Abstract
Despite its association with important agricultural crops, Phytophthora clade 8b is a poorly studied group of species. The clade currently consists of three officially described species (Phytophthora porri, P. brassicae and P. primulae) that are host-specific pathogens of leek, cabbages and Primula spp., respectively. However, over the past few decades, several other clade 8b-like Phytophthoras have been found on a variety of different host plants that were all grown at low temperatures in winter seasons. In this study, a collection of 30 of these isolates was subjected to a phylogenetic study using two loci (the rDNA ITS region and the mitochondrial cox1 gene). This analysis revealed a clear clustering of isolates according to their host plants. To verify whether these isolates belong to separate species, a detailed morphological study was conducted. On the basis of genetic and morphological differences and host specificity, we now present the official description of three new species in clade 8b: Phytophthora cichorii sp. nov., P. dauci sp. nov. and P. lactucae sp. nov. Two other groups of isolates (Phytophthora taxon castitis and Phytophthora taxon parsley) might also represent new species but the data available at this time are insufficient for an official description. This brings Phytophthora clade 8b to a group of six species that are all host-specific, slow-growing and specifically infect herbaceous crops at low temperatures.
Keywords: host specificity, low temperatures, phylogeny, Phytophthora, species description, vegetables
INTRODUCTION
Phytophthora is a genus of plant pathogenic, filamentous oomycetes, belonging to the eukaryotic kingdom Chromalveolata (Adl et al. 2005). Morphologically, they are very similar to the filamentous fungi (kingdom Fungi), but recent phylogenetic studies have proven them to be of a very different evolutionary origin. Oomycetes differ from Fungi in some important morphological and biochemical aspects and in the fact that they are diploid in their vegetative lifestyle, whereas most Fungi are haploid (Beakes et al. 2012).
Since the official description of Phytophthora infestans in 1876, the number of known Phytophthora species has increased steadily until a sharp increase in the number of new species descriptions occurred with the introduction of molecular tools in the last decade of the previous century. These new techniques (together with the use of specific selective media and proper isolation techniques) have made rapid and reliable identification of Phytophthora species possible. Therefore, large-scale surveys for new species have become more feasible, and many new species have been found in such surveys in recent years. Today, over 100 Phytophthora species have been officially described (Kroon et al. 2012), and practically all of them are plant pathogens.
According to the most recent phylogenetic studies (Blair et al. 2008, Cooke et al. 2000, Kroon et al. 2004), the genus Phytophthora consists of 10 clades. In this paper, we focus on subclade 8b. According to the phylogenetic studies cited above, this group consists of five species: the closely related P. porri (Foister 1931), P. primulae (Tomlinson 1952) and P. brassicae (Man in ’t Veld et al. 2002) and the more distantly related P. syringae (Klebahn 1909) and P. austrocedrae (Greslebin et al. 2007). However, Grünwald et al. (2011) recently described a new species in clade 8 and revised the subclade structure using the same loci that were used in the study by Blair et al. (2008). In this analysis P. porri, P. brassicae and P. primulae are placed in clade 8b, while P. syringae, P. austrocedrae and the newly described P. obscura form a new subclade 8d.
Phytophthora porri is known to cause the white tip disease of leek (Allium porrum), one of the most important diseases in leek cultivation in autumn and winter seasons in temperate regions (Declercq et al. 2009, 2011). Isolates pathogenic to cabbages (Brassicaceae) used to be regarded as a host-specific subspecies of P. porri, but were officially described as P. brassicae on the basis of genetic and morphological differences and host specificity by Man in ‘t Veld et al. (2002). Phytophthora primulae has been described as a root pathogen of primrose (Primula spp.), an ornamental plant (Tomlinson 1952).
During the past few decades, isolates morphologically similar to the species described above have been reported to cause diseases in a range of other hosts. Firstly, isolates similar to P. porri have been isolated from lettuce (Lactuca sativa). One report describes the occurrence of stem rot of lettuce in South Australia, resulting in complete wilting of the heads (Sitepu & Bumbieris 1981). Another report describes the same symptoms in Greece (Elena et al. 2006). Secondly, a disease named ‘rubbery brown rot’ occurred in Canada in stored carrots (Daucus carota; Stelfox & Henry 1978). Furthermore, in some regions in the north of France, a P. porri-like organism is associated with a carrot disease called ‘ring rot disease’ (Danielle Breton, pers. comm.). Thirdly, isolates similar to P. primulae were derived from parsley plants (Petroselinum crispum) in Greece. They caused stem base rot and consequently wilting of entire plants in consecutive years starting from 2002, resulting in significant yield losses (Elena & Grigoriou 2008). Fourthly, isolates belonging to a clade 8b-like species have been causing rotting in chicory roots (Cichorium intybus var. foliosum) in the UK (Kim Green & John Scrace, ADAS, pers. comm.) and in the Netherlands during the past decade. Fifthly, a P. porri-like organism provoking crown rot of strawberry (Fragaria × anannassa) was collected in Sweden. Last of all, a Japanese report from the 1960s describes leaf blight and bulb rot in scallion (Allium bakeri), caused by a P. porri-like species, as one of the most serious problems in scallion cultivation in some regions in Japan (Katsura et al. 1969). The same disease was described in Great Britain (Griffin & Jones 1977) and in South Africa (von Maltitz & von Broembsen 1984). These isolates may possibly represent an incipient species arising from interspecific hybridization between P. porri and a closely related species (Bertier et al. unpubl. data, Declercq et al. 2009). Because of their probable hybrid nature, these isolates will not be discussed in this paper. In all cases mentioned above, the disease occurred at low temperatures during winter seasons.
Despite its association with these important agricultural crops, up until now clade 8b has been an understudied group of species. A reason for this might be the recalcitrance of the clade 8b species: they are all very slow growing on culture media, and therefore extremely hard to detect and isolate from infected plants. In an attempt to fill this gap, we performed a multi-locus phylogenetic study on a collection of clade 8b isolates from the hosts mentioned above. From this phylogenetic study, it became clear that some groups of isolates showed considerable amounts of genetic variation from the known clade 8b species. To verify whether these groups of isolates belong to separate species, the isolates were subjected to a detailed morphological study. On the basis of genetic and morphological differences and host specificity, we now present the official description of three new species in clade 8b, namely Phytophthora cichorii sp. nov., P. dauci sp. nov. and P. lactucae sp. nov. Next to this, we confirm the existence of two possible new taxa, Phytophthora taxon parsley and Phytophthora taxon castitis.
MATERIALS AND METHODS
Isolate collection and maintenance
All 31 isolates used in this study are listed in Table 1. They were either freshly isolated from diseased plants or obtained from culture collections or from other researchers via personal contacts. The cultures were routinely maintained on V8 agar (V8A, 200 mL V8 juice (Campbell); 3 g CaCO3; 15 g agar and 800 mL of sterile water) or Corn Meal Agar (CMA-BD, Beckton Dickinson) or CBS cornmeal agar (CMA-CBS, Crous et al. 2009) and kept as V8A plugs in 10 % glycerol at −80 °C for long term storage. All cultures are available at the CBS Fungal Diversity Center in the Netherlands (CBS) or at the Benaki Phytopathological Institute in Greece.
Table 1.
List of the 31 Phytophthora isolates examined in this study.
