.10,18
Selection of Patients
In all, 10 studies with 400 patients made up the present review. Of these, 86 patients were children/adolescents aged from 5 to 15 years, and just >50% were female patients. Of the female patients, 45 were pregnant at the time the study was conducted.
EFFECTS OF INTERVENTIONS
Meta-analyses were performed with regard to the parameters “nasal symptom score,” “medicine consumption,” “mucociliary clearance time,” and “quality of life” in terms of the respective absolute improvement in comparison between the beginning and the end of the study. In addition, the relative improvements of the parameters “nasal symptom score” and “medicine consumption” were analyzed comparing the irrigation group and the nonirrigation group both at the beginning and at the end of the study. Furthermore, the mode of application (spray/irrigation) was specified in the analyses and was able to be evaluated.
Nasal Symptom Score
The primary parameter—nasal symptom score—was analyzed in 8 of the 10 relevant studies (Fig. 2).9,10,12–17 For the most part, the four cardinal symptoms consisting of obstruction, sneezing, itching, and secretion were assessed on scales of 0 to a maximum of 5 points (severe symptoms) either by the patients themselves in a diary or by the treating physician. Barbieri et al.9 concentrated solely on nasal obstruction and used a scale of 0–10 points. Rogkakou et al.17 took into consideration the daytime and nighttime symptoms rated on a scale of 0–3. Values for symptom scores indicated before and after SNI were extracted from texts and diagrams, and the improvement was calculated in percent. SNI induced no symptom improvement only in pregnant patients, but instead caused mild worsening by −8.125% because of the natural course of AR at the beginning of the pollen season.14 In all other studies, symptom improvement ranging from 3.150 to 70.492% resulted from the regular use of SNI.
Figure 2.
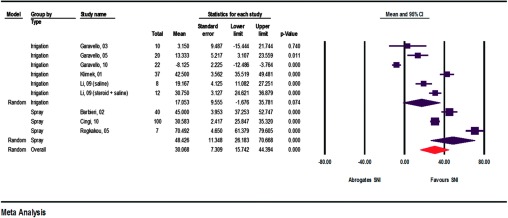
Absolute improvements in symptom scores (assessment of heterogeneity: irrigation, q = 194.2, df(Q) = 5, p < 0.001, I2 = 97.4, τ2 ≥ 522.3; spray, q = 59.7, df(Q) = 2, p < 0.001, I2 = 96.7, τ2 = 372.1; overall, q = 385.5, df(Q) = 8, p < 0.001, I2 = 97.9, τ2 = 586.2).
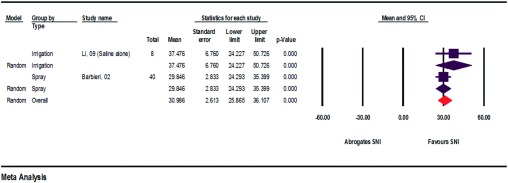
Medicine Consumption
Medicine consumption was taken into consideration in four of the relevant studies (Fig. 3).12–15 The study conducted by Klimek et al.15, however, could not be included in the meta-analysis because of incongruent data. All data used in the meta-analysis thus originate from only one author, which reduces the relevance of the results. The additional consumption of pharmaceuticals was able to be lowered with the aid of SNI by 24.154–100%. In the group of pregnant women, however, the opposite effect could again be observed here, too. Absolute improvement was −67.159%.
Figure 3.
Absolute improvements in medicine consumption (assessment of heterogeneity: irrigation, q = 25.3, df(Q) = 2, p < 0.000, I2 = 92.1, τ2 = 4214.9; overall, q = 25.3, df(Q) = 2, p < 0.000, I2 = 92.1, τ2 = 4214.9).
Mucociliary Clearance Time
The MCT, used as a parameter for indicating proper/improper nasal mucosa function, was examined in four studies before and after SNI (Fig. 4).9,10,16,18 In the process, only the studies performed by Barbieri et al.9 and Li et al.16 yielded adequate data for the meta-analysis. Improvements from 2.667 to 31.60% were ascertained.
Figure 4.
Absolute improvements in MCT (assessment of heterogeneity: spray, q = 0.4, df(Q) = 1, p < 0.542, I2 = 0.000, τ2 = 0.000; overall, q = 0.4, df(Q) = 1, p < 0.542, I2 = 0.000 τ2 = 0.000).
Quality of Life
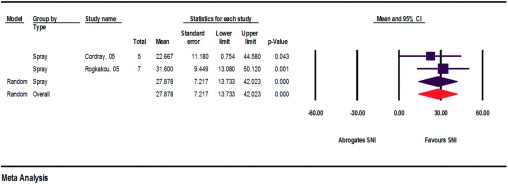
Cordray et al.11 and Rogkakou et al.17 surveyed the study participants before and after SNI about their subjective quality of life (Fig. 5). They conducted the survey using the validated Rhinitis Quality of Life Questionnaire (28 questions, seven areas, scale of 0–6) and the Rhinasthma Questionnaire (30 questions, scale of 0–5). The calculated improvement in quality of life was 29.846–37.476%.11,17
Figure 5.
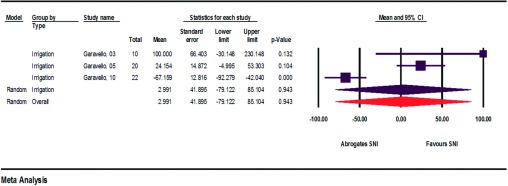
Absolute improvements in quality of life (assessment of heterogeneity: irrigation, q = 0.0, df(Q) = 0, p < 1.000, I2 = 0.0, τ2 = 0.0; spray, q = 0.000, df(Q) = 0, p < 1.000, I2 = 0.0, τ = 0.0; overall, q = 1.1, df(Q) = 1, p < 0.297, I2 = 7.7, τ2 = 1.5).
SNI Users versus Nonusers
The relative improvement in the symptom score, each comparing the SNI user group and the nonuser group, varied from 4.833 to 70% (Figs. 6 and 7). The average relative improvement was 32.268%. Generally, measured improvements in the symptom scores were always larger in the SNI user groups than in the nonuser groups. With respect to the pregnant patients, the SNI user group achieved more positive values than the nonuser group.12–15
Figure 6.
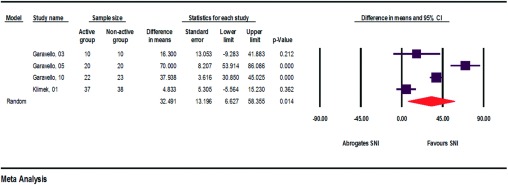
Relative improvements in symptom scores when comparing SNI users/nonusers (assessment of heterogeneity: q = 51.8, df(Q) = 3, p < 0.000, I2 = 94.2, τ2 = 631.8).
Figure 7.
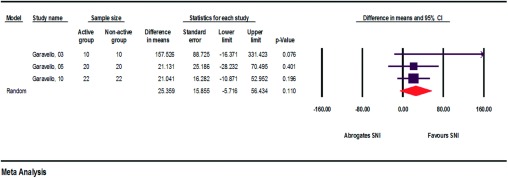
Relative improvements in medicine consumption when comparing SNI users/nonusers (assessment of heterogeneity: qe = 2.3, df(Q) = 2, p < 0.315, I2 = 13.4, τ2 = 128.3)/.
The relative reduction in medicine consumption, each comparing the SNI user group and the nonuser group, ranged from 21.041 to 157.526%. The average relative reduction was 66.566%. In all SNI user groups, medicine consumption decreased more than in the nonuser groups. Also, pregnant patients were able to limit their medicine consumption more when using irrigation than without it.12–14
Isotonic versus Hypertonic
In the relevant literature, the isotonic rinsing solution is generally preferred to the hypertonic version. The reasoning for this recommendation is that optimal mucociliary transport can only be ensured at a neutral pH.21
In the studies analyzed, only special salts (Salsomaggiore or seawater gel) and isotonic solutions were able to induce improvements in MCT. The use of a hypertonic solution resulted in mild worsening of MCT in patients with AR in contrast to the application in healthy subjects with chronic rhinosinusitis. Overall, improvements in the parameters after the use of special salts (Salsomaggiore, seawater gel, or Dead Sea salt) ranged from 30.6 to 45% and after the application of isotonic solutions from 19.2 to 70.5%. The use of hypertonic solutions yielded generally much lower results from −67.2 to 13.3%, with the exception of Garavello et al.12, who reported 100% improvement in 10 patients.
Form of Application
SNI was applied in the form of a spray9–11,17 in four studies and as irrigation in the other six studies.12–16,18 The use of spray resulted in improvements in the parameters ranging from 22.7 to 70.5%; improvements achieved with irrigation generally varied from −67.2 to 45.5%, whereas Garavello et al.12 reported 100% improvement in ten patients.
Safety
Subjects participating in the study by Cingi et al.10 were asked to make written documentation of any possibly occurring adverse effects. None of the patients reported any such effects after the application of SNI. This corresponds to the data in the available literature, in which permanent severe adverse effects have not been previously described.22
DISCUSSION
The meta-analysis of the studies that were included based on the systematic review showed the significant effectiveness of nasal irrigation treatment in AR. Nasal symptoms decreased by an average of 27.66%, and medicine consumption decreased by an average of 2.99% with regard to all patients. It dropped by 62.1% after excluding the study that had investigated the onset of symptoms in pregnant women. MCT improved by 31.19% under the use of isotonic saline solution. Quality of life rose by an average of 27.88%, which is comparable with the values achieved under pharmacotherapy.23 According to a recent evidence-based review, median percentage changes from baseline for total nasal symptom score for SAR were −22.2% for nasal antihistamines, −23.5% for oral antihistamines, −40.7% for intranasal steroids, and −15.0% for placebo.24
Therefore, nasal irrigation can be recommended for the treatment of AR. A simple and inexpensive nonpharmacologic form of therapy, nasal irrigation, can reduce medicine consumption and thereby cut down on costs caused by this disorder for patients and for the health care system.