| Species | Code | Alternative collections | Host | Origin | Year of isolation | Genbank accession number |
|
|---|---|---|---|---|---|---|---|
| Cox1 | ITS | ||||||
| Phytophthora porri | CBS 802.95 | PD 92/214 | Allium porrum | Netherlands | 1992 | KC478717 | KC478747 |
| CBS 114100 | – | Allium porrum | Denmark | 1992 | KC478718 | KC478748 | |
| CBS 116662 | Smilde GG | Allium porrum | UK | 1994 | KC478719 | KC478749 | |
| CBS 127099 | K06006(2) | Allium porrum | Belgium | 2006 | KC478720 | KC478750 | |
| CBS 127101 | S05029(1) | Allium porrum | Belgium | 2005 | KC478721 | KC478751 | |
| Phytophthora primulae | CBS 110167 | BBA 71108 | Primula eliator | Germany | 1999 | KC478722 | KC478752 |
| CBS 116663 | PD 99/2429 | Primula sp. | Netherlands | 1999 | KC478723 | KC478753 | |
| CBS 114346 | LYN 916-A | Primula polyantha | New Zealand | 2003 | KC478724 | KC478754 | |
| CBS 110162 | BBA 70403 | Primula sp. | Germany | 1997 | KC478725 | KC478755 | |
| CBS 620.97 | PD 97/875 | Primula acaulis | Germany | 1997 | KC478726 | KC478756 | |
| Phytophthora taxon parsley | BPIC 2584 | – | Petroselinum crispum | Greece | 2006 | KC478727 | KC478757 |
| CBS 114156 | – | Petroselinum crispum | Australia | 2003 | KC478728 | KC478758 | |
| Phytophthora taxon castitis | CBS 688.79 | – | Daucus carota | Canada | 1978 | KC478729 | KC478759 |
| CBS 131246 | CH112 | Fragaria x ananassa | Sweden | 1995 | KC478730 | KC478760 | |
| Phytophthora dauci sp. nov. | CBS 127102 | BorfSP370 | Daucus carota | France | 2009 | KC478731 | KC478761 |
| CBS 114039 | – | Daucus carota | Australia | 2003 | KC478732 | KC478762 | |
| Phytophthora brassicae | CBS 782.97 | Smilde HH | Brassica chinensis | Netherlands | 1994 | KC478733 | KC478763 |
| CBS 212.82 | P3273 | Brassica oleraceae | Netherlands | 1982 | KC478734 | KC478764 | |
| CBS 113350 | PD 94/166 | Brassica oleraceae | Netherlands | 1994 | KC478735 | KC478765 | |
| CBS 112277 | ICMP 14271 | Brassica oleraceae | New Zealand | 2001 | KC478736 | KC478766 | |
| CBS 127274 | B10001 | Brassica oleraceae | Belgium | 2010 | KC478737 | KC478767 | |
| Phytophthora lactucae sp. nov. | BPIC 1985 | – | Lactuca sativa | Greece | 2001 | KC478738 | KC478768 |
| BPIC 1986 | – | Lactuca sativa | Greece | 2001 | KC478739 | KC478769 | |
| BPIC 1987 | – | Lactuca sativa | Greece | 2002 | – | – | |
| BPIC 1988 | – | Lactuca sativa | Greece | 2002 | KC478740 | KC478770 | |
| BPIC 1991 | – | Lactuca sativa | Greece | 2003 | KC478741 | KC478771 | |
| BPIC 1992 | – | Lactuca sativa | Greece | 2003 | KC478742 | KC478772 | |
| Phytophthora cichorii sp. nov. | CBS 115029 | – | Cichorium intybus | Netherlands | 2004 | KC478743 | KC478773 |
| CBS 114345 | – | Cichorium intybus | Netherlands | 2003 | KC478744 | KC478774 | |
| CBS 115030 | – | Cichorium intybus | Netherlands | 2004 | KC478745 | KC478775 | |
| CBS 133815 | SCRACE5388 | Cichorium intybus | UK | 1999 | KC478746 | KC478776 | |
DNA-extraction, PCR and sequencing
Isolates were grown in clarified V8 broth (100 mL of clarified V8 juice, 3 g CaCO3 and 900 mL of sterile water) for 7–10 d at 15 °C in the dark. The mycelial mats were harvested by filtration, blotted dry, frozen in liquid nitrogen and pulverized using mortar and pestle. DNA was extracted using Qiagen’s DNeasy Plant Mini Kit (Hilden, Germany). Amplification and sequencing of the ITS region was performed using primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) (White 1990). PCR reactions were performed in a 25 μL mix containing 2.5 μL PCR buffer (10×, Qiagen), 0.5 μL dNTPs (10 mM, Qiagen), 1 μL of each primer (10 μM), 0.15 μL Taq polymerase (5U/μL; Fermentas), 17.85 μL milli-Q water and 2 μL of DNA template (25 ng/μL). The amplifications were done in a Flexcycler PCR Thermal Cycler (Analytikjena) programmed as follows: initial denaturation for 10 min at 94 °C; 35 cycles of denaturation for 1 min at 94 °C; annealing for 1 min at 60 °C; extension for 1 min at 72 °C; final extension for 10 min at 72 °C. For the cox1 gene, the degenerate primers Oom-CoI-Lev-up (5′-TCA WCW MGA TGG CTT TTT TCA AC-3′) and FM-85-mod (5′-RRH WAC KTG ACT DAT RAT ACC AAA-3′) were used as described by Bala et al. (2010).
Phylogenetic analysis
Sequence alignments for 44 isolates were made for the two loci (ITS and cox1) using ClustalW in Bio-Edit and manually edited afterwards. Of these 44 isolates, 30 isolates represented the clade 8b strains that were sequenced in this study; the other 14 isolates represent species of the other clade 8 subclades (8a, 8c and 8d) and their sequences were derived from Q-bank (www.q-bank.eu) or from GenBank (www.ncbi.nlm.nih.gov). For each locus, two different phylogenetic analyses were performed, one using Maximum Likelihood analysis as implemented in the MEGA5 software (Tamura et al. 2011) and the other using Bayesian Inference of Phylogeny (MrBayes v. 3.1.2; Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003). Model selection was done by jModeltest (Guindon & Gascuel 2003, Posada 2008) with the Akaike Information Criterion (AIC). The model that best fitted the data and that was available in MEGA5 and MrBayes was the General Time Reversible model with gamma distributed rate variation (GTR + G). For the Maximum Likelihood phylogenetic analysis, the data were described as coding (cox1) or non-coding (ITS). A bootstrap consensus tree inferred from 1 000 replicates was built. For the Bayesian analysis, two analyses were run simultaneously for 1 000 000 generations with three heated chains and one cold chain. The majority-rule consensus tree was calculated after discarding the first 250 000 generations (25 %) from each run as burn-in.
Both alignments, trees, as well as the phylogenetic source files are available on TreeBASE (study number 13805).
Determination of growth curves
For the study of temperature growth relationships, CMA-Oxoid medium was used (Oxoid, Basingstoke, Hampshire, England). This medium differs from the other CMA agars used in this study in that it is a clear medium that allows better judgement of colony diameter. Inoculum plugs were taken from the margin of an actively growing, young colony. The plugs were transferred to the centre of a series of 13 Petri dishes that were incubated in darkness at 18 °C. In most cases, cultures showed some growth after one day. If not, the cultures were incubated one or several days longer. After this initial incubation, Petri dishes were transferred to a series of incubators. Thirteen incubators ranging in temperature from 0–36 °C with increments of 3 °C were used. One additional incubator was set at 40 °C. After an hour, two perpendicular lines were drawn on the back of the Petri dish, intersecting beneath the inoculum plug. Radial growth was determined after 24 h, 48 h and 1 wk by marking the margin of the colony along these lines in all four directions.
Colony morphology
Colony morphology of isolates was studied on V8-CBS, PDA-CBS (Crous et al. 2009) and CMA-Oxoid. Round inoculum plugs with a diameter of 5 mm were taken from the margin of young, actively growing colonies. The mycelium plugs were placed in the centre of Petri dishes with the aforementioned media and were placed at 18 °C and 24 °C in darkness. Photographs were taken after one week of incubation.
Morphology
Sporangium production was studied on infected hemp and bell pepper seeds in sterile pond water. Autoclaved bell pepper and hemp seeds were placed on the edge of young Phytophthora colonies, and incubated for one or several days at 18 °C in the dark until the seeds were colonised by the oomycete. The colonised seeds were transferred to new Petri dishes with sterile filtered pond water, and were incubated at 18 °C. Isolates that sporulated poorly were also incubated at other temperatures and light conditions. However, this did not lead to better results.
CMA-CBS agar was used for the production of gametangia. Homothallic isolates produced oogonia after incubation in darkness at 18 °C for one or several weeks. Isolates that failed to produce oogonia in single culture were mated with heterothallic isolates of known mating type to test if they were heterothallic.
Gametangia used for the morphological description of heterothallic isolates were produced by the following procedure. A plug of mycelium approximately a centimetre in diameter was transferred to an empty Petri dish and covered with a polycarbonate filter. A mycelium plug of a culture of the opposing mating type was put on top and the Petri dish was sealed with parafilm, and incubated in the dark for 1–2 wk. Experiments with plugs without mycelium on one side were used as a control to confirm that the membrane was impermeable to hyphae. When two-celled antheridia were produced, the entire globose structure was measured when determining antheridial size, even though by biological function, only the top cell is the antheridium. Production of hyphal swellings and chlamydospores occurred both in the water cultures used for sporangium production and on CMA-CBS agar. Nomenclature and descriptions linked to taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
RESULTS
Phylogenetic analysis
The number of variable sites among the 30 clade 8b isolates in the alignments differed between the two loci. The cox1 gene showed the highest variability with 79/716 variable characters. The ITS region had a lower variability with 39/855 variable characters. However, there was enough phylogenetic signal to resolve reliable clustering with Bayesian and Maximum Likelihood analysis in both ITS and cox1 phylogenies. In Fig. 1 and 2, the Maximum Likelihood bootstrap consensus trees derived from the ITS and cox1 alignments, respectively, are shown. The majority consensus rule trees derived from the Bayesian analysis showed a nearly identical clustering for both loci. Bayesian Posterior Probability values of clusters are shown only when higher than 0.90. Maximum Likelihood bootstrap measures are shown only when higher than 70.
Fig. 1.
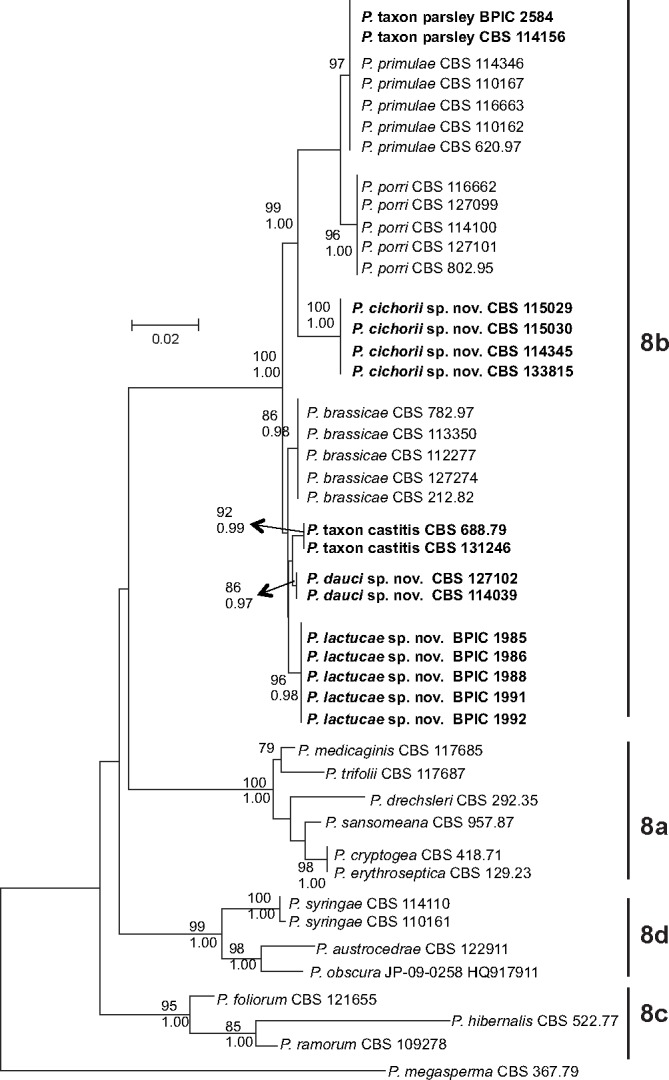
ITS phylogeny of Phytophthora clade 8. Phylogenetic tree derived from ITS sequence data. The bootstrap consensus tree from the Maximum Likelihood analysis, with its according branch lengths, is presented. The Maximum Likelihood bootstrap support values are shown only for those branches with a bootstrap support higher than 70 (top). Bayesian Posterior Probability values are shown only for those branches having support values higher than 0.90 (bottom). The tree is rooted with P. megasperma isolate CBS 367.67 (clade 6). Sequences from species from subclades 8a, 8c and 8d are derived from Q-bank (http://www.q-bank.eu; CBS number is shown) or from GenBank (GenBank accession number is shown).
Fig. 2.
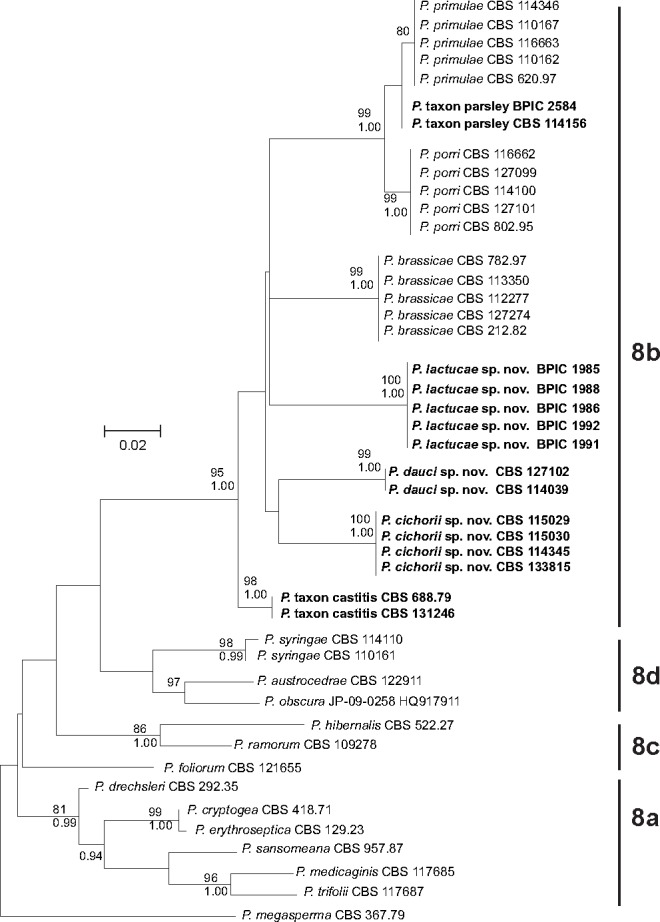
Cox1 phylogeny of Phytophthora clade 8. Phylogenetic tree derived from cox1 sequence data. The bootstrap consensus tree from the Maximum Likelihood analysis, with its according branch lengths, is presented. The Maximum Likelihood bootstrap support values are shown only for those branches with a bootstrap support higher than 70 (top). Bayesian Posterior Probability values are shown only for those branches having support values higher than 0.90 (bottom). The tree is rooted with P. megasperma isolate CBS 367.67 (clade 6). Sequences from species from subclades 8a, 8c and 8d are derived from Q-bank (http://www.q-bank.eu; CBS number is shown) or from GenBank (GenBank accession number is shown).
From the phylogenetic analyses, it is clear that all isolates examined (Table 1) belong to clade 8b and are closely related to the three described clade 8b species P. porri, P. brassicae and P. primulae. However, there is a clear phylogenetic difference between groups of isolates derived from different host plants (Fig. 1, 2). The P. porri isolates from leek form a distinct group in both phylogenies with high support from both analyses. Their closest relatives are the P. primulae isolates and the isolates from parsley. The two isolates from parsley only form a separate cluster from the primrose isolates in the cox1 phylogeny. In more detail, the ITS sequences of the parsley isolates are 100 % identical to those of P. primulae; in the cox1 gene however, there are two point mutations compared to P. primulae. The P. brassicae isolates form a cluster in both phylogenies with high support from both analyses, supporting its reclassification as a species separate from P. porri. The four isolates from chicory form a separate cluster in both phylogenies receiving high support from both analyses. The five isolates from lettuce also form a separate cluster in both phylogenies with high support from both analyses. Isolates from carrot fell into two distinct groups. The first group contains two isolates from carrot (CBS 127102 and CBS 114039) and forms a distinct cluster with high support in both phylogenies. The third isolate from carrot (CBS 688.79) seems to be genetically different and clusters together with another isolate that was derived from strawberry (CBS 131246).
To avoid confusion, isolates from the first group will be referred to as P. dauci, while isolates from the clade containing the strawberry and carrot isolate will be referred to as P. taxon castitis. Similarly, isolates from chicory will be referred to as P. cichorii, isolates from lettuce will be referred to as P. lactucae and the isolates from parsley as P. taxon parsley.
Our phylogenetic data also supports the revised clade 8 structure as proposed by Grünwald et al. (2011), in which P. syringae, P. austrocedrae and the newly described P. obscura form a distinct subclade 8d.
In our study, the cox1 gene proved more useful for distinguishing the separate taxa than the ITS region, while the latter provided a better resolution at a higher taxonomical level, namely that of the subclades.
Morphological and physiological characters
In the phylogenetic analysis, several highly supported clades were found. The morphology and physiology of these isolates was studied to determine whether there was support for these clades representing new species. Morphological measurements and observations are summarized in Table 2. For comparison with the other clade 8b species, we refer to Q-bank (www.q-bank.eu). Temperature growth relationships for P. cichorii, P. lactucae, P. dauci, P. taxon castitis and P. taxon parsley are shown in Fig. 3a–e, respectively. Morphology for each of the new species and for P. taxon castitis and P. taxon parsley is shown in Fig. 5, 6, 7, 8, 9. Colony morphology photographs are shown in Fig. 4.
Table 2.
Overview of morphological data of the new Phytophthora clade 8b species.