No adequate differentiation has been made between mild, moderate, and severe AR in the available literature. Additional studies must clarify whether symptomatic therapy with nasal irrigation alone can suffice in mild AR before pharmacologic therapy with antihistamines or topical nasal steroids, e.g., is begun. In moderate to severe forms, nasal irrigation can be applied in addition to pharmacologic treatment and/or immunotherapy.
When comparing the results of adult patients and children, it becomes apparent that in children <15 years of age, improvement reaches a maximum of 20% and it is up to 45.5% in adults. This difference may be caused by the different ways the immature body can react or caused by less compliance and thereby a less intensive irrigation treatment in children.
The spectrum of what is called “nasal irrigation” is broad, reaching from applying a nasal spray to rinsing the nose with 250 mL of saline solution. It seems obvious that this makes a difference in the subsequent effects.
The application of spray with a much smaller volume yielded more distinct improvements in the parameters ranging between 23 and 45% than the use of nasal irrigation with larger volumes (200–400 mL), which resulted in improvements between 3.2 and 45.5%.
Today, however, no comparative controlled studies and no clear data are available in the literature that define the optimal method of nasal irrigation (spraying or nasal douching), the preferred type of saline solution (sodium chloride, Emser salt, or seawater salt; buffered or nonbuffered; isotonic or hypertonic), and the best frequency for the individual indication (AR, acute rhinosinusitis, chronic rhinosinusitis, dry nose, or after endonasal sinus surgery). The question as to the most advantageous form of application therefore remains unanswered.
The mechanism of action is still unknown. Today, it has been assumed that mucosal function improves because of
Direct physical cleansing by flushing out thick mucus, crusts, debris, allergens, air pollutants, etc.25
The removal of inflammatory mediators.26
Better mucociliary clearance by improving ciliary beat frequency.27,28
It is conceivable that the mechanical stimulus involved in the spray application of saltwater plays a role in the achieved effect by causing neuronal changes in the immunologic process. This could explain the greater effect of the spray application.
There are only two limitations of use: injuries in the anterior part of the nose or recurrent bleeding caused by nasal irrigation. Because nasal douches are available for children (e.g., Nasanita junior; Siemens & Co., Bad Ems, Germany), age poses no restriction. Younger children can perform nasal douching with the help of their parents; older children can do it by themselves.
Nsouli et al.29 has hypothesized that daily nasal irrigation in healthy people could lead to an increase in the number of upper respiratory tract infections. Several circumstances, however, do not support this hypothesis. First, the study has not appeared in a peer-reviewed journal to date, thereby putting their hypothesis into serious question. Second, their conclusion is incorrect due to several logical flaws already apparent in the abstract. Third, no article in the literature published up to now indicates a risk of nasal irrigation for upper respiratory tract infections. On the contrary, some controlled studies are, in fact, available, all of which report a reduction in upper respiratory tract infections after long-term nasal irrigation with isotonic saline solutions.30–32
This observation is in line with a recent randomized, placebo-controlled, double-blind study showing that nasal irrigation with thermal water improved the microbiological features of patients with nonallergic chronic rhinosinusitis and significantly reduced total nasal resistance.33
A limitation of the present systematic review and meta-analysis is the heterogeneity of the current studies regarding type, amount, and timing of nasal irrigation and the use of different saline solutions. Nevertheless, the effect is consistent throughout the various methods, such that conducting a meta-analysis seems justified.
CONCLUSION
Nasal irrigation with saline solution in AR results in the improvement of symptoms, quality of life, and MCT. The consumption of antiallergic medication can also be decreased. Nasal irrigation represents a safe and inexpensive, nonpharmacologic form of treatment. However, additional studies need to be performed in the future to clarify the questions as to the optimal salt concentration and mode of application.
ACKNOWLEDGMENTS
The authors thank Gena Kittel for proofreading the article.