| Phytophthora cichorii | Phytophthora dauci | Phytophthora lactucae | Phytophthora taxon castitis | Phytophthora taxon parsley | |
|---|---|---|---|---|---|
| Number of isolates examined | 3 | 2 | 6 | 2 | 2 |
| Cardinal growth t° | |||||
| minimum | 0 °C | 0 °C | 0 °C | 0 °C | 0 °C |
| maximum | 24 °C | 21 °C | 24 °C | 27 °C | 24 °C |
| optimum | 15–21 °C | 9–18 °C | 15–21 °C | 15–24 °C | 15–21 °C |
| Growth rate at 21 °C (mm/day) | 0.6–1.1 | 0.8–1.0 | 0.7–2.1 | 2.0–2.6 | 1.5–2.0 |
| Sporangia | |||||
| length × width: | |||||
| range | 30.6–142.9 × 20.7–63.1 | 27.4–89.9 × 24–56.2 | 27.6–101.3 × 18.3–53.5 | 23.6–97.9 × 14.9–49.5 | 25.2–107.7 × 17.8–46.5 |
| isolate means | 69.0–74.9 × 37.5–42 | 52.6–58.8 × 37.2–40.1 | 55.3–61.1 × 35.5–38.5 | 50.8–55.4 × 32.6–35.6 | 52.8–54.3 × 29.4–30.6 |
| length/width ratio: | |||||
| range | 1.2–3.2 | 1.1–2.0 | 1.2–2.4 | 1.2–2.6 | 1.2–3.5 |
| isolate means | 1.7–1.8 | 1.4–1.5 | 1.5–1.6 | 1.4–1.7 | 1.7–1.9 |
| discharge pore width: | |||||
| range | 6.0–16.9 | 6.5–11.7 | 6.0–15.5 | – | 5.2–10.7 |
| isolate means | 8.9–11.0 | 9.1–9.5 | 9.5–10.3 | – | 7.9–8.0 |
| Mating system | heterothallic | homothallic | homothallic | sterilea | homothallic |
| Antheridia | |||||
| type | amphigynous | paragynous | paragynous, some amphigynous | – | both amphigynous and paragynous |
| length × width: | |||||
| paragynous range | – | 12.1–21.9 × 8.5–16.8 | 10.7–25.2 × 7.5–16.5 | – | 11.8–30.7 × 8.5–18.3 |
| paragynous isolate means | – | 16.0–17.1 × 11.6–12.5 | 16.1–17.5 × 9.8–11.9 | – | 18.1–19.3 × 13.2–14.0 |
| amphigynous range | 16.4–37.7 × 13.2–26.4 | – | rare | – | 10.2–21.3 × 10–16.9 |
| amphigynous isolate means | 23.4–27.4 × 19.8–19.8 | – | rare | – | 15.8–16.5 × 13.6–14.2 |
| Oogonia | |||||
| length × width: | |||||
| range | 23.7–49.5 × 19.2–45.9 | 25.3–37.2 × 25–37.2 | 22–44.1 × 21–44.1 | – | 24.3–44.7 × 23.4–42.6 |
| isolate means | 34.5–37 × 30.3–35.4 | 31.9–32.3 × 31.2–31.6 | 33.5–36.4 × 33.0–36.4 | – | 34.4–37.6 × 33.9–37.3 |
| oogonium wall (isolate means) | 1.0–1.2 | 1.0–1.1 | 1.3–1.7 | – | 1.1–1.4 |
| oospore width: | |||||
| range | 21.6–42.0 | 20.6–30.6 | 19.0–38.7 | – | 19.4–37.3 |
| isolate means | 27.5 | 26.3–27.6 | 27.2–31.0 | – | 28.9–30.9 |
| oospore wall (isolate means) | 1.2 | 1.1–1.3 | 1.1–1.6 | – | 0.9–1.4 |
| Hyphal swellings | |||||
| length × width: | |||||
| range | 16.0–56.5 × 13.5–42.3 | 17.1–59.9 × 14.3–41.4 | 14.3–49.8 × 10.4–39.3 | 14.6–61.8 × 12.6–52.1 | 11.3–61.5 × 10.3–38.7 |
| isolate means | 25.1–32.2 × 19.1–26.2 | 30.3–30.8 × 22.6–25.1 | 26.0–31.6 × 21.8–26.4 | 32.5 × 28.8 | 24.1–28.1 × 19.8–20.7 |
| Chlamydospores | |||||
| length × width: | |||||
| range | – | 23.7–57.4 × 20.9–56.0 | 22.0–51.4 × 20.4–52.4 | 29.1–73.2 × 29.1–73.2 | – |
| average | – | 31.9–38.3 × 30.8–37.2 | 31.7–37.2 × 29.7–35.3 | 36.4–51.4 × 36.4–51.4 | – |
a Oogonia not produced under normal circumstances or in mating test, oogonia were produced by Ho (1983) after exposing a culture to X-rays.
Fig. 3.
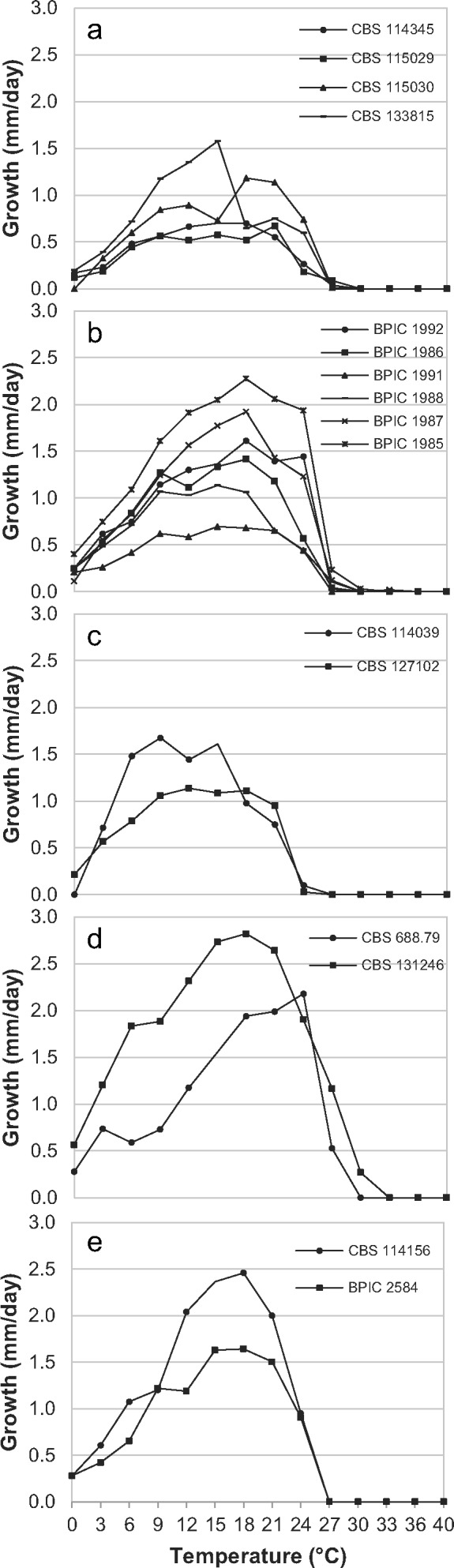
Temperature growth relationships of a. P. cichorii; b. P. lactucae; c. P. dauci; d. P. taxon castitis; e. P. taxon parsley isolates.
Fig. 5.
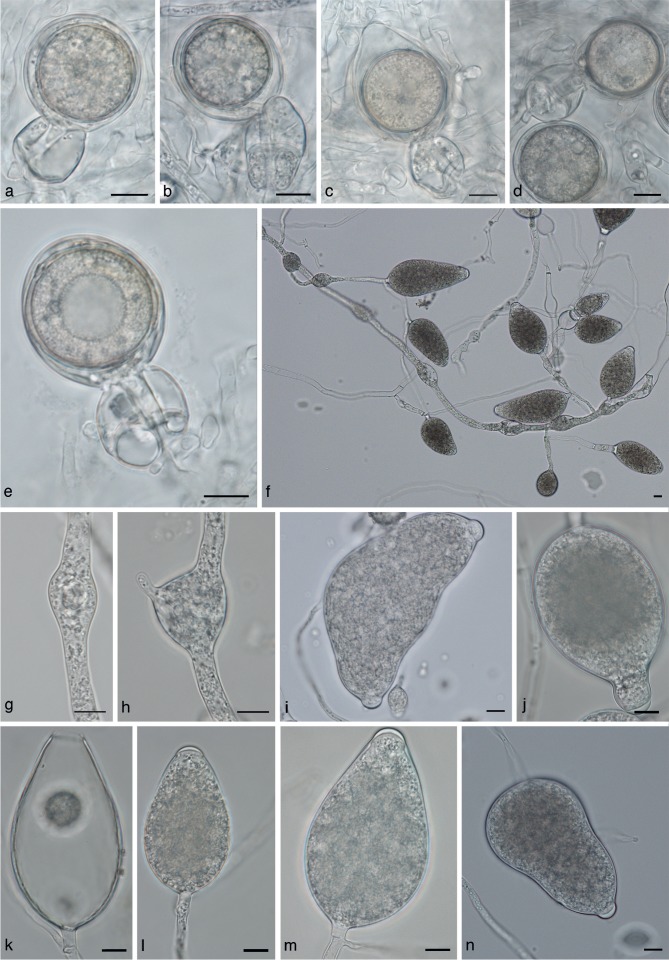
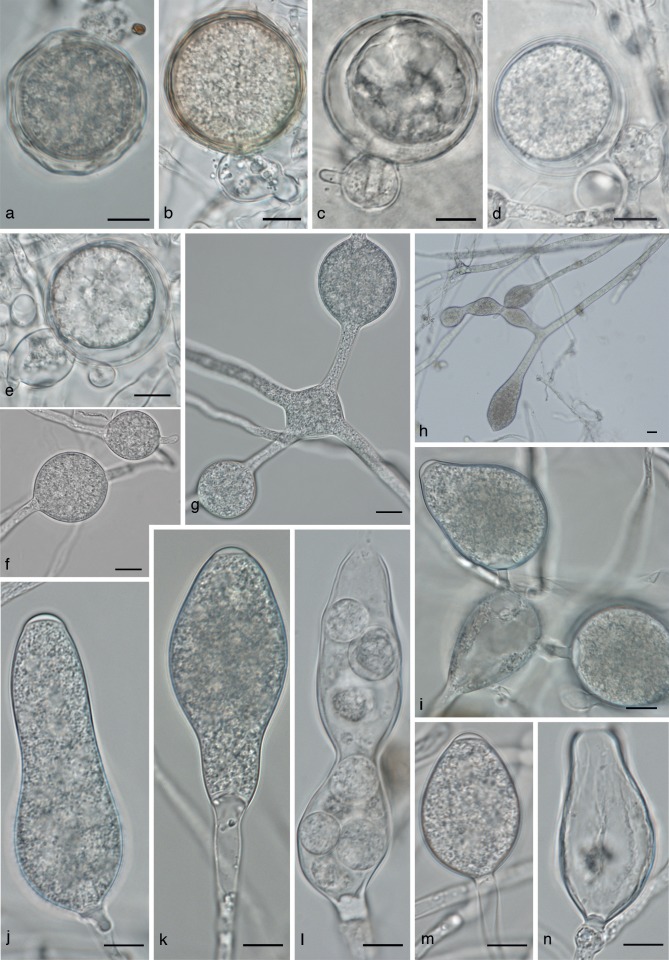
Phytophthora cichorii morphology. a–e. Oogonia with ‘2-celled’ amphigynous antheridia; f. sporangia and hyphal swellings at low magnification; g, h. intercalary hyphal swellings; i–n. sporangia; i. bipapillate sporangium; j. sporangium with elongated neck; k. empty sporangium with wide discharge pore. — Scale bars = 10 μm.