Footnotes
RK Weber has received funding of his research and consultancy fees from Pari GmbH and Siemens & Co, both located in Germany. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Bousquet J, Khaltaev N, Cruz AA, et al. ARIA. Allergy 63(suppl 86):8–160, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 5:758–764, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein JA. Allergic and mixed rhinitis: Epidemiology and natural history. Allergy Asthma Proc 31:365–369, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc 31:375–380, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 Revision. J Allergy Clin Immunol 3:466–476, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Fokkens WJ. Nasal corticosteroids, first choice in moderate to severe allergic rhinitis. What prevents general practitioners from using them? Allergy 8:724–726, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Koberlein J. What does guideline-conforming therapy achieve in the treatment of allergic rhinoconjunctivitis?. Dtsch Med Wochenschr 133:S79–S83, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Prospective studies having at least evidence level IIa. www.cochrane.de/de/willkommen-auf-unseren-webseiten; last accessed July 15, 2011.
- 9. Barbieri M, Salami A, Mora F, et al. Behavior of serum IgE and IgA in patients with allergic rhinitis treated with iodine bromide thermal water. Acta Otorhinolaryngol Ital 4:215–219, 2002. [PubMed] [Google Scholar]
- 10. Cingi C, Unlu HH, Songu M, et al. Seawater gel in allergic rhinitis: Entrapment effect and mucociliary clearance compared with saline. Ther Adv Respir Dis 1:13–18, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J 7:426–430, 2005. [PubMed] [Google Scholar]
- 12. Garavello W, Romagnoli M, Sordo L, et al. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: A randomized study. Pediatr Allergy Immunol 2:140–143, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Garavello W, Di Berardino F, Romagnoli M, et al. Nasal rinsing with hypertonic solution: An adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol 4:310–314, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Garavello W, Somigliana E, Acaia B, et al. Nasal lavage in pregnant women with seasonal allergic rhinitis: A randomized study. Int Arch Allergy Immunol 2:137–141, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Klimek LJV, Hundorf F, Hommel G, Hörmann K. Lässt sich der Medikamentenverbrauch bei Patienten mit saisonaler Rhinitis allergica durch Nasenspülbehandlungen mit isoosmotischer Emser Salzlösung reduzieren? Allergologie 24:309–315, 2001. [Google Scholar]
- 16. Li H, Sha Q, Zuo K, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec 1:50–55, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Rogkakou A, Guerra L, Massacane P, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis—A randomized controlled study. Eur Ann Allergy Clin Immunol 9:353–356, 2005. [PubMed] [Google Scholar]
- 18. Ural A, Oktemer TK, Kizil Y, et al. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: Clinical study. J Laryngol Otol 5:517–521, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1:1–12, 1996. [DOI] [PubMed] [Google Scholar]
- 20. Oremus M, Wolfson C, Perrault A, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord 3:232–236, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Passali D, Damiani V, Passali FM, et al. Atomized nasal douche vs nasal lavage in acute viral rhinitis. Arch Otolaryngol Head Neck Surg 9:788–790, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Hildenbrand T, Weber R, Heubach C, et al. Nasal douching in acute rhinosinusitis. Laryngorhinootologie 6:346–351, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Wilson A, O'Byrne P, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: A systematic review and meta-analysis. Am J Med 5:338–344, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: An evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol 104:13–29, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Michel O. Nasal irrigation in case of rhinosinusitis. Laryngorhinootologie 85:448–458, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest 5:1487–1492, 1994. [DOI] [PubMed] [Google Scholar]
- 27. Boek WM, Keles N, Graamans K, et al. Physiologic and hypertonic saline solutions impair ciliary activity in vitro. Laryngoscope 109:396–399, 1999. [DOI] [PubMed] [Google Scholar]
- 28. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 4:500–503, 1997. [DOI] [PubMed] [Google Scholar]
- 29. Nsouli TM, Schluckebier CD, McSorley-Gerard EJ, et al. Long-term use of nasal saline irrigation: harmful or helpful? Ann Allery Asthma Immunol 103(suppl 3):A3–A146, 2009. (Abstracts of the 2009 annual meeting of the American College of Allergy, Asthma, and Immunology, Miami, FL, November 5–10, 2009.) [Google Scholar]
- 30. Rabago D, Zgierska A, Mundt M, et al. Efficacy of daily hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngol Head Neck Surg 133:3–8, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Schmidt Th, Bitzer EM, Dörning H, et al. Tägliches Nasespülen reduziert Atemwegsbeschwerden—Eine randomisierte crossover-studie. Gesundheitswesen 66, 2004. (DOI: 10.1055/s-2004-833862.) [Google Scholar]
- 32. Tano L, Tano K. A daily nasal spray with saline prevents symptoms of rhinitis. Acta Otolaryngol 124:1059–1062, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Ottaviano G, Marioni G, Staffieri C, et al. Effects of sulfurous, salty, bromic, iodic thermal water nasal irrigations in nonallergic chronic rhinosinusitis: A prospective, randomized, double-blind, clinical, and cytological study. Am J Otolaryngol 32:235–239, 2011. [DOI] [PubMed] [Google Scholar]