Fig. 6.
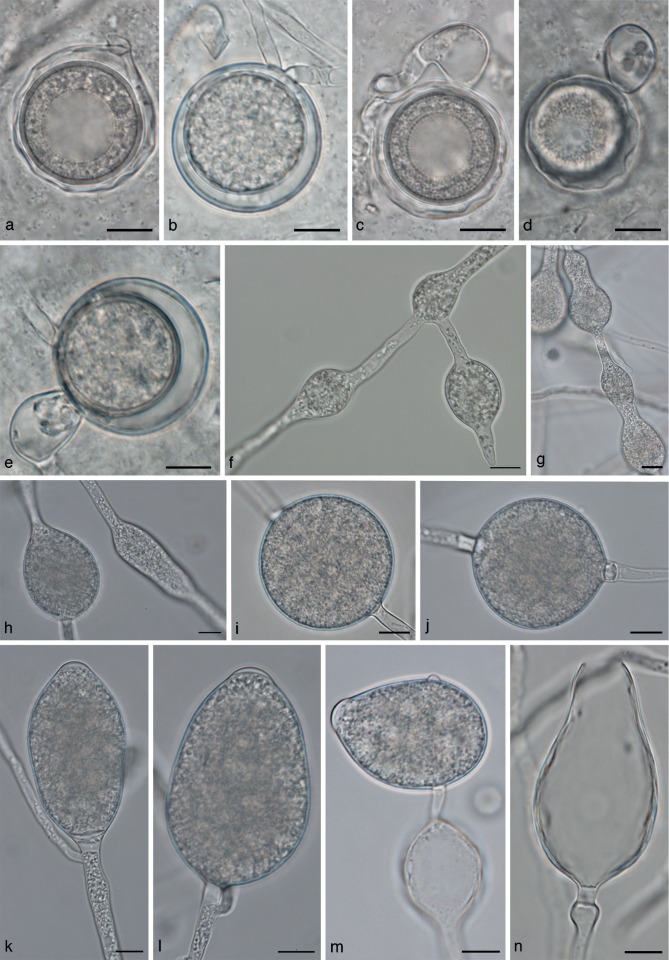
Phytophthora dauci morphology. a–e. Oogonia; a, c, d. oogonia with ‘wavy’ oogonium walls; b. intercalary oogonium; c–e. paragynous antheridia; f–h. hyphal swellings; i, j. chlamydospores; k–n. sporangia; n. empty sporangium with wide discharge pore; m. sporangium with lateral attachment, with a hyphal swelling in the subtending hypha. — Scale bars = 10 μm.
Fig. 7.
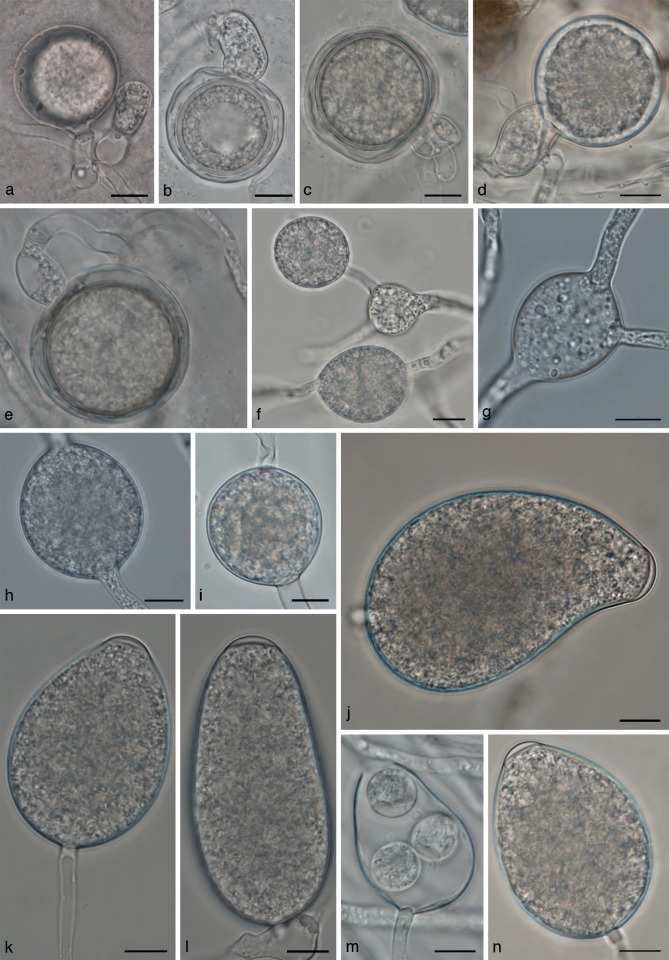
Phytophthora lactucae morphology. a–e. Oogonia; a, e. paragynous antheridia; c, d. amphigynous anteridia; f–h. hyphal swellings; i. chlamydospore; j–n. sporangia; m. discharged sporangium, several zoospores have encysted within the sporangium. — Scale bars = 10 μm.
Fig. 8.
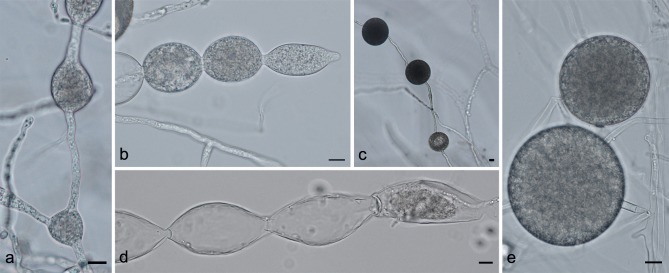
Phytophthora taxon castitis morphology. a. Hyphal swellings; b. catenulate hyphal swellings with a terminal sporangium; c, e. chlamydospores; d. empty swellings with a terminal empty sporangium. — Scale bars = 10 μm.
Fig. 9.
Phytophthora taxon parsley morphology. a. Oogonium with ‘wavy’ wall; b, d, e. oogonia with paragynous antheridia; c. oogonium with amphigynous antheridium; d. oogonium with two antheridia; f–h. hyphal swellings; i–n. sporangia; j. elongated sporangium; k. sporangium with a tapering base; l. constricted sporangium that failed to discharge all its zoospores. — Scale bars = 10 μm.
Fig. 4.
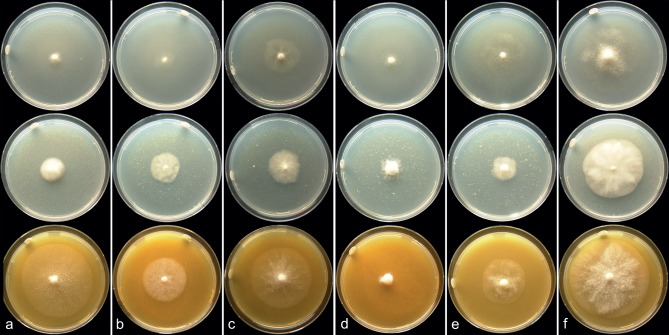
Colony morphology. Colony morphology on CMA-Oxoid (top row), PDA (middle row) and V8 agar (bottom row) after 1 wk of incubation at 18 °C; a. P. cichorii (CBS 115029); b. P. dauci (CBS 127102); c. P. lactucae (BPIC 1985); d. P. taxon castitis (CBS 688.79); e. P. taxon parsley (CBS 114156); f. P. porri (CBS 114100).
Concerning the production of sexual structures, the following findings should be mentioned. Isolates belonging to P. taxon castitis failed to produce gametangia in our experiments in both single culture and in mating tests. The P. cichorii isolates tested were heterothallic.
Isolates CBS 115029 and CBS 114345 produced oogonia in a polycarbonate filter separated mating setup with P. capsici CBS 128.23, a strain of A2 mating type. Isolate CBS 115030 failed to produce oogonia, but did induce oogonium formation in an A2 strain of P. capsici CBS 128.23 indicating that it is silent heterothallic under the conditions tested. Strain CBS 114345 produced only aborted oospores. The mating system of CBS 133815 was not determined.
All isolates belonging to P. dauci and P. lactucae were homothallic. For P. lactucae, two isolates produced almost exclusively aborted oospores (BPIC 1988, BPIC 1992). Only a limited number of gametangia could be measured for these isolates but for those measured, oogonium sizes fell within the size ranges reported for other P. lactucae isolates.
Not all isolates produced sporangia in our experiments; two of the six P. lactucae isolates failed to sporulate or produced only a few sporangia (BPIC 1987, BPIC 1991). Isolate CBS 688.79 (P. taxon castitis) sporulated very poorly.
Taxonomy
Phytophthora cichorii Bertier, H. Brouwer, De Cock & D.E.L. Cooke, sp. nov. — MycoBank MB803102; Fig. 5
Etymology. Named after the host plant, Cichorium intybus (chicory).
This species is heterothallic with amphigynous, often 2-celled, antheridia. Antheridia measure 23.4 ± 3.5 × 19.8 ± 1.7 μm in size. Oogonia are smooth walled and 34.5 ± 4.7 μm long by 30.3 ± 2.7 μm wide; oospores are aplerotic to almost plerotic and on average 27.5 ± 2.4 μm wide. The semi-papillate sporangia are 70.3 ± 19.1 × 42.0 ± 9.4 μm in size. Hyphal swellings measure on average 25.1 ± 5.9 × 19.1 ± 3.5 μm. Chlamydospores were not observed. The minimum temperature for growth is 0 °C, maximum 24 °C and optimum temperature 18–21 °C. Average growth rate at 21 °C on CMA-Oxoid is 0.8 mm/day. No colony pattern was observed on all media examined (V8, PDA and CMA-Oxoid).
Observations on other isolates examined — Colonies on V8, CMA-Oxoid and PDA without a discernible colony pattern. The species grows very poorly on CMA-Oxoid, with diffuse colony edges. On both V8 and PDA medium, colonies have a well-defined edge. Growth on V8 was submerged, with very sparse aerial mycelium. On PDA agar colonies form a thick, cottony, aerial mycelium (Fig. 4a). Growth occurred from 0–24 °C. No growth occurred at 27 °C and higher. Growth rate at 21 °C was 0.6–1.1 mm/d (range of isolate means).
Phytophthora cichorii is heterothallic, antheridia are amphigynous, most antheridia are ‘2-celled’ (Fig. 5a–e). Size ranges for antheridia are 16.4–37.7 × 13.2–26.4 μm, with isolate means of 23.4–27.4 × 19.8 μm. Sizes of oogonia range from 23.7–49.5 × 19.2–45.9 μm, with a range of isolate means of 34.5–37 × 30.3–35.4 μm. Oogonial wall thickness ranges from 0.6–1.8 μm with a range of isolate means of 1.0–1.2 μm. Oogonia are smooth walled and lack ornamentation (Fig. 5a–e). Oospores measure 21.6–42.0 μm diam (av. 27.5 μm).
The size range for the semipapillate sporangia is 30.6–142.9 × 20.7–63.1 μm, with a range of isolate means of 69.0–74.9 × 37.5–42.0 μm. Discharge pore width is 8.9–11.0 μm (range of isolate means). Larger sporangia with distorted shapes were seen but are rare. Sporangia often had thick basal plugs (Fig. 5m). Sporangia are usually persistent on the hyphae, but a few detached sporangia were seen. Sporangia are ovoid to obpyriform, sometimes with distorted shapes or constrictions. Sporangia with elongated necks (Fig. 5j) and bipapillate sporangia (Fig. 5l) occur occasionally. Internal proliferation was not observed. Hyphal swellings occur in water and in rare cases in agar. The size range for hyphal swellings is 16.0–56.5 × 13.5–42.3 μm with a range of isolate means of 25.1–32.2 × 19.1–26.2 μm.
Specimens examined. Great Britain, from root of Cichorium intybus var. foliosum, 1999, J. Scrace, CBS 133815. – The Netherlands, from root of Cichorium intybus var. foliosum, 2004, holotype Herb. CBS H-21127 (dried culture), culture ex-holotype CBS 115029; 2003, CBS 114345; 2004, CBS 115030.
Notes — Phytophthora cichorii can be distinguished from related species by ITS and cox1 sequence data, and morphologically by its heterothallic mating behaviour, and the occurrence of 2-celled antheridia.
Phytophthora dauci Bertier, H. Brouwer & De Cock, sp. nov. — MycoBank MB803103; Fig. 6
Etymology. Named after the host plant, Daucus carota (carrot).
The species is homothallic with paragynous antheridia measuring 16.0 ± 1.7 × 11.6 ± 1.3 μm. Oogonia are globose, often intercalary and 32.3 ± 2.2 × 31.6 ± 2.3 μm in size. Oospores measure on average 27.6 ± 2.2 μm; oogonium walls are smooth or wavy. Sporangia are semipapillate and 58.8 ± 11.6 × 40.1 ± 7.2 μm in size. Hyphal swellings present, on average, 30.8 ± 6.8 × 22.6 ± 5.5 μm. Chlamydospores are found on CMA-CBS agar in low numbers and are thin-walled, often slightly subglobose and measure 31.9 ± 4.1 × 30.8 ± 4.7 μm. Minimum growth temperature 0 °C; maximum 21 °C; optimum 12–21 °C. Average growth rate at 21 °C on CMA-Oxoid is 1.2 mm/d. Colonies on V8, CMA-Oxoid and PDA are without a discernible colony pattern.
Observations on other isolates examined — Colonies on V8, CMA-Oxoid and PDA are without a discernible colony pattern. The species grows very poorly on CMA-Oxoid, with diffuse colony edges. On both V8 and PDA medium, colonies have a well-defined edge. Growth on V8 was submerged, with very sparse aerial mycelium. On PDA agar colonies form a thick, cottony, aerial mycelium (Fig. 4b).
Growth occurred from 0–21 °C. No growth occurred at 24 °C and higher. Growth rate at 21 °C was 0.8–1.0 mm/d (range of isolate means). Antheridia are paragynous and mostly diclinous; size ranges are 12.1–21.9 × 8.5–16.8 μm, with a range of isolate means of 16.0–17.1 × 11.6–12.5 μm.
Intercalary antheridia are quite common. Phytophthora dauci is homothallic. Compared to other clade 8b species, oogonium production is very low. Oogonia were produced in CMA-CBS agar and were sparsely and evenly distributed through the medium. Oogonium production did not increase when paired with A1 or A2 strains. Oogonia often had wavy walls, but smooth walls also occurred (Fig. 6a–e).
The size range for oogonia was 25.3–37.2 × 25–37.2 μm, with a range of means of 31.9–32.3 × 31.2–31.6 μm. Oogonia are often found in intercalary positions in the hyphae, which in many cases could be easily mistaken for laterally attached oogonia, as the subtending and outgrowing hyphae are usually close together on the same side of the oogonium (Fig. 6b). Oogonium wall thickness ranges from 0.6–1.8 μm with a range of isolate means of 1.0–1.1 μm. Oospore diameter ranges from 20.6–30.6 μm, with average diameters of 26.3–27.6 μm for the tested isolates. Oospore wall thickness ranges from 0.6–1.9 μm, with a range of isolate means of 1.1–1.3 μm. Sporangium size ranges from 27.4–89.9 × 24–56.2 μm with a range of isolate means of 52.6–58.8 × 37.2–40.1 μm. Discharge pore width ranges from 6.5–11.7 μm (av. 9.1 and 9.5 μm for the two isolates). Hyphal swellings in agar cultures range in size from 17.1–59.9 × 14.3–41.4 μm, with a range of isolate means of 30.3–30.8 × 22.6–25.1 μm. Chlamydospores are quite rare, they were occasionally found in low numbers in CMA-CBS agar cultures. The size range of chlamydospores is 23.7–57.4 × 20.9–56.0 (range of isolate means 31.9–38.3 × 30.8–37.2).
Specimens examined. Australia, from root of Daucus carota, 2003, CBS 114039. – France, from root of Daucus carota, 2009, D. Breton, holotype CBS H-21128 (dried culture), culture ex-holotype CBS 127102.
Notes — Phytophthora dauci can be distinguished from other related species by its low maximum temperature for growth, and ITS and cox1 sequence data.
Phytophthora lactucae Bertier, H. Brouwer & De Cock, sp. nov. — MycoBank MB803104; Fig. 7
Etymology. Named after the host plant, Lactuca sativa (lettuce).
Phytophthora lactucae is homothallic, antheridia are mostly paragynous, but amphigynous antheridia occur as well. Paragynous antheridia are 16.1 ± 2.1 × 10.5 ± 1.1 μm in size. Antheridia were mostly monoclinous or distantly monoclinous.
The globose oogonia averaged 33.5 ± 4.0 × 33.0 ± 4.0 μm in size. Oospores measure on average 28.2 ± 3.8 μm. Sporangia are semipapillate and ovoid to ellipsoid or irregular in shape and on average 55.5 ± 12.3 × 35.5 ± 7.1 μm in size. Hyphal swellings averaged 27.6 ± 5.4 × 23.8 ± 5.7 μm. The minimum temperature for growth on CMA-Oxoid is 0 °C, and the maximum 24 °C. Optimum temperature is 21 °C. Average growth rate at 21 °C on CMA-Oxoid is 3.1 mm/d. Colonies on V8 had a faint chrysanthemum pattern; no discernible pattern on V8 or CMA-Oxoid.
Observations on other isolates examined — Colony morphology is somewhat variable, colonies on CMA-Oxoid have no discernible pattern. Colonies have sparse aerial or submerged mycelium. Colony patterns on PDA are more variable and range from no discernible pattern to chrysanthemum with dense, velvety mycelium. Colony edges were either sharp or vague. Colonies on V8 have no discernible pattern or a very faint chrysanthemum pattern. Aerial mycelium is sparse and fluffy to velvety (Fig. 4c).
Growth occurred from 0–24 °C. No growth occurred at 27 °C and higher. Growth rate at 21 °C was 0.7–2.1 mm/d (range of isolate means).
Phytophthora lactucae is homothallic. Antheridia are predominantly paragynous, but some amphigynous antheridia occur in all isolates tested. Paragynous antheridia are 10.7–25.2 × 7.5–16.5 μm in size with isolate means of 16.1–17.5 × 9.8–11.9 μm. Oogonia are globose, measuring 22–44.1 × 21–44.1 μm, with a range of isolate means of 33.5–36.4 × 33.0–36.4 μm. Oogonium wall thickness varies from 0.6–2.7 μm with a range of isolate means of 1.3–1.7 μm. Oospore diameter is 19.0–38.7 μm, with isolate means of 27.1–31 μm. Oospore wall thickness varies from 0.5–2.5 μm, with isolate means of 1.1–1.6 μm.
The semipapillate sporangia are mostly ovoid in shape, but ellipsoid, slightly obpyriform and asymmetrical shapes are also found (Fig. 7j–n). Tapered bases are rare. Sizes ranged from 27.6–101.3 × 18.3–53.5 μm, with isolate means of 55.3–61.1 × 35.5–38.5 μm. Discharge pore width ranged from 6–15.5 μm with isolate means of 9.5–10.3 μm.
Hyphal swellings are produced in agar and in water cultures, occur in sparse clusters and are globose to somewhat angular or irregular in shape (Fig. 7f–h). Hyphal swellings are 14.3–49.8 × 10.4–39.3 μm in size with isolate means of 26–31.6 × 21.8–26.4 μm. Chlamydospores are rare and measure 22.0–51.4 × 20.4–52.4 μm, with isolate means of 31.7–37.2 × 29.7–35.3 μm. Only isolates BPIC 1987 and BPIC 1988 produced chlamydospores occasionally in larger numbers, for other isolates only a few chlamydospores were seen. Chlamydospores were thin-walled.
Specimens examined. Greece, Marathon, Attika, from stem of Lactuca sativa cv. Paris Island Cos, 2001, K. Elena, holotype CBS H-21129 (dried culture), culture ex-holotype BPIC 1985; 2001, K. Elena, BPIC 1986; 2002, K. Elena, BPIC 1987; 2002, K. Elena, BPIC 1988; 2003, K. Elena, BPIC 1991; 2003, K. Elena, BPIC 1992.
Notes — Phytophthora lactucae is morphologically similar to other homothallic species from clade 8b; it can be distinguished from P. brassicae by antheridial type, with P. brassicae having predominantly amphigynous antheridia instead of paragynous antheridia; from P. dauci by the lower maximum temperature for growth of that species; from P. primulae by the higher growth rate and maximum temperature for growth of that species. Phytophthora lactucae can also be distinguished from all other clade 8b isolates based on ITS and cox1 sequence data.
Phytophthora taxon castitis — Fig. 8
Etymology. From the Latin ‘castita’ meaning chastity; referring to the sexual dormancy of the isolates.
Two isolates are available for this potential new species, one from strawberry and one from carrot (Table 1). For both isolates, the minimum temperature for growth was 0 °C; the optimum growth temperature was 18–24 °C. No growth occurred at 30 °C or higher. Isolate CBS 688.79 from carrot is characterised by very abundant production of chlamydospores, both in agar and in water cultures (Fig. 8c, e). Chlamydospores are globose and very large, ranging from 29.1–73.2 μm diam with an average of 51.4 μm. Isolate CBS 131246 produced smaller chlamydospores, with an average of 36.4 μm. They can be intercalary or terminal. Hyphal swellings ranged in size from 14.6–61.8 × 12.6–52.1 μm (av. 32.5 × 28.8 μm). Sporangia were only sporadically produced and were 23.6–97.9 × 14.9–49.5 μm in size (av. 50.8–55.4 × 32.6–35.6 μm). In case of CBS 688.79, sporangia were often found at the end of long chains of hyphal swellings, or swollen, constricted hyphae. In many cases, only the very terminal tip of these chains of swellings would contain cytoplasm. During development of these catenulate swellings, in some cases septa are formed, delineating the subtending, empty part of the hypha from the living part as the cytoplasm moves along with the growing tip. This leaves behind a long string of empty hyphal swellings (Fig. 8b, d). These chains of swellings have also been described for P. primulae, and are quite common in that species (Tomlinson 1952). Neither isolate produced oogonia in single culture or when mated with other strains in our study.
Phytophthora taxon parsley — Fig. 9
Two P. primulae-like isolates were available from parsley (Table 1). Minimum growth was 0 °C. Optimum growth was between 15–21 °C. The maximum temperature for growth was 24 °C.
The morphology of these isolates was mostly similar to the P. primulae isolates. A notable difference was the ratio of paragynous to amphigynous anteridia. These were produced in roughly equal amounts in the isolates from parsley, while P. primulae was originally described as having only paragynous antheridia (Tomlinson 1952). Both examined isolates from primrose (CBS 116663 and CBS 114346) did indeed produce mostly paragynous antheridia, but a few amphigynous antheridia were found in both isolates.
Other morphological characters did not differ much between the parsley and P. primulae isolates. Sporangial shapes for the parsley isolates varied from ovoid and ellipsoid to elongated with distorted shapes, often with constrictions (Fig. 9i–n). Sporangia can, but do not always, have a tapered base. The average size for sporangia was 52.8–54.3 × 29.4–30.6 μm with a size range of 25.2–107.7 × 17.8–46.5 μm. Discharge pores of empty sporangia ranged in diameter from 5.2–10.7 μm; averages for the two isolates were 7.9–8.0 μm.
Larger sporangia may occur as larger, already discharged sporangia were found that could no longer be measured accurately. One of the typical features of P. primulae is chains of constricted hyphal swellings, like those that are described above for Phytophthora taxon castitis (Fig. 9f–h). These were rare but were seen on a few occasions and were also observed in one of the parsley isolates by Elena & Grigoriou (2008). Hyphal swellings size ranges were 11.3–61.5 × 10.3–38.7 μm. The average size for CBS 114156 was 24.1 × 19.8 μm and for BPIC 2584 28.1 × 20.7 μm. Chlamydospores were not observed. Oogonia measured 24.3–44.7 × 23.4–42.6 μm with a range of isolate means of 34.4–37.6 × 33.9–37.3 μm. Oospores were 19.4–37.3 μm diam with isolate means of 28.9 μm and 30.9 μm for the two isolates. Both isolates produced amphigynous and paragynous antheridia in roughly equal amounts. Paragynous antheridia were 11.8–30.7 × 8.5–18.3 μm in size. Amphigynous antheridia ranged from 10.2–21.3 × 10.0–16.9 μm. The range of isolate means was 18.1–19.3 × 13.2–14 μm and 15.8–16.5 × 13.6–14.2 μm, respectively for the two aforementioned antheridia types.
DISCUSSION
Our publication provides further evidence for the revised structure of clade 8 as proposed by Grünwald et al. (2011). All isolates discussed in this paper are closely related to the three known clade 8b species P. brassicae, P. porri and P. primulae and together they form a distinct clade from the newly raised subclade 8d. Moreover, a difference in host-plant preference is clear. The species of subclade 8d are all pathogens of woody perennials, while the species in clade 8b are pathogens of herbaceous plants.
The description of P. cichorii, P. dauci and P. lactucae brings the total number of clade 8b species to six. The Phytophthora taxon castitis and the Phytophthora taxon parsley isolates may represent additional new species, but formal description of these taxa would be premature. In the case of the Phytophthora taxon parsley isolates, difference in growth rate, maximum temperature for growth and ratio of amphigynous and paragynous antheridia were found when compared to P. primulae isolates. Elena & Grigoriou (2008) also found evidence for differences in host range. The genetic differences between the parsley isolates and isolates from primrose, however, are small. A study involving more isolates, or a more in-depth study of gene flow between the two groups of isolates may offer more insight into the taxonomic relationship between the Phytophthora taxon parsley and P. primulae isolates. In the case of Phytophthora taxon castitis, there is also genetic and morphological evidence that these isolates could be considered a separate species. Sporulation of this species, however, was very poor. Only a few sporangia were produced, and gametangia were not observed. More data, if possible using fresh isolates from the field, are needed if the species is to be properly described.
Phytophthora cichorii is the only clade 8b species known to have a heterothallic mating system. Heterothallic species produce oospores when isolates of compatible mating type of the same or different species are paired in cultures. Two mating types are known, A1 and A2. Heterothallic species do not require exchange of genetic material with the mating partner for successful formation of oospores, after induction of gametangium formation by mating hormone from the mating partner all oospores may be formed by selfing without further interaction with the other isolate (Ko 1978, Judelson 2007). Phytophthora cichorii gametangia in our study were formed by pairing isolates with compatible isolates of P. capsici using the polycarbonate filter technique developed by Ko (1978). Phytophthora capsici belongs to clade 2, and is unrelated to the clade 8b species. The reason for our use of strain P. capsici CBS 128.23 is that it was found to be a good mating partner for a wide variety of A1 strains from different phylogenetic clades (Brouwer et al., unpubl. data).
Oomycete strains may degenerate when kept under artificial conditions for a prolonged period of time. Strains of clade 8b seem to be especially likely to develop such problems. During this study, several P. brassicae and P. porri strains studied by de Cock et al. (1992) and Man in ’t Veld et al. (2002) were considered. Some of these no longer produced oogonia, or only produced aborted oogonia, often with distorted shapes. The failure of several isolates belonging to the new species presented in this paper to produce oogonia is likely to be caused by this phenomenon and should not be taken as evidence of (partial) sterility of these species, as has been reported for other Phytophthora spp. (Jung et al. 2011).
The lack of gametangium formation by some isolates may also depend on other poorly understood factors. One of the Phytophthora taxon castitis isolates (CBS 688.79) has been studied quite intensively in the past. This isolate failed to produce gametangia when it was originally isolated (Stelfox & Henry 1978), and in an earlier study by de Cock et al. (1992) and as mentioned above, also failed to produce oogonia in our study, both in single culture and in mating tests. Ho (1983), however, could induce oogonium formation by irradiating a young culture with X-rays. The irradiated culture produced oogonia just before the culture completely dried out, after four months of storage.
There are more cases where Phytophthora isolates that appear sterile may produce oogonia under specific conditions. Phytophthora thermophila isolates failed to produce oogonia in single culture or mating test, but one isolate produced oogonia in single culture when flooded with non-sterile soil filtrate (Jung et al. 2011). These cases provide evidence that isolates or species that appear sterile can actually have a functioning, but dormant, sexual system. Gametangium formation in such species may be triggered by internal or external conditions that are poorly understood. The same could be true for other species that are, up to now, considered to be sexually sterile. Elucidation of pathways and genes involved in gametangium formation combined with information from genome sequencing projects may provide another way of determining the sexual status of Phytophthora species.
With the discovery of these new species, some interesting common ecological features of this clade are becoming clear. One is their ability to persist at very low temperatures. All isolates studied have a minimum growth temperature of ≤ 3 °C, most isolates even show some growth at 0 °C. Moreover, optimum and maximum growth temperatures are among the lowest observed in the whole genus (www.q-bank.eu), making them well adapted to infect winter-grown plants.
A second feature is their slow growth. The new species characterized in this study have maximum growth rates between 0.5 and 2.8 mm/d. This slow growth rate makes it very hard to isolate these species from diseased plants, since they are easily overgrown by secondary pathogens. These secondary pathogens can cause a lot of problems, but are not the primary cause. Moreover, secondary pathogens can be mistakenly treated as being the primary cause and as a result, the disease will not be effectively managed. Because these species are not easy to isolate, it is likely that the presence of disease caused by these phytophthoras in the field is underestimated.
Another interesting feature is host specificity. The new species described here have so far only been isolated from their respective host plants, lettuce, chicory and carrot, indicating host plant driven speciation. For P. lactucae, pathogenicity tests confirmed this host specificity (Elena et al. 2006). Speciation influenced by geographic isolation is less likely since for all species in clade 8b, isolates have been found in different countries and in most cases even in different continents. An exception is P. lactucae, for which only isolates from Greece are available. Another culture isolated from lettuce in Australia existed (BRIP 15683; Sitepu & Bumbieris 1981) but this isolate has been lost.
Combining all this information, we can conclude that Phytophthora clade 8b is a clade containing cold-tolerant species that are specifically adapted to cause disease at low temperatures on a range of important agricultural crops, mostly vegetables.
Acknowledgments
This work was funded by a PhD grant of the Agency for Innovation by Science and Technology in Flanders (IWT) given to LB and by the Dutch Ministry of Agriculture, Nature and Food Quality through a FES program. We would like to thank Danielle Breton for kindly providing isolate CBS 127102 and John Scrace for kindly providing isolate CBS 133815 to the authors.
REFERENCES
- Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, et al. 2005. The new higher level classification of Eukaryotes with emphasis on the taxonomy of Protists. Journal of Eukaryotic Microbiology 52: 399–451 [DOI] [PubMed] [Google Scholar]
- Bala K, Robideau GP, Désaulniers N, Cock AWAM de, Lévesque CA. 2010. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia 25: 22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beakes G, Glockling S, Sekimoto S. 2012. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 249: 3–19 [DOI] [PubMed] [Google Scholar]
- Blair JE, Coffey MD, Park SY, Geiser DM, Kang S. 2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45: 266–277 [DOI] [PubMed] [Google Scholar]
- Cock A de, Neuvel A, Bahnweg G, Cock J de, Prell H. 1992. A comparison of morphology, pathogenicity and restriction fragment patterns of mitochondrial DNA among isolates of Phytophthora porri Foister. Netherlands Journal of Plant Pathology 98: 277–289 [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology 30: 17–32 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. 2009. Fungal Biodiversity, CBS Laboratory Manual Series 1. CBS-KNAW Fungal Biodiversity Centre, The Netherlands [Google Scholar]
- Declercq B, Buyten E van, Claeys S, Cap N, Nies J de, et al. 2009. Molecular characterization of Phytophthora porri and closely related species and their pathogenicity on leek (Allium porrum). European Journal of Plant Pathology 127: 341–350 [Google Scholar]
- Declercq B, Devlamynck J, Vleesschauwer D de, Cap N, Nies J de, et al. 2011. New insights in the life cycle and epidemics of Phytophthora porri on leek. Journal of Phytopathology 160: 67–75 [Google Scholar]
- Elena K, Grigoriou A. 2008. First report of Phytophthora primulae in Greece: identification based on morphology and DNA analysis and determination of its host range. Annals of the Benaki Phytopathological Institute 1: 46–54 [Google Scholar]
- Elena K, Grigoriou A, Antonopoulos FD. 2006. Phytophthora porri causing stem rot of lettuce in Greece: First report in Europe. Annals of the Benaki Phytopathological Institute 20: 88–100 [Google Scholar]
- Foister CE. 1931. The White Tip disease of leeks and its causal fungus Phytophthora porri n. sp. Transactions of the Botanical Society of Edinburgh 30: 257–281 [Google Scholar]
- Greslebin AG, Hansen EM, Sutton W. 2007. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentina). Mycological Research 111: 308–316 [DOI] [PubMed] [Google Scholar]
- Griffin MJ, Jones OW. 1977. Phytophthora porri on autumn-sown salad onions. Plant Pathology 26: 149–150 [Google Scholar]
- Grünwald NJ, Werres S, Goss EM, Taylor CR, Fieland VJ. 2011. Phytophthora obscura sp. nov., a new species of the novel Phytophthora subclade 8d. Plant Pathology 61: 610–622 [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704 [DOI] [PubMed] [Google Scholar]
- Ho HH. 1983. Phytophthora porri from stored carrots in Alberta. Mycologia 75: 747–751 [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Judelson HS. 2007. Sexual reproduction in plant pathogenic oomycetes: biology and impact on disease. In: Heitman J, Kronstad JW, Taylor JW, Casselton LA. (eds), Sex in fungi: Molecular determination and evolutionary implications: 445–458 ASM Press, USA [Google Scholar]
- Jung T, Stukely MJC, Hardy GEStJ, White D, Paap T, et al. 2011. Multiple new Phytophthora species from ITS clade 6 associated with natural ecosystems in Australia: evolutionary and ecological implications. Persoonia 26: 13–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura K, Isaka M, Miyagoshi M. 1969. Phytophthora porri Foister, the causal fungus of the leaf blight and bulb rot of scallion, Allium bakeri. Annals of the Phytopathological Society of Japan 35: 55–61 [Google Scholar]
- Klebahn H. 1909. Die neue Zweig- und Knospenkrankheit. In: Krankheiten des Flieders: 18–75 [Google Scholar]
- Ko WH. 1978. Heterothallic Phytophthora: evidence for hormonal regulation of sexual reproduction. Journal of General Microbiology 107: 15–18 [Google Scholar]
- Kroon L, Bakker FT, Bosch GBM van den, Bonants PJM, Flier WG. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology 41: 766–782 [DOI] [PubMed] [Google Scholar]
- Kroon L, Brouwer H, Cock AWAM de, Govers F. 2012. The genus Phytophthora anno 2012. Phytopathology 102: 348–364 [DOI] [PubMed] [Google Scholar]
- Maltitz PM von, Broembsen SL von. 1984. Phytophthora porri on onions in South-Africa. Plant Disease 68: 732 [Google Scholar]
- Man in ’t Veld W, Cock AWAM de, Ilieva E, Levesque A. 2002. Gene flow analysis of Phytophthora porri reveals a new species: Phytophthora brassicae sp. nov. European Journal of Plant Pathology 108: 51–62 [Google Scholar]
- Posada D. 2008. jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution 25: 1253–1256 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Sitepu D, Bumbieris M. 1981. Stem rot of lettuce caused by a low temperature Phytophthora porri in South Australia. Australasian Plant Pathology 10: 59–60 [Google Scholar]
- Stelfox D, Henry AW. 1978. Occurrence of rubbery brown rot of stored carrots in Alberta. Canadian Plant Disease Survey 58: 87–91 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5; Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JA. 1952. Brown core root rot of Primula caused by Phytophthora primulae n. sp. Transactions of the British Mycological Society 35: 221–235 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: A guide to methods and applications: 315–322 Academic Press, San Diego [Google Scholar]